ABSTRACT
The CD47-Signal regulatory protein α (SIRPα) singling axis acts as a crucial regulator that limits the phagocytic activity of professional phagocytes such as macrophages. Recent studies have demonstrated that the interaction between CD47 on tumor cells and SIRPα on macrophages is implicated in the ability of tumors to evade immunosurveillance. Targeting the CD47-SIRPα interaction is therefore considered to be a promising approach for cancer therapy. Herein, we review some of studies displaying the potential clinical application of antibodies and other modalities that target the CD47-SIRPα interaction. Current limitations of the CD47-SIRPα-targeted immunotherapeutic approaches are also discussed as well as other avenues for future study to improve the current strategies in targeting the CD47-SIRPα signaling axis for cancer immunotherapy.
1. Introduction
Tumor cells influence immune cell functions in the tumor microenvironment via cell-cell contact, metabolites, and secretory factors such as cytokines and growth factors, and have the ability to evade antitumor immune responses. Cell surface molecules, including programmed cell death protein 1 (PD-1), programmed death-ligand 1 (PD-L1), and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), that function as negative regulators of immune activity have been shown to be involved in tumor evasion of immunosurveillance. Specifically, tumor cells are able to dampen the tumor-killing capacity of tumor-specific cytotoxic T cells (CTLs) through the interaction of PD-L1 on tumors to PD-1 on CTLs. By contrast, blockade of the PD-1-PD-L1 interaction using antibodies enhances CTL-mediated antitumor effects and provides impressive therapeutic outcomes in patients with a wide range of solid and hematological cancers. Therefore, a variety of cell surface molecules that negatively regulate adaptive and innate immunity are considered potential targets for cancer immunotherapy.
2. The CD47-SIRPα signaling for regulation of phagocytes
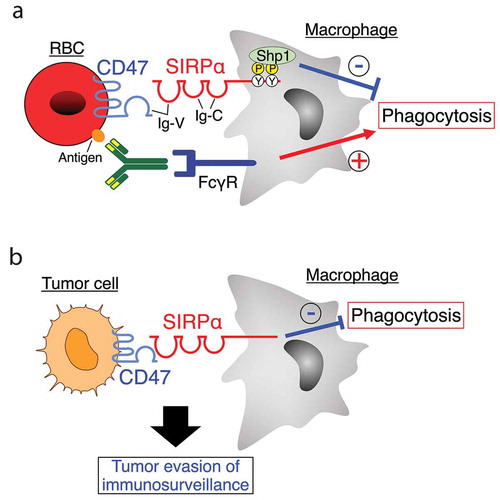
The CD47-SIRPα signaling axis consists of two transmembrane proteins, CD47 and signal regulatory protein α (SIRPα), that interact through their ectodomains [Citation1] (). CD47 is an immunoglobulin (Ig) superfamily protein, with an Ig-V-like extracellular domain, five putative membrane-spanning segments, and a short cytoplasmic tail. By contrast, SIRPα consists of an extracellular region bearing an N-terminal Ig-V-like domain and two Ig-C-domains, and of a cytoplasmic region with two immunoreceptor tyrosine-based inhibition motifs (ITIMs), which are putative phosphorylation sites and binding sites of the protein tyrosine phosphatase Shp1 (). CD47 is expressed in most cells, including red blood cells (RBCs) and platelets, whereas SIRPα is particularly abundant in neurons and myeloid hematopoietic cells, such as macrophages, neutrophils, and dendritic cells (DCs) [Citation1]. Ablation of CD47 in RBCs has been shown to promote phagocytosis of RBCs by splenic macrophages, suggesting that CD47 is a ‘don’t eat me’ signal against macrophages [Citation2]. Moreover, ablation of the SIRPα cytoplasmic region resulted in enhanced macrophage-mediated phagocytosis of antibody-opsonized (but not of non-opsonized) RBCs [Citation3]. Therefore, the interaction of CD47 on RBCs with SIRPα on macrophages likely promotes the recruitment and activation of Shp1 at the tyrosine-phosphorylated ITIMs of SIRPα, resulting in the inhibition of Fcγ receptor (FcγR)-mediated phagocytic activity of macrophages toward antibody-opsonized RBCs [Citation3,Citation4] (). In contrast, wild-type splenic macrophages exhibited significant phagocytotic activity against non-opsonized CD47-deficient RBCs, whereas blockade of the CD47-SIRPα interaction by anti-SIRPα antibodies slightly enhanced the splenic macrophage-mediated phagocytosis of non-opsonized wild-type RBCs (unpublished observation). Thus, CD47 on RBCs likely prevents macrophage-mediated phagocytosis of RBCs via as of yet unknown mechanisms.
