ABSTRACT
Introduction
Chronic pain affects approximatively 30–50% of the population globally. Pathologies such as migraine, diabetic neuropathy, nerve injury and treatment with chemotherapeutic agents, can induce chronic pain. Members of the transient receptor potential (TRP) channels, including the TRP ankyrin 1 (TRPA1), have a major role in pain.
Areas covered
We focus on TRPA1 as a therapeutic target for pain relief. The structure, localization, and activation of the channel and its implication in different pathways to signal pain are described. This paper underlines the role of pharmacological interventions on TRPA1 to reduce pain in numerous pain conditions. We conducted a literature search in PubMed up to and including July 2020.
Expert opinion
Our understanding of the molecular mechanisms underlying the sensitization of central and peripheral nociceptive pathways is limited. Preclinical evidence indicates that, in murine models of pain diseases, numerous mechanisms converge on the pathway that encompasses oxidative stress and Schwann cell TRPA1 to sustain chronic pain. Programs to identify and develop treatments to attenuate TRPA1-mediated chronic pain have emerged from this knowledge. Antagonists explored as a novel class of analgesics have a new and promising target in the TRPA1 expressed by peripheral glial cells.
1. Introduction – Transient Receptor Potential channels
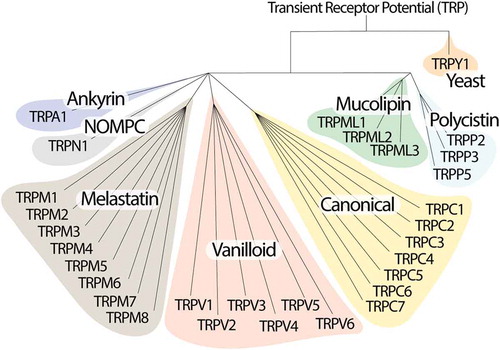
Transient Receptor Potential (TRP) channels belong to a large family of ion channels with more than 50 subtypes, mostly conserved from nematodes to humans, where they play an important role in homeostatic functions [Citation1,Citation2]. TRPs are grouped in 7 subfamilies based on their amino acid sequence homology: canonical or classic (TRPC1-7), vanilloid (TRPV1-6), melastatin (TRPM1-8), non-mechanoreceptor potential C (NOMP-like, TRPN1), long TRP ankyrin (a solitary member is the transmembrane protein 1 [TRPA1]), and the more distant relatives, polycystins (TRPP1-5) and mucolipins (TRPML1-3) [Citation2,Citation3]. An eighth sub-family labeled TRP yeast (TRPY) is not included in either of these groups because of its distant relation with the other channels [Citation4] (). In mammals, the TRP superfamily consists of 6 subfamilies and 28 members that mainly act as nonselective cation permeable channels.
TRPs possess a primary structure that is common to all members, consisting of six transmembrane domains (TM1-TM6), and one hydrophilic loop between TM5 and TM6, forming the pore permeable to monovalent cations and calcium ions [Citation2]. Both the amino (NH2) and carboxy (COOH) -terminal regions, of variable length, face the intracellular side [Citation5]. Whereas the COOH-terminus possesses highly conserved domains, in most TRP channels, the NH2-terminus region is characterized by the presence of ankyrin repeats (33-residue motifs consisting of pairs of antiparallel α-helices connected by β-hairpin motifs), which are associated to different features, such as the regulation of channel assembly in tetramers and interaction with ligands and protein partners [Citation6]. It has been reported that the functional TRP channels are formed by four subunits of a complete TRP structure assembled in a homo- and hetero- tetramer, and heteromultimers [Citation7]. As TRP channels are permeable to calcium and other monovalent ions, they allow an inward cation current that can modulate cell function, mainly intracellular calcium-dependent pathways [Citation6]. In addition, TRP channels can act as integrators of several signaling systems, like those modulated by cell surface receptors, including G protein-coupled receptors (GPCRs) and growth factor receptors [Citation8]. In summary, beyond the structure and cation permeability, TRPs possess an impressive variety of activation modes by mechanical, thermal and chemical stimuli, such as exogenous chemical compounds, lipids, oxidative stress, acids, pheromones, osmolarity, mechanical stimulation, light, and temperature [Citation9]. In addition, regulatory mechanisms, such as transcription, alternative splicing, glycosylation and phosphorylation, and tissue distribution, modulate their activity.
Here, we summarize recent findings regarding the implication of the TRPA1 channel in sustaining pain transmission. To provide a complete overview on the role of TRPA1 as a therapeutic target for chronic pain, a robust literature search was performed on PubMed. To identify the main findings in the field, we used the following search terms: TRPA1, TRPA1 activation, TRPA1 and nociceptive pain, neuropathic pain, cancer pain and migraine.
