1. Introduction
Long non-coding RNAs (lncRNAs) are defined as transcripts longer than 200 nucleotides, typically lacking an open reading frame and thus not being translated into proteins. LncRNAs show tissue, developmental stage, and disease-specific expression, which make them interesting therapeutic targets [Citation1]. LncRNAs are important regulators of gene expression under physiological and pathological conditions. They control gene expression on various levels via chromatin modification, regulation of transcription, or posttranscriptional processing [Citation2]. LncRNAs were comprehensively studied in the field of cancer in the last decade. Today a huge set of lncRNAs is known to be involved in cancer initiation or progression by affecting all hallmarks of cancer. Dysregulated lncRNAs were found in nearly every type of cancer, driving tumor progression by regulating cancer cell proliferation, migration, invasion, or epithelial to mesenchymal transition (EMT) [Citation3]. Thus, lncRNAs are considered as promising new therapeutic targets in the future. In this editorial we will discuss the potential of CCAT2 as target for future anticancer treatment.
2. Body
2.1. ncRNAs as therapeutic approach
Since the focus of ncRNA research lied on microRNAs in the past, it is not surprising that the first promising therapeutic approaches are based on miRNAs [Citation4]. Mimics for the tumor suppressive miR-34 and miR-16 reached phase I clinical trials in the treatment of liver cancer and mesothelioma. Current delivery systems for RNA-based therapeutics reach from viral vectors over lipid-based and polymeric vectors to inorganic materials [Citation5]. These systems are improved steadily and will be capable of delivering RNAs, targeting all kinds of ncRNAs, to specific cells in the next few years. Thus, the need for promising therapeutic targets among lncRNAs is high.
2.2. CCAT2 in colorectal cancer
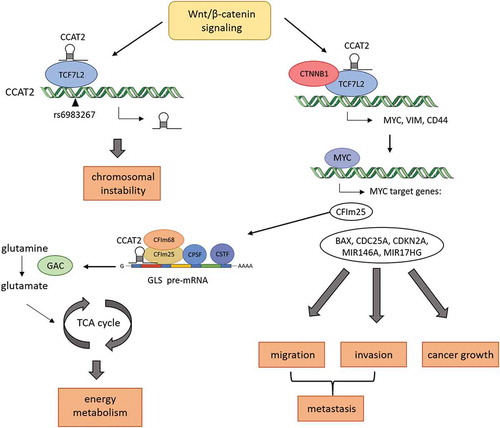
Ling et al., who first described the lncRNA colon cancer associated transcript 2 (CCAT2) in colorectal cancer (CRC), show that its genomic location resides at the chromosomal region 8q24, that is amplified in many solid cancers [Citation6]. This region is highly conserved between different organisms and encompasses the MYC gene, which is a very popular oncogene, several genes for lncRNAs and multiple single nucleotide polymorphisms (SNP) [Citation7]. The SNP rs6983267 overlapping CCAT2 exists in two allelic forms containing either T or G. Previous studies showed that the G allele is associated with increased risk for colorectal, prostate, ovarian, and inflammatory breast cancer [Citation6]. It is also known to contain enhancer elements that have binding affinity to TCF7L2 (transcription factor 7-like 2), which acts as a transcription factor together with CTNNB1 for WNT target genes [Citation6]. Ling et al. further analyzed the importance of CCAT2 in CRC and its underlying mechanism and found that CCAT2 is significantly upregulated in CRC compared to normal mucosal tissue, especially in microsatellite stable samples. Using CCAT2 overexpressing cell lines, they could show that CCAT2 promotes growth under low-adherence conditions, colony formation, and migration in vitro. Additionally, CCAT2 increases tumor growth of xenografts subcutaneously injected in nude mice, as well as the incidence of liver metastasis and the number of metastases after intrasplenic injection. High levels of CCAT2 could also be associated with metastasis occurrence in CRC and breast cancer patients [Citation6]. Interestingly high levels of CCAT2 could induce chromosomal instability, which results in an increased number of polyploid cells. This might be due to increased numbers of centromeres tearing chromosomes apart and leading to abnormal chromosome breaking and fusion. High levels of CCAT2 are associated with upregulated expression of MYC and its downstream target genes BAX, CDC25A, CDKN2A, MIR146A and the miRNA cluster gene MIR17HG in CRC. Knockdown of CCAT2 reduced expression of MYC and its target genes, which supports a CCAT2-dependent regulation of MYC. MYC is a well-known target of Wnt-signaling through binding of TCF7L2 to its promoter region. Ling et al. used a luciferase reporter assay to detect increased WNT activity in CCAT2 overexpressing cells; hence, the expression of WNT target genes like CD44 and vimentin was upregulated. An RNA immunoprecipitation assay confirmed the direct interaction of CCAT2 with TCF7L2, suggesting that CCAT2 increases transcriptional activity of MYC via binding TCF7L2. The authors used LiCl to induce Wnt activity and found an upregulation of CCAT2 expression, which could be abolished via TCF7L2 knockdown. Thus, CCAT2 seems to be regulated via a Wnt – TCF7L2 feedback loop [Citation6].