Figure 1. The CD47-SIRPα signaling for regulation of phagocytes. (a) CD47 is a transmembrane protein with an immunoglobulin (Ig)-V–like extracellular domain, five putative membrane-spanning segments, and a short cytoplasmic tail. Signal regulatory protein α (SIRPα) consists of an N-terminus Ig-V–like domain and two Ig-C–domains in its extracellular region as well as two immunoreceptor tyrosine-based inhibition motifs (ITIMs) in the cytoplasmic region. The tyrosine-phosphorylated ITIMs of SIRPα bind and thereby activate the protein tyrosine phosphatase Shp1. Ligation of SIRPα on macrophages by CD47 on antibody-opsonized red blood cells (RBC) promotes tyrosine phosphorylation of SIRPα and its subsequent association with Shp1, which in turn inhibits RBC phagocytosis by macrophages induced by the interaction of RBC-bound antibodies with the Fcγ receptor (FcγR) on macrophages. (b) The CD47-SIRPα interaction between tumor cells and macrophages negatively regulates macrophage-mediated phagocytosis of tumor cells, contributing to tumor evasion of immunosurveillance
3. Blockade of CD47 or SIRPα promotes tumor killing by professional phagocytes
Given that CD47-SIRPα signaling prevents macrophage phagocytosis of target cells, this axis is also implicated in tumor immunosurveillance by professional phagocytes such as macrophages () and is a potential target for cancer immunotherapy. Indeed, CD47 was found to be highly expressed in various hematological malignancies and solid tumors relative to their normal counterparts, and such increased expression was correlated with poor prognosis in patients with these malignancies [Citation5–8]. In contrast, blockade of the CD47-SIRPα interaction by anti-CD47 antibodies has been reported to enable the phagocytosis of acute myeloid leukemia (AML) stem cells, but not of CD34+ normal cells, by human macrophages in vitro [Citation5]. It also eliminated AML cells and AML stem cells in human AML stem cell-engrafted mice. Anti-CD47 antibodies and an engineered SIRPα recombinant protein with high binding affinity to CD47 have been shown to enhance macrophage-mediated antibody-dependent cellular phagocytosis (ADCP) of tumor cells opsonized with tumor-targeting antibodies, such as rituximab or trastuzumab, and were able to synergize with these antibodies to eliminate tumor cells in human xenograft tumor models [Citation6–9]. Blocking the CD47-SIRPα interaction using anti-SIRPα antibodies was also observed to enhance tumor-targeting antibody (e.g. rituximab or trastuzumab)-mediated killing of tumor cells by macrophages or neutrophils in vitro and to suppress tumor growth in vivo [Citation10–14]. Moreover, an anti-mouse SIRPα antibody suppressed tumor formation by murine renal cell carcinoma or melanoma cells, both of which are abundant in SIRPα [Citation11]. The inhibitory effect of the anti-mouse SIRPα antibody appeared to be mediated by dual mechanisms: direct induction of ADCP of tumor cells by macrophages, and blockade of CD47-SIRPα signaling, a negative regulator of phagocytosis. Simultaneous blockade of SIRPα and colony-stimulating factor 1 receptor (CSF-1 R) by anti-SIRPα antibody-coated nanoparticles containing a CSF-1 R inhibitor (AK750) also promoted macrophage-mediated phagocytosis of tumors by inhibiting the SIRPα-CD47 interaction and was shown to enhance repolarization of macrophages from a pro-tumorigenic M2 to an antitumorigenic M1 phenotype in the tumor microenvironment [Citation15]. AK750 treatment of murine melanoma and breast cancer models displayed greater antitumor effects than monotherapy with anti-SIRPα antibodies or CSF-1 R inhibitors [Citation15].
In addition to the blockade by antibodies of the CD47-SIRPα interaction, silencing of CD47 expression in non-small cell lung cancer PC-9 cells using liposomes that carry CD47-targeted siRNA was found to promote human monocyte-mediated cytotoxicity toward tumor cells in vitro [Citation16]. These liposomes reduced tumor growth and metastasis in immunocompetent mice transplanted with syngeneic mammary carcinoma cells. A glutaminyl cyclase inhibitor stimulated the antibody-dependent cellular cytotoxicity of neutrophils against tumor cells opsonized with tumor-targeting antibodies in vitro by inhibiting the N-terminal glutamate cyclization of CD47, which is crucial for CD47-SIRPα binding [Citation17]. Therefore, it is feasible that targeting of CD47 or SIRPα contributes to promote phagocyte-mediated tumor killing by inactivating CD47-SIRPα signaling ( and ).