2. Transient Receptor Potential Ankyrin 1 (TRPA1)
The history of the TRPA1 channel started in 1999 when it was first identified in lung fibroblasts [Citation10], and was named ‘ankyrin-like protein with transmembrane domains protein 1’ (ANKTM1). Only later was it recognized as a TRP member for its homology with other components of the channel superfamily [Citation11]. The human trpa1 gene is located in chromosome 8q13, consists of 27 exons, and spans 55,701 base pairs [Citation10]. One particularity of this receptor is that the trpa1 gene is found in many animal species (vertebrates and invertebrates), including Caenorhabditis elegans, fruit flies, zebrafish, chickens, mice, rats and dogs, in addition to humans. One curiosity is that, while in mammals only one gene for this receptor is present, other animal classes contain multiple TRPA1 homologues (4 in fruit fly, 2 in C. elegans, 2 in zebrafish, 4 in the sea squirt, Ciona intestinalis) [Citation1,Citation12].
Like other TRPs, TRPA1 possesses the NH2 and COOH- terminal parts facing the intracellular space. Its name derives from an elongated number (14–19) of an ankyrin repeat region within the NH2-terminal. The receptor is a nonselective channel permeable to calcium, sodium, and potassium, with a much higher permeability to calcium compared to other TRPs. TRPA1 functions as a sensor of cell damage signals and takes part in many diseases. TRPA1 is implicated in cold sensations related to painful stimuli [Citation13]. Several studies modulating TRPA1 activity have shown that this receptor is involved in inflammatory and immune responses, and in the conversion of physical and chemical stimuli in irritative (itching) or pain sensations [Citation14–16].
2.1. TRPA1 structure
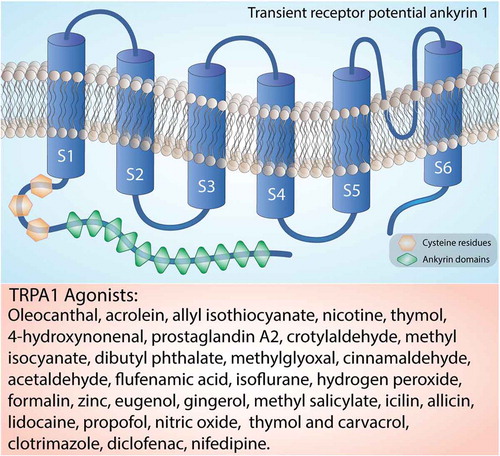
The structure of human TRPA1 has been determined using cryoelectron microscopy [Citation17]. The mostly conserved COOH-terminal region contains positively charged domains for interaction with negatively charged ligands, including inorganic polyphosphates or phosphoinositides, able to modulate TRPA1 activation [Citation18]. As mentioned, on the NH2-terminal portion, which constitutes more than half of the protein (around 64%) [Citation19], TRPA1 possesses an elongated ankyrin repeat domain (ARD 14–18) with the role of controlling the protein-protein interaction, as well as the channel insertion and regulation in the plasma membrane [Citation20,Citation21]. The initial region of the NH2-terminal portion of the protein, located before the first transmembrane segment, is responsible for allosteric regulation of TRPA1 and consists of a prominent ankyrin repeat domain (amino acids 1–639) and a linker (amino acids 640–720), formed by a β-hairpin loop followed by two α-helix and the pre-S1-helix, which connects the ankyrin repeats to the first transmembrane segment [Citation22,Citation23]. The NH2-terminal portion also contains a large number of cysteine and lysine residues that are important for forming disulfide bridges, which are critical for changing the structure upon receptor-agonist binding and are targets for electrophilic activators of TRPA1 [Citation13]. TRPA1 also contains a calcium-binding EF-hand domain, commonly found in other calcium-interacting proteins [Citation13]. Intracellular calcium ions can directly activate the channel, or potentiate agonist-induced responses, probably through this mechanism [Citation13] ().