2.3. Involvement of CCAT2 in cancer energy metabolism
In a recently published study, Redis et al. described a CCAT2-dependent regulation of the cancer energy metabolism via interaction with the splicing process of glutaminase [Citation8]. Overexpression of CCAT2 in CRC cell lines showed higher glucose uptake, lactate secretion, and oxygen consumption in vitro and in vivo by subcutaneous injection into nude mice. Due to the increased metabolic activity, the intermediates of the TCA cycle must be replenished via anaplerotic reactions and glutamine is the main source for that. Glutamine is subsequently deaminated by glutaminase (GLS). Redis et al. showed that glutamine uptake was not altered in CCAT2 overexpressing cells but GLS activity was upregulated. Glycolysis and glutaminolysis are both regulated by MYC, which is a target of CCAT2.Two alternative splicing forms of GLS are known, KGA (glutaminase kidney isoform) and GAC (glutaminase isoform C). GAC has a higher catalytic activity than KGA and therefore may be more relevant for replenishing intermediates of the TCA cycle. GAC expression was upregulated in CCAT2 overexpressing cells and could be shown to promote migration, metastasis, and enhanced energy metabolism. CFIm25 is a subunit of the CFlm complex, which is associated with the GLS splicing. CCAT2 binds CFlm25 and interacts with GLS pre-mRNA, which suggests that CCAT2 enhances the alternative splicing of GLS to GAC. Additionally, MYC acts as transcription factor for CFIm25, which describes another regulatory pathway in this complex network. A complete overview of the above described mechanisms is provided in .
Figure 1. Regulatory network of CCAT2 in colorectal cancer (CRC). CCAT2-induced chromosomal instability in CRC. CCAT2 enhances the expression of MYC, VIM, and CD44 via direct interaction with TCF7L2 (transcription factor 7 like 2) of the Wnt/ß-catenin pathway. CCAT2 itself is regulated via a TCF7L2-dependent feedback pathway. MYC target genes subsequently induce cancer growth, migration, invasion, and metastasis. Another MYC target gene called CFIm25 is part of the CFIm complex, which is involved in splicing of pre-mRNAs. CFIm25 assists in the alternative splicing of glutaminase (GLS) into the C isoform (GAC), which has increased activity for the deamination of glutamine to glutamate. Glutamate replenishes the intermediates of the TCA cycle, boosting energy metabolism.
2.4. The role of CCAT2 in breast cancer
Cai et al. described an upregulation of CCAT2 in breast cancer tissue, which could be associated with poor overall survival [Citation9]. Knockdown of CCAT2 inhibits proliferation and invasion in vitro as well as tumorigenesis in vivo. Additionally, knockdown of CCAT2 reduced ß-catenin levels in nucleus and cytoplasm and reduced the expression of WNT downstream targets including MYC. The ß-catenin inhibitor FH535 could even improve the effect in combination with CCAT2 siRNA. In a following publication, Cai et al. found an interesting connection between CCAT2 and tamoxifen (TAM) resistant breast cancer [Citation10]. TAM was the favored adjuvant first line treatment for ER+breast cancer for several years, due to its induction of apoptosis and necrosis. However, cancer cells developed resistance to TAM after some time and the cancer recurred. CCAT2 is highly upregulated in TAM-resistant cells and knockdown of CCAT2 increased apoptosis of TAM resistant cells after TAM application. It has been shown that the ERK/MAPK pathway is activated in TAM resistant cells. Blockage of this pathway led to downregulation of CCAT2, indicating that the TAM resistance mechanism operates via an ERK/MAPK – CCAT2 – axis.
2.5. CCAT2 in other cancer entities
A study performed by Li et al. could show that CCAT2 knockdown inhibits progression of bladder cancer [Citation11]. CCAT2 is upregulated in bladder cancer and is positively correlated with histological grade and TNM stage. Furthermore, it promotes cell proliferation and migration in bladder cancer cells (in vitro) but inhibits apoptosis. A tetracycline inducible shRNA system targeting CCAT2 reduced proliferation and migration and induced apoptosis, providing a possible treatment strategy. Huang et al. identified CCAT2 upregulation in ccRCC tissue and a positive correlation with poor overall survival, tumor size, and stage [Citation12]. Knockdown of CCAT2 inhibited proliferation, migration, and invasion but induced apoptosis in vitro. Furthermore, CCAT2 shRNA transfected cells showed reduced tumorigenicity in nude mice. The reason for these effects is suppressed WNT activity via downregulation of ß-catenin, c-myc, and cyclin D1. Similar results were obtained by Guo et al. in glioma tissue and cell lines [Citation13]. Another group showed CCAT2 upregulation associated with poor overall survival and TNM stage in hepatocellular carcinoma (HCC) [Citation14]. Knockdown of CCAT2 inhibited cell migration and invasion in vitro. CCAT2 knockdown also reduced expression of EMT markers like vimentin and snail2, whereas the epithelial marker E-cadherin is increased. These results suggest that CCAT2 promotes invasion and migration via snail2-induced EMT. Zheng et al. published data from a prostate cancer study, which matches the findings in HCC [Citation15].