Figure 2. Blockade of CD47 or SIRPα promotes tumor killing by professional phagocytes. The phagocytosis of target tumor cells by professional phagocytes, such as macrophages, is regulated by activating and inhibitory signals, specifically via the interaction of receptors and their ligands. Calreticulin (CRT) and signaling lymphocytic activation molecule family 7 (SLAMF7) on tumor cells have been demonstrated to serve as an activating signal by interacting with low-density lipoprotein receptor-related protein 1 (LRP-1) and SLAMF7 on macrophages, respectively [Citation18,Citation19]. When bound by antibodies, antigens (tumor-associated antigens) on tumor cells are recognized by the Fcγ receptor (FcγR) on macrophages and initiate activating signals. Blockade of CD47 or signal regulatory protein α (SIRPα) by agents such as antibodies disrupts the inhibitory signal mediated by the interaction of CD47 on tumor cells with SIRPα on macrophages, which in turn promotes phagocytosis induced by activating signals. Ab, antibody
![Figure 2. Blockade of CD47 or SIRPα promotes tumor killing by professional phagocytes. The phagocytosis of target tumor cells by professional phagocytes, such as macrophages, is regulated by activating and inhibitory signals, specifically via the interaction of receptors and their ligands. Calreticulin (CRT) and signaling lymphocytic activation molecule family 7 (SLAMF7) on tumor cells have been demonstrated to serve as an activating signal by interacting with low-density lipoprotein receptor-related protein 1 (LRP-1) and SLAMF7 on macrophages, respectively [Citation18,Citation19]. When bound by antibodies, antigens (tumor-associated antigens) on tumor cells are recognized by the Fcγ receptor (FcγR) on macrophages and initiate activating signals. Blockade of CD47 or signal regulatory protein α (SIRPα) by agents such as antibodies disrupts the inhibitory signal mediated by the interaction of CD47 on tumor cells with SIRPα on macrophages, which in turn promotes phagocytosis induced by activating signals. Ab, antibody](/cms/asset/61827c7b-b17c-467c-b103-a596ccccbc3d/iett_a_1811855_f0002_oc.jpg)
Figure 3. Antitumor effect of CD47 or SIRPα blockade involves both innate and adaptive immune responses. Blockade of the interaction of signal regulatory protein α (SIRPα) on macrophages (MΦ) or dendritic cells (DCs) with CD47 on tumor cells using CD47- or SIRPα-targeting agents, such as anti-CD47 or anti-SIRPα antibodies (Abs), promotes the phagocytosis of tumor cells and tumor cell-derived substances by these antigen-presenting cells as well as the consequent priming of tumor-specific cytotoxic T cells [Citation20,Citation21]. Disruption by antibodies to CD47 or SIRPα of the CD47-SIRPα interaction between neutrophils and tumor cells also enhances the neutrophil-mediated killing of antibody-opsonized tumor cells [Citation10]. Moreover, blocking CD47 on tumor-specific cytotoxic T cells and natural killer (NK) cells with anti-CD47 antibodies increases their cellular cytotoxicity against tumor cells [Citation25,Citation28]
![Figure 3. Antitumor effect of CD47 or SIRPα blockade involves both innate and adaptive immune responses. Blockade of the interaction of signal regulatory protein α (SIRPα) on macrophages (MΦ) or dendritic cells (DCs) with CD47 on tumor cells using CD47- or SIRPα-targeting agents, such as anti-CD47 or anti-SIRPα antibodies (Abs), promotes the phagocytosis of tumor cells and tumor cell-derived substances by these antigen-presenting cells as well as the consequent priming of tumor-specific cytotoxic T cells [Citation20,Citation21]. Disruption by antibodies to CD47 or SIRPα of the CD47-SIRPα interaction between neutrophils and tumor cells also enhances the neutrophil-mediated killing of antibody-opsonized tumor cells [Citation10]. Moreover, blocking CD47 on tumor-specific cytotoxic T cells and natural killer (NK) cells with anti-CD47 antibodies increases their cellular cytotoxicity against tumor cells [Citation25,Citation28]](/cms/asset/7de3fe71-ab61-44c1-b56b-ab23ebe35886/iett_a_1811855_f0003_oc.jpg)
The phagocytosis of tumor cells by professional phagocytes, such as macrophages and neutrophils, is regulated by activating and inhibitory signals. The phagocyte-mediated phagocytosis of tumor cells that follows the blocking of the CD47-SIRPα interaction appears to depend on activating signals (). Indeed, an engineered SIRPα protein containing Fc fragments was shown to effectively induce the phagocytosis of several hematopoietic and solid tumor cell lines by macrophages, compared to a corresponding engineered protein without Fc fragments [Citation9]. Anti-CD47 blocking antibodies have also been shown to promote the killing of tumor cells by neutrophils in vitro, whereas antibodies lacking Fc fragments (anti-CD47 F(ab’)2 fragments) did not [Citation10]. These findings suggest that binding of anti-CD47 blocking antibodies