Figure 2. Structure of the TRPA1 channel. The TRPA1 channel is a homotetramer with each subunit containing six transmembrane helices, a series of ankyrin repeats, and intracellular NH2- and COOH-termini. The transmembrane helices are labeled S1-S6. Orange box reports a list of the main TRPA1 agonists
2.2. TRPA1 localization
Initially, TRPA1 was described as a nociceptive channel with a plentiful presence in subpopulations of primary sensory neurons of the dorsal root (DRG), vagal (VG) and trigeminal (TG) ganglia, and responding to noxious compounds and low temperatures [Citation11,Citation24]. TRPA1 is mainly expressed in unmyelinated C-fibers and thinly myelinated Aδ-fibers, and only occasionally large myelinated fibers [Citation11]. The expression can vary between species. At least 25% of the TRPA1 expressing neurons are peptidergic, and therefore able to release substance P (SP) and the calcitonin gene-related peptide (CGRP). However, the colocalization of TRPA1 with non-peptidergic neuron markers, such as isolectin B4 (IB4), the purinergic P2X3 receptor, and the Na(V)1.8 channel, has also been observed [Citation25-26–Citation27]. TRPA1 is present in 30–50% of TRPV1-expressing neurons and rarely exists in neurons which do not express TRPV1 [Citation28]. There is also evidence for a TRPV1-TRPA1 interaction, which suggests that the two proteins might form a heteromeric channel [Citation29].
Although TRPA1 is mainly located in nociceptive neurons of the peripheral nervous system (PNS), it is also found at different sites of the central nervous system (CNS) [Citation30]. Studies in rat brain have shown TRPA1 protein expression in the hippocampus, brainstem, olfactory bulb, striatum and amygdala [Citation31-36–Citation37]. In humans, TRPA1 has been detected in the cortex, caudate nucleus, putamen, globus pallidus, substantia nigra, hippocampus, cerebellum, amygdala, and hypothalamus [Citation38]. However, the role of TRPA1 in the CNS is still unknown. TRPA1 expression has also been reported in chick [Citation39], mouse, and human retina [Citation40]. Emerging evidence has identified TRPA1 in a variety of additional extra-neuronal tissues where it contributes to different regulatory and proinflammatory pathways. These include the mouse inner ear and the organ of Corti [Citation41], rat vascular endothelial cells [Citation42], enterochromaffin cells of human and rat colon, cells of the respiratory tract [Citation43,Citation44], human keratinocytes and melanocytes, human synoviocytes, and human dental pulp and gingival fibroblasts [Citation45]. The TRPA1 receptor can also be found in epithelial cells, mast cells, and pancreatic β cells [Citation46-52–Citation53]. More recent studies have reported the presence of TRPA1 in glial cells, such as astrocytes [Citation54], oligodendrocytes [Citation55] and Schwann cells [Citation56,Citation57]. It is worth noting that the presence of different TRPs, including TRPA1, has been observed in tumor tissues, including breast, pancreas, lung, liver, colon and gastroesophageal cancer, and has been proposed as a biomarker in cancer diagnosis and prognosis [Citation58].
2.3. Agonists and modes of activation of TRPA1
TRPA1 agonists can be divided into two main groups: thiol-reactive electrophiles that modify the channel covalently, and compounds that modulate the channel differently. The first group includes a number of naturally occurring molecules often found in alimentary sources, including herbs and spices, such as allyl isothiocyanate (AITC) [Citation14,Citation59], allicin [Citation24,Citation60], and cinnamaldehyde [Citation14]. Additional agonists characterized by remarkable chemical heterogeneity include volatile irritants, such as acrolein and crotonaldehyde [Citation61,Citation62], chemicals of industrial origin [Citation63-64–Citation65], general anesthetics (e.g. isoflurane [Citation66], lidocaine [Citation67], propofol [Citation68]), and laboratory chemicals (e.g. formalin [Citation69-70–Citation71]). Endogenous activators include oxidative stress components (e.g. nitric oxide [Citation72], zinc [Citation73,Citation74], hydrogen peroxide [Citation63-64–Citation65], and methylglyoxal [Citation22,Citation75,Citation76]). These TRPA1 agonists covalently modify the channel after association. A second group of TRPA1 agonists includes non-reactive compounds which are unable to modify the channel covalently and include plant origin, such as menthol [Citation77], thymol and carvacrol [Citation78,Citation79]. Additional non-covalent activators are nicotine [Citation80] and Δ9-tetrahydrocannabinol [Citation59,Citation81], and some common drugs, such as clotrimazole [Citation82], nifedipine [Citation83], and non-steroidal anti-inflammatory drugs, such as diclofenac [Citation84] and acyl-glucuronide ibuprofen [Citation85].