A summary of CCAT2-associated clinical properties and its regulated function across several types of cancer is listed in .
Table 1. List of cancers with described CCAT2 involvement. CCAT2 was upregulated in all of the listed cancers. This table shows which clinical properties are correlated with upregulation of CCAT2 in the given type of cancer. Furthermore, it shows what kind of properties associated with cancer hallmarks are increased or inhibited and which signaling pathway is connected with these changes (OS = overall survival, TNM = tumor node metastasis staging, EMT = epithelial to mesenchymal transition).
3. Expert opinion
Research on lncRNAs is currently booming and it will go on for at least the next decade. The progress in this field seems to follow the path of miRNAs, which was a very influential and successful one so far. The research topics will move more and more to application-based approaches in the next few years. This assumes that delivery systems for anticancer drugs are improved with great emphasis. Pitfalls regarding the drug application and stability as well as specific uptake of drugs by cancer cells are among the prior issues to be solved. The success of anticancer treatment targeting ncRNAs will also depend on the outcome of the miRNA-based approaches currently in clinical trials. Furthermore, lack of conservation among many lncRNAs often hinders the evaluation of physiological function. Some lncRNAs have been described to have both oncogenic and tumor suppressive function in different types of tissue. As summarized in , CCAT2 seems to have mainly oncogenic function via induced Wnt-signaling across several types of cancer, indicating functional conservation in this lncRNA.
CCAT2 is upregulated in almost every type of cancer investigated so far and it shows oncogenic effects (reduced overall survival, advanced tumor stage, etc.) in all these cancers. Nevertheless, detailed insights into its function have been published only for CRC until now. Thus, drug development studies must focus on CRC primarily. Preclinical studies on CCAT2 described so far, focused mainly on xenotransplantation models using cancer cells modified prior to injection into mice. In vivo anticancer treatment strategies based on drugs targeting CCAT2 are currently missing. To strengthen potential applications, follow-up papers with in-depth investigation of initially described phenotypes and treatment approaches in mouse models are necessary.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Ling H, Vincent K, Pichler M, et al. Junk DNA and the long non-coding RNA twist in cancer genetics. Oncogene. 2015;34:5003–5011.
- Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155.
- Rigoutsos I, Lee SK, Nam SY, et al. N-BLR, a primate-specific non-coding transcript leads to colorectal cancer invasion and migration. Genome Biol. 2017;18:98.
- Shah MY, Ferrajoli A, Sood AK, et al. microRNA therapeutics in cancer - an emerging concept. EBioMedicine. 2016;12:34–42.
- Slaby O, Laga R, Sedlacek O. Therapeutic targeting of non-coding RNAs in cancer. Biochem J. 2017;474:4219–4251.
- Ling H, Spizzo R, Atlasi Y, et al. CCAT2, a novel noncoding RNA mapping to 8q24, underlies metastatic progression and chromosomal instability in colon cancer. Genome Res. 2013;23:1446–1461.
- Shen P, Pichler M, Chen M, et al. To Wnt or lose: the missing non-coding linc in colorectal cancer. Int J Mol Sci. 2017;18:2003.
- Redis RS, Vela LE, Lu W, et al. Allele-specific reprogramming of cancer metabolism by the long non-coding RNA CCAT2. Mol Cell. 2016;61:640.
- Cai Y, He J, Zhang D. Long noncoding RNA CCAT2 promotes breast tumor growth by regulating the Wnt signaling pathway. Onco Targets Ther. 2015;8:2657–2664.
- Cai Y, He J, Zhang D. Suppression of long non-coding RNA CCAT2 improves tamoxifen-resistant breast cancer cells’ response to tamoxifen. Molekuliarnaia biologiia. 2016;50:821–827.
- Li J, Zhuang C, Liu Y, et al. shRNA targeting long non-coding RNA CCAT2 controlled by tetracycline-inducible system inhibits progression of bladder cancer cells. Oncotarget. 2016;7:28989–28997.
- Huang JL, Liao Y, Qiu MX, et al. Long non-coding RNA CCAT2 promotes cell proliferation and invasion through regulating Wnt/beta-catenin signaling pathway in clear cell renal cell carcinoma. Tumour Biol. 2017;39:1010428317711314.
- Guo H, Hu G, Yang Q, et al. Knockdown of long non-coding RNA CCAT2 suppressed proliferation and migration of glioma cells. Oncotarget. 2016;7:81806–81814.
- Xu Y, Wang B, Zhang F, et al. Long non-coding RNA CCAT2 is associated with poor prognosis in hepatocellular carcinoma and promotes tumor metastasis by regulating Snail2-mediated epithelial–mesenchymal transition. Onco Targets Ther. 2017;10:1191–1198.
- Zheng J, Zhao S, He X, et al. The up-regulation of long non-coding RNA CCAT2 indicates a poor prognosis for prostate cancer and promotes metastasis by affecting epithelial-mesenchymal transition. Biochem Biophys Res Commun. 2016;480:508–514.