or an engineered SIRPα protein containing Fc fragments to activating FcγR on phagocytes is implicated in triggering an activating signal and inducing the killing of tumor cells during CD47-SIRPα blockade. In addition, calreticulin (CRT), which is highly expressed on several human tumor cells, including AML, non-Hodgkin lymphoma, glioblastoma, and bladder cancer cells, interacts with LRP-1 (low-density lipoprotein receptor-related protein 1) on macrophages and has been shown to act as a crucial mediator of an activating signal in the induction of phagocytosis during CD47-SIRPα blockade by anti-CD47 antibodies [Citation18]. Similarly, SLAMF7 (signaling lymphocytic activation molecule family 7) on macrophages was found to initiate an activating signal necessary for efficient phagocytosis of several hematopoietic tumor cells during CD47-SIRPα blockade [Citation19]. As SLAMF7 is a homotypic receptor, the interaction of SLAMF7 on macrophages and tumor cells was likely involved in the induction of phagocytosis [Citation19]. In light of these observations, it would be useful to combine simple blockade of the CD47-SIRPα interaction with the potentiation of activating signals in macrophages in order to improve the therapeutic efficacy of CD47- or SIRPα-targeting agents. This notion is supported by evidence showing that compared to monotherapy with CD47/SIRPα-targeting agents or tumor-targeting antibodies, combination therapy with these agents increases phagocytosis of tumor cells by macrophages in vitro, and promotes the elimination of tumor cells in vivo [Citation6,Citation9,Citation11].
4. The antitumor effects of CD47-SIRPα blockade involve adaptive immunity
Antigen-presenting cells (APCs), including DCs, macrophages, and neutrophils, are thought to play key roles in the adaptive immune response to tumors. Given that the therapeutic efficacy of CD47- or SIRPα-targeting agents was significantly decreased by depletion of CD8+ T cells, but not CD4+ T cells, in immunocompetent mice models of cancer [Citation11,Citation20], CD47-SIRPα blockade could affect tumor-specific CD8+ T-cell activation induced by APCs. Indeed, it was demonstrated that mouse bone marrow-derived macrophages cultured with ovalbumin (OVA)-expressing human colon cancer cells in the presence of anti-human CD47 blocking antibodies promoted OVA-specific CD8+ T-cell proliferation and suppressed priming OVA-specific CD4+ T cells in vitro [Citation21]. Transfer of such macrophages into mice infused with OVA-specific CD8+ T cells also enhanced the proliferation of OVA-specific CD8+ T cells and protected the mice from OVA-expressing tumor challenge. In a separate study, coculture of OVA-specific CD8+ T cells with mouse bone marrow-derived CD11c+ DCs that had been precultured with OVA-expressing mouse tumor cells in combination with anti-mouse CD47 blocking antibodies resulted in the promotion of OVA-specific CD8+ T-cell proliferation and production of interferon gamma (IFNγ) [Citation20]. Similarly, DCs isolated from tumor-bearing immunocompetent mice treated with anti-mouse CD47 antibodies were capable of priming tumor-specific CD8+ T cells. Anti-mouse CD47 blocking antibodies also promoted the production of type I interferons in DCs via the stimulator of interferon genes pathway in tumor-bearing mice, and this increased type I interferon production was crucial for anti-mouse CD47 antibody-dependent enhancement of the priming of tumor-specific CD8+ T cells by DCs and control of tumor growth [Citation20]. These findings suggest that CD47-SIRPα blockade potentiates the phagocytosis of tumor cells and tumor cell-derived substances by macrophages or DCs, thereby enhancing the priming of tumor-specific cytotoxic T cells by these APCs and the tumor-specific cytotoxic T-cell-mediated antitumor responses in vivo ().
Given that CD47-SIRPα blockade exerts its therapeutic efficacy against tumors in part by linking innate and adaptive immune responses to tumors, combined therapy with CD47- or SIRPα-targeting agents and T-cell immune checkpoint inhibitors, such as PD-1 and its ligand PD-L1, may enhance both innate and adaptive antitumor responses. In support of this notion, compared with CD47-SIRPα or PD-1-PD-L1 blockade alone, combined blockade of these axes by specific antibodies, nanobodies, or bispecific agents suppresses the growth of tumors formed by B16 melanoma or MC38 colon cancer cells in syngeneic immunocompetent mice [Citation11,Citation22,Citation23]. In addition, treatment with liposomes containing CD47 and PD-L1 siRNA reduced the expression of CD47 and PD-L1 in tumor cells in vitro and had greater antitumor efficacy in a syngeneic tumor mouse model than treatment with liposomes containing either CD47 or PD-L1 siRNA alone [Citation16]. However, the exact mechanism by which blockade of both the CD47-SIRPα and PD-1-PD-L1 axes reduces the tumor burden in vivo remains to be elucidated.