In addition, agonists of GPCRs, such as prostaglandins, bradykinin, histamine, and trypsin can induce intracellular changes that directly or indirectly result in TRPA1 activation [Citation13]. TRP channel targeting stimulates calcium-dependent pathways, as these receptors allow the divalent cation influx from the extracellular space [Citation2]. On the other hand, activation of phospholipase C (PLC) coupled receptors stimulates TRP channels, including TRPA1 [Citation86]. Intracellular calcium, released by the endoplasmic reticulum, is an essential TRPA1 co-activator [Citation59,Citation87-90–Citation91]. Intracellular pathways involving protein kinase A (PKA) have been shown to increase TRPA1 expression in the plasma membrane [Citation92]. In particular, the receptor insertion on the membrane is regulated by PKA phosphorylation by increasing its expression in the lipidic layer [Citation92]. Calcium release from intracellular stores and calcium influx from TRPV1 targeting also results in TRPA1 activation [Citation13]. Ischemia usually increases proton concentrations, promoting TRPA1 activation and the ensuing increase in intracellular calcium concentration [Citation55].
2.4. TRPA1 antagonists
The list of TRPA1 naturally occurring antagonists, which can be more appropriately labeled as partial agonists or weak inhibitors, includes camphor [Citation93], camphor laurel, basil, wormwood, rosemary, 1,8-cineole, lemon eucalyptus, sage [Citation5], and menthol, which, depending on the concentration, can either activate or inhibit the channel [Citation77]. Gadolinium, amiloride, gentamicin and ruthenium red are nonselective TRPA1 antagonists [Citation47].
The search for more selective TRPA1 channel antagonists has led to the development of molecules with optimization of existing scaffolds. Xanthine derivatives, including HC-030031, were the first identified as TRPA1 antagonists [Citation71]. HC-030031 has proven effective in vivo at 100 and 300 mg/kg [Citation71]. Later, other xanthinic derivatives were recognized as TRPA1 selective antagonists, including Chembridge-5861528, which is about 10 times more potent than HC-030031 [Citation94,Citation95]. Another antagonist is the oxime derivative, A-967079 (WO/2009/089082) [Citation96]. A wide range of trichloro(sulfanyl)ethyl benzamides has also been produced and made available as potent TRPA1 antagonists, showing activity in human, but not in rat, TRPA1 [Citation97]. More recently, additional TRPA1 antagonists have been reported. These include GRC 17536 [Citation98], and in 2010, a series of heterocyclic amides revealed properties of TRPA1 inhibition (2010/141805 A1) [Citation99]. However, the promising compounds in this series suffer from poor solubility.
In 2011–2012, powerful antagonists of the TRPA1 channel were found among the derivatives of decalin (WO/2011/043954) and derivatives of proline (WO/2012/152983 A1) [Citation100,Citation101]. In 2013 and 2017, heterocyclic amides were also tested as TRPA1 antagonists (WO2013/108857 A1, WO/2017/135462 A1) [Citation102]. Recently, some arylamide derivatives, carbamate compounds, azabenzofuran and 5-(2-(trifluoromethyl) phenyl)-indazoles, have been reported as TRPA1 antagonists with potency, metabolic stability, and considerable solubility [Citation103,Citation104]. In 2017, bicyclic heterocycle derivatives were patented as TRPA1 receptor antagonists (WO/2017/060488 A1). In 2018, the possibility of using ophthalmic preparations for the dispensing of eye drops for the treatment of eye diseases using TRPA1 antagonists was reported (WO/2018/009717 A1).
Among the various antagonists that have shown selectively toward TRPA1, only five have been tested in clinical trials for the treatment of pain or other conditions. These include: GRC 17536 (Glenmark Pharmaceuticals) [Citation105], which, after a positive press release on its efficacy in painful diabetic neuropathy, has been the object of further publications; CB-189625 (Hydra Biosciences in partnership with Cubist Pharmaceuticals) was advanced into a phase-1 clinical trial for acute surgical pain [Citation106], however, the study was discontinued due to disappointing pharmacokinetics; HX-100 (Hydra Biosciences) was tested for painful diabetic neuropathy and allergic asthma [Citation107], however, no results have been reported so far; ODM-108 (Orion Pharma) was investigated for the treatment of neuropathic pain, however, the study failed, apparently for the poor pharmacodynamic features of the compound [Citation108]; GDC-0334 (Genentech/Roche) which, developed for the treatment of asthma, has been tested in a phase-1 trial [Citation109] but further development of the molecule was halted in 2019. A summary of clinical studies for TRPA1 antagonists is reported in .
Table 1. Status summary of TRPA1 antagonists
3. Classifying pain
Pain may be classified according to different criteria based on the pathophysiological mechanism (nociceptive, inflammatory or pathological pain) [Citation110-112–Citation113], duration (acute, chronic, episodic, or end of dose pain), etiology (malignant or nonmalignant) or the anatomical position [Citation114]. Pain elicited by a physiological protective system from noxious stimuli is called nociceptive pain. Nociceptive pain is a short-lived condition generated by the body in response to a potentially harmful environmental stimulus; this generates reflexes that protect the individual from potential damage. Nociceptive pain is divided into two categories: somatic nociceptive pain, which is well localized usually at the level of the dermis, described as pungent, lacerating and burning, and visceral pain, which usually arises as a diffuse and poorly defined sensation perceived in the midline of the body, at the lower sternum, or upper abdomen [Citation115].