5. Expert opinion
Preclinical studies have demonstrated that CD47- or SIRPα-targeting agents enable innate and adaptive immunity to eradicate tumor cells in part by blocking the CD47-SIRPα interaction. Recently, in a phase Ib clinical trial, combination therapy with rituximab and Hu5F9-G4 (magrolimab), a therapeutic anti-human CD47 antibody, was shown to be effective in patients with refractory non-Hodgkin lymphoma [Citation24]. Agents that block CD47 or SIRPα are therefore being considered as innate immune checkpoint inhibitors. However, the mechanism by which CD47 or SIRPα blockade exerts antitumor effects in vivo remains to be fully understood. In addition, the effect of CD47- or SIRPα-targeting agents on normal cells and tissues, which may result in adverse effects and reduced therapeutic efficiency in the body, should be considered.
Mechanisms underlying the antitumor effects of CD47 or SIRPα blockade have been investigated with a focus on CD47 on tumors and SIRPα on macrophages, neutrophils, and DCs. However, blockade of CD47 on immune cells, including T cells and natural killer (NK) cells, controls antitumor responses (). For instance, blocking CD47 on tumor-specific cytotoxic T cells with anti-CD47 blocking antibodies, as well as knockdown of CD47 in tumor-specific cytotoxic T cells, increased the T-cell-mediated cytotoxicity against tumor cells in vitro [Citation25]. Blocking antibodies to human CD47 or SIRPα, as well as recombinant human SIRPα ectodomain proteins, was also found to inhibit human T-cell proliferation and production of IFNγ in allogeneic mixed lymphocyte reactions (MLRs) [Citation12,Citation26,Citation27], whereas an anti-human SIRPα antibody, ADU-1805, did not impede them [Citation12]. CD47 blockade in T cells by CD47- or SIRPα-targeting agents may thus have a positive effect on cytotoxic T-cell-mediated cytotoxicity and a negative impact on T-cell priming by APCs. This inhibitory effect of CD47 blockade may also limit the antitumor activity of tumor-specific cytotoxic T cells and raises the issue of whether combination therapy with CD47- or SIRPα-targeting agents and T-cell immune checkpoint inhibitors provides a mechanistic rationale. Similar to T cells, NK cells were found to contribute to the antitumor effect of CD47 blockade. An anti-CD47 blocking antibody, which prevented the binding of CD47 to its ligands, SIRPα and thrombospondin-1, abrogated the thrombospondin-1-mediated inhibitory effect on the proliferation of a human NK cells in vitro [Citation28]. The antibody also suppressed the growth of tumors formed by mouse B16 melanoma cells in syngeneic mice and promoted tumoral NK cell recruitment and expression of granzyme B and IFNγ [Citation28], suggesting that blockade of CD47 induces tumor elimination in part by enhancing NK functions. In addition, CD47 blockade on DCs may also control antitumor immune responses mediated by DCs, given that CD47 on SIRPα+ DCs is crucial for homeostasis of CD4+ DCs and T cells in the spleen [Citation29]. Therefore, further investigation of the impact of CD47 or SIRPα blockade on various types of immune cells in vitro and in vivo would aid elucidation of its limitations and potential in cancer immunotherapy and the benefit of combination therapy with CD47- or SIRPα-targeting agents and other antitumor drugs, in particular immune checkpoint inhibitors.
The adverse effect of CD47-targeting agents on normal cells and tissues may need to be considered. Binding of Hu5F9-G4 or the Fc-fused SIRPα recombinant protein CV1-IgG4 to healthy RBCs promoted macrophage-meditated phagocytosis of RBCs, resulting in mild to severe anemia in cynomolgus monkeys and patients with non-Hodgkin lymphoma or advanced solid tumors [Citation9,Citation24,Citation30,Citation31]. Consistent with the abundant expression of CD47 on platelets, thrombocytopenia was also observed in Hu5F9-G4-treated patients with non-Hodgkin lymphoma [Citation24]. To reduce the on-target effects of CD47-targeting agents on normal cells, bispecific agents have been developed. For example, bispecific antibody targeting of CD47 together with a tumor-associated antigen (TAAs; e.g., CD19, CD20, and mesothelin) selectively bound to CD47 and TAA double-positive tumor cells, rather than CD47 single-positive cells, promoted tumor killing in vitro and in vivo [Citation32–34]. In particular, the CD47/CD19 bispecific antibody NI-1701 resulted in no signs of anemia or thrombocytopenia following single or multiple administrations to cynomolgus monkeys [Citation32]. Similarly, epithelial cell adhesion molecule (EpCAM)-coated cation liposomes containing CD47-targeted siRNA were effectively taken up into EpCAM-expressing tumor cells, silenced CD47 expression, and enhanced the killing activity of phagocytes against the tumor cells in vitro [Citation16]. Liposome treatment induced the attenuation of tumor growth and metastasis in mice bearing EpCAM-positive tumors, and mice that received the liposomes displayed no significant decrease in RBCs and platelets [Citation16]. Further investigation of these bispecific agents is warranted in preclinical and clinical studies. The identification of tumor-associated antigens appropriate for each cancer type that should be combined with CD47 would be needed to further develop bispecific agents with high affinity for tumor cells and few adverse effects. Besides the adverse effects of CD47-targeting agents on normal cells, another issue with the clinical use of CD47-targeting agents, particularly antibodies to human CD47, has been reported. For patients treated with anti-CD47 antibodies but who need RBC transfusion because of their primary malignancies, the anti-CD47 antibody in the plasma of the patients likely interferes with pretransfusion RBC compatibility testing owing to its binding capacity to RBCs [Citation35,Citation36]. Establishing methods to eliminate the effect of antibodies targeting CD47 in plasma samples from patients treated with the antibodies would be necessary to ensure safe RBC transfusion.
SIRPα-targeting agents may trigger adverse effects on the nervous system, given that SIRPα is highly expressed in neuronal and glial cells and is involved in regulating the nervous system [Citation37,Citation38]. Although intraperitoneal injection of anti-mouse SIRPα antibodies in mice induced no apparent behavior abnormality (unpublished observation), the effects of SIRPα-targeting agents on the nervous system should be examined. SIRPα-targeting agents may also cross-react with other SIRP family members, such as SIRPβ and SIRPγ [Citation7,Citation39], and modulate their functions because of the high sequence similarity of their extracellular domains. Several anti-mouse or human SIRPα antibodies that inhibit the CD47-SIRPα interaction were found to cross-react with other SIRP family members [Citation11,Citation12,Citation14]. SIRPβ is present on the cell surface of macrophages and DCs in humans and mice, but unlike SIRPα, it does not bind to CD47 [Citation7,Citation39]. Ligation of mouse SIRPβ with anti-SIRPβ monoclonal antibodies promoted the phagocytosis of antibody-opsonized RBCs by mouse macrophages [Citation40] but suppressed the phagocytic activity of mouse DCs against Leishmania major parasites [Citation41]. SIRPγ is expressed in human T cells and activated NK cells and is considered to be involved in regulating T-cell functions via its interaction with CD47 [Citation42]. Anti-SIRPγ antibodies that inhibit the SIRPγ-CD47 interaction suppressed human CD4+ T-cell proliferation in an allogeneic MLR [Citation42]. Thus, blockade of SIRPβ or SIRPγ may have the potential to inhibit antitumor responses mediated by macrophages, DCs, and T cells. Votes et al. recently generated an anti-human SIRPα antibody, ADU-1805, that cross-reacts with SIRPγ but minimally binds to SIRPβ [Citation12]. The antibody blocked the CD47-SIRPα interaction and promoted the killing of antibody-opsonized tumor cells by macrophages and neutrophils, but did not affect T-cell proliferation in vitro [Citation12]. In addition, the administration of a single dose of the antibody did not induce any signs of anemia, thrombocytopenia, or other toxicities in cynomolgus monkeys [Citation12]. Furthermore, Hazama et al. identified a macrocyclic peptide comprising 15 amino acids that binds to mouse SIRPα, but not to mouse SIRPβ, and promotes macrophage-mediated ADCP against tumor cells by blocking the CD47-SIRPα interaction [Citation43]. The peptide enhanced tumor-targeting antibody-mediated suppression of tumor growth or metastasis in mouse models of cancers, but did not elicit apparent adverse effects such as anemia and thrombocytopenia [Citation43]. Agents with high selectivity and affinity for SIRPα may thus have a favorable hematological safety profile as antitumor drugs compared with CD47-targeting agents, while the results of clinical studies using SIRPα-targeting agents are anticipated. In addition, given that SIRPβ and SIRPγ are implicated in phagocytosis or cytotoxicity in macrophages, DCs, T cells, or NK cells, targeting these molecules potentially constitutes a novel cancer immunotherapy.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
One reviewer is employed by a company has interests in anti-SIRPα antibody R&D.