Pain perception resulting from harmful cellular damage following surgical, traumatic, or disease-related injuries is defined as inflammatory pain. Inflammatory pain is adaptive and protective and is mainly caused by activation of the immune system following tissue injury or infection. In general, the intensity of pain is proportional to the extent of tissue damage and the release of inflammatory mediators, which are critical to initiate and sustain pain. Pathological pain does not occur as a symptom of a disorder, but rather represents a disease state of the nervous system, which can occur after damage to the nervous system (i.e. neuropathic pain) or in such conditions in which there is no specific damage or inflammation (i.e. dysfunctional pain). Acute pain has a short duration, is a consequence of tissue injuries, and tends to reduce in intensity over time. Chronic pain is more prolonged (it usually lasts at least 12 weeks), can be acute or dull, presents as a burning or pain sensation in the affected areas, can be present in all parts of the body, can be continuous or intermittent, can be difficult to diagnose, causes are not always clear, and it can greatly affect the patient’s quality of life [Citation116].
A variety of receptors in sensory neurons directly encode harmful stimuli that generate propagated action potentials to signal pain. These stimuli consist of physical (mechanical forces, temperature variations) and exogenous (irritants of vegetal origin such as capsaicin, menthol or isothiocyanates) and endogenous (protons, prostaglandins, kinins, and many others) chemical agents. Multi damage events may act indirectly on the nociceptors, via the release of inflammatory substances and the accumulation of inflammatory cells, which favor nociceptor sensitization to any stimulus [Citation117]. In this context, TRP channels have been identified as potential targets and multimodal sensors of irritative and pain signals. In addition, TRP channels may cooperate to encode a specific pain signal, as in the case of noxious heat, which requires the simultaneous contribution of TRPV1, TRPM3, and TRPA1 [Citation118]. More importantly, robust evidence indicates TRPA1 as a major player in mediating the prolonged hypersensitivity to thermal, chemical, and mechanical stimuli detected in models of nociceptive, inflammatory and neuropathic pain [Citation11,Citation15,Citation119-121–Citation122].
4. TRPA1 and nociceptive pain
TRPA1 activators encompass an unprecedented series of inflammatory agents and mediators, including protons, nucleotides and nucleosides, enzymes (proteases), fatty acid derivatives (prostaglandins), biogenic amines (histamine, norepinephrine and serotonin), cytokines, chemokines, neurotrophins, and other peptides (bradykinin, endothelin). Nociceptors have an intrinsic activation threshold, and normally harmful stimuli of either mechanical, thermal or chemical origin must overcome this threshold to initiate action potentials to signal pain [Citation117]. The involvement of TRPA1 in inflammatory hypersensitivity was initially suggested by the observation that TRPA1, via a PLC/calcium signaling pathway, contributes to bradykinin excitatory effects [Citation14], thus representing an essential downstream target to induce nociceptor hypersensitivity [Citation123].
The role of TRPA1 in inflammatory nociception has been described in a seminal work showing that either pharmacological blockade or gene deletion of the channel markedly reduced both the first and second phases of the nociceptive response induced by formalin in rat and mouse paw [Citation71]. In addition to the acute nociceptive response, TRPA1 is involved in mechanical and thermal (cold) hypersensitivity, even days or weeks after the administration of the harmful stimulus, when damaging agents are removed and inflammation presumably resolved. Indeed, several studies have documented that acute pharmacological inhibition, using various TRPA1 inhibitors or TRPA1 gene deletion, reduced both the mechanical and cold hypersensitivity associated with a persistent inflammation in models of osteoarthritis, induced by complete Freund adjuvant, carrageenan, monosodium iodoacetate, and monosodium urate [Citation124-131–Citation132].
5. TRPA1 and neuropathic pain
Unlike inflammatory pain, neuropathic pain is not associated with an overt tissue inflammatory condition, but rather is caused by a lesion or dysfunction of the somatosensory system, including peripheral nerve fibers (Aβ, Aδ and C fibers), and the ensuing altered transmission of sensory signals to the spinal cord and brain. Neuropathic pain affects 7–10% of the general population [Citation133], and its incidence is likely to increase due to the rising age of the global population. Multiple causes of neuropathic pain have been described in the central and peripheral nervous systems, including brain and spinal cord injury, neurodegenerative diseases, multiple sclerosis, diabetes, HIV infection, leprosy, chemotherapeutics, and alcohol abuse [Citation134-141–Citation142].