Peer reviewers on this manuscript have no other relevant financial or other relationships to disclose.
Additional information
Funding
References
- Murata Y, Kotani T, Ohnishi H, et al. The CD47-SIRPα signalling system: its physiological roles and therapeutic application. J Biochem. 2014 Jun;155(6):335–344.
- Oldenborg PA, Zheleznyak A, Fang YF, et al. Role of CD47 as a marker of self on red blood cells. Science. 2000 Jun 16;288(5473):2051–2054.
- Okazawa H, Motegi S, Ohyama N, et al. Negative regulation of phagocytosis in macrophages by the CD47-SHPS-1 system. J Immunol. 2005 Feb 15;174(4):2004–2011.
- Oldenborg PA, Gresham HD, Lindberg FP. CD47-signal regulatory protein α (SIRPα) regulates Fcγ and complement receptor-mediated phagocytosis. J Exp Med. 2001 Apr 2;193(7):855–862.
- Majeti R, Chao MP, Alizadeh AA, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009 Jul 23;138(2):286–299.
- Chao MP, Alizadeh AA, Tang C, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010 Sep 3;142(5):699–713.
- Matlung HL, Szilagyi K, Barclay NA, et al. The CD47-SIRPα signaling axis as an innate immune checkpoint in cancer. Immunol Rev. 2017 Mar;276(1):145–164.
- Weiskopf K. Cancer immunotherapy targeting the CD47/SIRPα axis. Eur J Cancer. 2017 May;76:100–109.
- Weiskopf K, Ring AM, Ho CC, et al. Engineered SIRPα variants as immunotherapeutic adjuvants to anticancer antibodies. Science. 2013 Jul 5;341(6141):88–91.
- Zhao XW, van Beek EM, Schornagel K, et al. CD47-signal regulatory protein-α (SIRPα) interactions form a barrier for antibody-mediated tumor cell destruction. Proc Natl Acad Sci U S A. 2011 Nov 8;108(45):18342–18347.
- Yanagita T, Murata Y, Tanaka D, et al. Anti-SIRPα antibodies as a potential new tool for cancer immunotherapy. JCI Insight. 2017 Jan 12;2(1):e89140.
- Voets E, Parade M, Lutje Hulsik D, et al. Functional characterization of the selective pan-allele anti-SIRPα antibody ADU-1805 that blocks the SIRPα-CD47 innate immune checkpoint. J Immunother Cancer. 2019 Dec 4;7(1):340.
- Ring NG, Herndler-Brandstetter D, Weiskopf K, et al. Anti-SIRPα antibody immunotherapy enhances neutrophil and macrophage antitumor activity. Proc Natl Acad Sci U S A. 2017 Dec 5;114(49):E10578–E10585.
- Sim J, Sockolosky JT, Sangalang E, et al. Discovery of high affinity, pan-allelic, and pan-mammalian reactive antibodies against the myeloid checkpoint receptor SIRPα. MAbs. 2019 Aug/Sep;11(6):1036–1052.
- Kulkarni A, Chandrasekar V, Natarajan SK, et al. A designer self-assembled supramolecule amplifies macrophage immune responses against aggressive cancer. Nat Biomed Eng. 2018 Aug;2(8):589–599.
- Lian S, Xie R, Ye Y, et al. Simultaneous blocking of CD47 and PD-L1 increases innate and adaptive cancer immune responses and cytokine release. EBioMedicine. 2019 Apr;42:281–295.
- Logtenberg MEW, Jansen JHM, Raaben M, et al. Glutaminyl cyclase is an enzymatic modifier of the CD47-SIRPα axis and a target for cancer immunotherapy. Nat Med. 2019 Apr;25(4):612–619.
- Chao MP, Jaiswal S, Weissman-Tsukamoto R, et al. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med. 2010 Dec 22;2(63):63ra94.
- Chen J, Zhong MC, Guo H, et al. SLAMF7 is critical for phagocytosis of haematopoietic tumour cells via Mac-1 integrin. Nature. 2017 Apr 27;544(7651):493–497.
- Liu X, Pu Y, Cron K, et al. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat Med. 2015 Oct;21(10):1209–1215.
- Tseng D, Volkmer JP, Willingham SB, et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci U S A. 2013 Jul 2;110(27):11103–11108.
- Sockolosky JT, Dougan M, Ingram JR, et al. Durable antitumor responses to CD47 blockade require adaptive immune stimulation. Proc Natl Acad Sci U S A. 2016 May 10;113(19):E2646–E2654.
- Liu X, Liu L, Ren Z, et al. Dual targeting of innate and adaptive checkpoints on tumor cells limits immune evasion. Cell Rep. 2018 Aug 21;24(8):2101–2111.
- Advani R, Flinn I, Popplewell L, et al. CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin’s lymphoma. N Engl J Med. 2018 Nov 1;379(18):1711–1721.
- Soto-Pantoja DR, Terabe M, Ghosh A, et al. CD47 in the tumor microenvironment limits cooperation between antitumor T-cell immunity and radiotherapy. Cancer Res. 2014 Dec 1;74(23):6771–6783.
- Waclavicek M, Majdic O, Stulnig T, et al. T cell stimulation via CD47: agonistic and antagonistic effects of CD47 monoclonal antibody 1/1A4. J Immunol. 1997 Dec 1;159(11):5345–5354.
- Seiffert M, Brossart P, Cant C, et al. Signal-regulatory protein α (SIRPα) but not SIRPβ is involved in T-cell activation, binds to CD47 with high affinity, and is expressed on immature CD34+CD38−hematopoietic cells. Blood. 2001 May 1;97(9):2741–2749.
- Nath PR, Pal-Nath D, Mandal A, et al. Natural killer cell recruitment and activation are regulated by CD47 expression in the tumor microenvironment. Cancer Immunol Res. 2019 Sep;7(9):1547–1561.
- Saito Y, Respatika D, Komori S, et al. SIRPα+ dendritic cells regulate homeostasis of fibroblastic reticular cells via TNF receptor ligands in the adult spleen. Proc Natl Acad Sci U S A. 2017 Nov 21;114(47):E10151–E10160.
- Liu J, Wang L, Zhao F, et al. Pre-clinical development of a humanized anti-CD47 antibody with anti-cancer therapeutic potential. PLoS One. 2015 Sep 21;10(9):e0137345.
- Sikic BI, Lakhani N, Patnaik A, et al. First-in-human, first-in-class phase I trial of the anti-CD47 antibody Hu5F9-G4 in patients with advanced cancers. J Clin Oncol. 2019 Apr 20;37(12):946–953.
- Buatois V, Johnson Z, Salgado-Pires S, et al. Preclinical development of a bispecific antibody that safely and effectively targets CD19 and CD47 for the treatment of B-cell lymphoma and leukemia. Mol Cancer Ther. 2018 Aug;17(8):1739–1751.
- Dheilly E, Moine V, Broyer L, et al. Selective blockade of the ubiquitous checkpoint receptor CD47 is enabled by dual-targeting bispecific antibodies. Mol Ther. 2017 Feb 1;25(2):523–533.
- Piccione EC, Juarez S, Liu J, et al. A bispecific antibody targeting CD47 and CD20 selectively binds and eliminates dual antigen expressing lymphoma cells. MAbs. 2015;7(5):946–956.
- Velliquette RW, Aeschlimann J, Kirkegaard J, et al. Monoclonal anti-CD47 interference in red cell and platelet testing. Transfusion. 2019 Feb;59(2):730–737.
- Brierley CK, Staves J, Roberts C, et al. The effects of monoclonal anti-CD47 on RBCs, compatibility testing, and transfusion requirements in refractory acute myeloid leukemia. Transfusion. 2019 Jul;59(7):2248–2254.
- Matozaki T, Murata Y, Okazawa H, et al. Functions and molecular mechanisms of the CD47-SIRPα signalling pathway. Trends Cell Biol. 2009 Feb;19(2):72–80.
- Sato-Hashimoto M, Nozu T, Toriba R, et al. Microglial SIRPα regulates the emergence of CD11c+ microglia and demyelination damage in white matter. Elife. 2019 Mar;26:8.
- van Beek EM, Cochrane F, Barclay AN, et al. Signal regulatory proteins in the immune system. J Immunol. 2005 Dec 15;175(12):7781–7787.
- Hayashi A, Ohnishi H, Okazawa H, et al. Positive regulation of phagocytosis by SIRPβ and its signaling mechanism in macrophages. J Biol Chem. 2004 Jul 9;279(28):29450–29460.
- Lahoud MH, Proietto AI, Gartlan KH, et al. Signal regulatory protein molecules are differentially expressed by CD8− dendritic cells. J Immunol. 2006 Jul 1;177(1):372–382.
- Piccio L, Vermi W, Boles KS, et al. Adhesion of human T cells to antigen-presenting cells through SIRPβ2-CD47 interaction costimulates T-cell proliferation. Blood. 2005 Mar 15;105(6):2421–2427.
- Hazama D, Yin Y, Murata Y, et al. Macrocyclic peptide-mediated blockade of the CD47-SIRPα interaction as a potential cancer immunotherapy. Cell Chem Biol. 2020 Jul;1:S2451–9456(20)30231-2.