Several lines of evidence support TRPA1 implication in models of neuropathic pain, with the first report obtained by spinal nerve ligation in mice [Citation143]. Downregulation of TRPA1 expression in L5/DRG, and upregulation in L4/DRG, suggested that a compensatory mechanism could occur after nerve injury. Subsequent studies showed a similar expression pattern using other nerve injury models, such as sciatic nerve injury by chronic constriction or transection [Citation144-145–Citation146]. All these data revealed a possible analgesic strategy by blocking/inhibiting TRPA1. Some TRPA1 antagonists given by different routes of administration have been used to reduce sensory hypersensitivity in rodent experimental models. Systemic blockade of TRPA1 decreased allodynia evoked by peripheral nerve injury [Citation147], and intrathecal injection of TRPA1 antagonist attenuated the hypersensitivity in animals with a spinal nerve ligation model [Citation148]. Further evidence has been obtained by genetic channel deletion [Citation149,Citation150], which inhibited mechanical allodynia [Citation151], thus strengthening the hypothesis that TRPA1 plays a major role in mechanical hypersensitivity following nerve damage. In addition, TRPA1 inhibition may decrease postoperative pain, as the administration of a channel antagonist into the injured surgical area attenuated mechanical allodynia. Furthermore, an implication of CNS TRPA1 has been proposed, given that mechanical allodynia decreased after spinal administration of channel antagonists [Citation94].
TRPA1 has been shown to contribute to peripheral neuropathic pain, not caused by mechanical trauma, such as the pain associated with diabetic neuropathy [Citation95,Citation152]. Peripheral neuropathy is a common characteristic complication of diabetes mellitus, thus causing cutaneous pain, often of burning quality. TRPA1 antagonism has been reported to reduce mechanical allodynia and hypersensitivity in a rodent model of streptozotocin-induced diabetes [Citation153]. Furthermore, oxidative stress and glucose metabolism byproducts, such as 4-hydroxy-2-nonenal (4-HNE) and methylglyoxal, largely increased in diabetes, can activate TRPA1, thus contributing to hyperalgesia [Citation22,Citation154,Citation155].
A common adverse-effect of anticancer drugs is a chemotherapeutic-induced peripheral neuropathy (CIPN), the symptoms of which usually encompass paresthesia and dysesthesia to the extremities, spontaneous pain, and mechanical and thermal hypersensitivity, provoking a prolonged and disabling condition [Citation156]. TRPA1 is markedly implicated in mechanical and cold allodynia evoked in mouse models of CIPN and therefore represents a potential therapeutic target for the human condition. Anticancer drugs that have been reported to directly target TRPA1 to elicit mechanical and thermal hypersensitivity or nociceptive responses are oxaliplatin, paclitaxel, bortezomib, and the aromatase inhibitors exemestane, letrozole and anastrozole [Citation127,Citation157,Citation158].
6. TRPA1 and cancer pain
Pain is a common and devastating symptom of cancer, which often impairs the quality of life more than cancer itself, and affects about 70–90% of cancer patients [Citation159]. As the cancer grows, tumor microenvironment composed by cancer cells, inflammatory and immune cells, and structural cells, progressively infiltrates the neighboring tissues and sends specific chemical signals, thus contributing to the development of a proalgesic neural environment [Citation160,Citation161]. Remodeling of the sensory fibers has been speculated to contribute to the generation of the spontaneous breakthrough episodes often observed in cancer patients, as similar neuroma-like structures have been reported in conditions characterized by spontaneous ectopic pain episodes, such as complex regional pain syndrome [Citation162,Citation163]. A very recent observation showed that oxidative stress and TRPA1 are essential in mediating mechanical and cold allodynia in a mouse model of metastatic cancer pain evoked by injection of melanoma cells in the hind paw as genetic deletion/pharmacological antagonism of TRPA1 or antioxidants attenuated pain-like responses [Citation164].
7. TRPA1 and dysfunctional pain
Dysfunctional pain, which falls into the category of chronic pain, has been proposed recently (2010) [Citation113,Citation163-164–Citation165]. Several clinical disorders have been associated with the onset of pain defined as dysfunctional, including primary headaches, fibromyalgia, temporomandibular disorder, back pain, interstitial cystitis, pelvic pain, and irritable bowel syndrome. Compared to other categories of pain, dysfunctional pain does not seem to have a well-defined cause, thus complicating diagnosis and treatment. One common feature of dysfunctional pain is the presence of unexplained pain as the main symptom which, however, significantly reduces the patient’s quality of life. Currently available pharmacological treatments for dysfunctional pain conditions are often not satisfactory, thus highlighting the need for the discovery of new therapeutic targets.
Several lines of evidence support the implication of TRPA1 in models of conditions characterized by dysfunctional pain. Pharmacological inhibition of the TRPA1 channel or its deletion from primary sensory neurons have been shown to switch off periorbital mechanical allodynia in a mouse model of migraine induced by glyceryl trinitrate administration [Citation166]. In a model of temporomandibular disorder induced by masseter muscle inflammation, TRPA1 and TRPV1 inhibition was reported to mitigate spontaneous pain [Citation167]. Increased levels of methylglyoxal were found in the plasma of patients suffering from low back pain (LBP). In a rat model of LBP, methylglyoxal accumulation was reported in dorsal root ganglia [Citation168], and TRPA1 can be implicated in this model as methylglyoxal is a channel agonist [Citation75].
Epigenetic regulation of TRPA1 [Citation169] seems to contribute to mechanical pain sensitivities in some multisomatoform disorders, including fibromyalgia, which is characterized by distressing and functionally disabling somatic symptoms with chronic pain as the most frequent and clinically relevant complaint. The gastrointestinal tract is among the most studied internal organs with regards to the role of TRPA1 in visceral inflammation and nociception. TRPA1 was found to mediate mechanical hypersensitivity to colonic distension in chemically induced colitis [Citation170,Citation171]. The dextran sulfate sodium (DSS)-evoked inflammatory bowel disease in rodents has been associated with sensitization of colonic TRPA1, which, promoting the release of SP, sustains inflammation [Citation172]. More recently, TRPA1 implication has been reported in the somatic mechanical hypersensitivity associated with DSS-induced colitis [Citation173]. Furthermore, histamine sensitizes TRPA1 and TRPV4, thus promoting visceral pain in patients suffering from irritable bowel syndrome [Citation174]. TRPA1 implication in the visceral pain associated to inflammatory conditions may suggest a major role for the channel in irritable bowel disease, thus representing an important candidate for the development of new treatments for this condition.
8. TRPA1 and migraine pain
Migraine pain is a major medical concern, due to the large prevalence of the disease in the general population. Although the underlying pathway responsible for migraine pain remains unknown, the introduction of small molecules that antagonize the CGRP receptor (CGRP-R) and monoclonal antibodies against CGRP or CGRP-R in acute and prophylactic migraine treatment indicates that the neuropeptide plays a major role in this disease [Citation175,Citation176]. The component of neurogenic inflammation produced by CGRP released from the terminals of trigeminal neurons seems to be one of the main mechanisms of migraine headaches. As TRPA1 stimulation may cause CGRP release, many migraine triggers activate TRPA1, and some drugs already used for migraine treatment can desensitize or inhibit TRPA1 [Citation177], the channel may be considered a therapeutic target for migraine. Exposure to environmental and smoke irritants, such as acrolein and other chemicals known as TRPA1 agonists, elicits CGRP release and neurogenic inflammation in cranial districts [Citation178]. All these effects can be inhibited by CGRP or TRPA1 antagonists [Citation178]. Glyceryl trinitrate, which is known to elicit migraine attacks in patients [Citation179] releases nitric oxide (NO), which induces pain-like responses via TRPA1 [Citation72]. Glyceryl trinitrate elicits periorbital allodynia via TRPA1 NOX1/2 activation within the soma of trigeminal nociceptors and the ensuing ROS and CGRP release [Citation166]. In addition, hydrogen sulfide, another gaseous stimulant of TRPA1, might contribute to the mechanism of migraine [Citation180].
9. Glial cell TRPA1 and pain
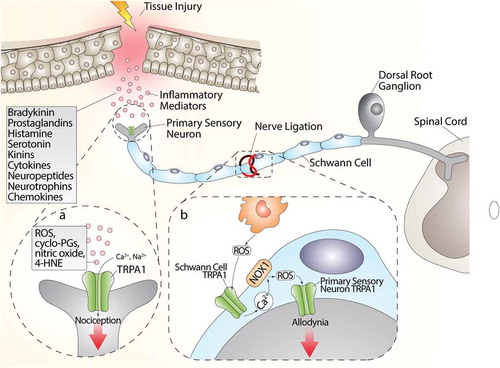
While there is broad evidence that direct TRPA1 stimulation in nociceptors mediates acute pain responses, recent findings have shifted the focus to the TRPA1 expressed in glial cells as the critical factor to sustain chronic pain. It has long been known that neuropathic pain caused by nerve injury (Wallerian degeneration) is associated with a robust infiltration of macrophages within the damaged nerve trunk [Citation56,Citation181-182–Citation183]. Findings obtained in a mouse model of type-II trigeminal neuralgia produced by infraorbital nerve constriction [Citation183] have proposed that nociceptor TRPA1, targeted by the oxidative burst generated invading macrophages, conveys the pain signal to the brain. However, a subsequent study in a different mouse model of neuropathic pain (partial sciatic nerve ligation) provided evidence that macrophage-dependent oxidative stress does not directly target TRPA1 in nociceptors, but rather activates the channel in Schwann cells that ensheath the nerve fibers [Citation56]. Then, via an NADPH oxidase 1 (NOX1)-dependent pathway, Schwann cell TRPA1 amplifies and sustains the oxidative stress signal, which exerts a dual function. An outwardly directed release of oxidants maintains macrophage recruitment inside the injured nerve trunk, and an inwardly directed oxidative stress targets nociceptor TRPA1 to signal pain [Citation56] ().
Figure 3. (a) Tissue injury generates a series of inflammatory agents, including reactive oxygen (ROS), nitrogen (nitric oxide) and carbonylic (4-hydroxy-2-nonenal, 4-HNE) species, bradykinin, prostaglandins, histamine, serotonin, kinins, cytokines, neuropeptides, and neurotrophins and chemokines. Some of these agents, such as ROS, cyclopentenone-prostaglandins (cyclo-PGs), nitric oxide, and 4-HNE, directly gate the channel, whereas other agents indirectly modulate TRPA1 activity, thus promoting intracellular signaling cascades. Activation of both pathways contributes to the generation of acute pain. (b) The injured nerve trunk releases proinflammatory chemokines, which recruit activated macrophages within the lesioned area. Phagocyte-dependent oxidative stress (ROS) activates TRPA1 in Schwann cells, which evokes a calcium (Ca2+)-dependent, NADPH oxidase 1 (NOX1)-mediated amplification of hydrogen peroxide (H2O2) release, which targets nociceptor TRPA1 to signal mechanical allodynia
However, the contribution of activated macrophages is not necessarily required to activate the proalgesic Schwann cell TRPA1 pathway. In fact, in a model of alcohol-evoked painful peripheral polyneuropathy [Citation57], we found that oral ethanol is converted by Schwann cell alcohol dehydrogenase-2 (ADH-2) into acetaldehyde, which, in an autocrine manner, targets Schwann cell TRPA1 to generate ROS and 4-HNE, which in turn activates TRPA1 in nociceptors to signal pain [Citation57]. In this case, macrophage contribution is not required, given that Schwann cells, via their own enzymatic machinery (ADH-2), generate the oxidative stress burst necessary to act on TRPA1 to sustain neuroinflammation.
10. Conclusions
Chronic pain is a major health problem, which leads to a decrease in the quality of life for many people worldwide. Strong efforts are being made toward limiting opiate use and pursuing safer treatments. Given its expression in many different types of tissues and cells, and its pleiotropic biological profile, TRPA1 represents an attractive therapeutic target in a variety of human diseases. Robust preclinical evidence accumulated in the last 20 years indicates TRPA1 as a potential target for the treatment of pain. Recent investigations have underlined the crucial role of the feed-forward mechanism that encompasses Schwann cell TRPA1 and oxidative stress in peripheral nerve fibers to sustain mechanical allodynia. These novel findings open new potential strategies for the treatment of chronic pain.
11. Expert opinion
Tissue insult may generate an acute painful effect, associated with early neural and cellular responses aimed at removing harmful stimuli, thereby limiting further tissue injury, and favoring repair. Response to tissue injury may evolve into a chronic status of allodynia and hyperalgesia, typically represented by hypersensitivity to both mechanical and thermal stimuli. In addition to central mechanisms, this hypersensitivity is also driven by over-activity of the peripheral nociceptors via sensitization of their nerve terminals. Several different etiologic agents, including physical trauma, neurotoxins, cancer and chemotherapeutic treatment, infections, and immune and metabolic diseases, produce pain symptoms, in which different molecular mechanisms contribute to the sensitization of small-diameter unmyelinated C-fibers and medium-diameter thinly myelinated Aδ-fibers. Although large advances have been made in pain research during the past decade, there has been little translation of preclinical results into clinical practice. Currently, very few novel therapeutic opportunities have been offered to patients, and older drugs have considerable side effects and incomplete efficacy. For these reasons, patients are frequently undertreated, and new, safer and more effective analgesic drugs are clearly needed.
TRPA1, also known as the proalgesic wasabi receptor, belongs to the larger TRP family of channels, which regulates numerous homeostatic functions and pathological processes. TRPA1, while maintaining a conserved sensitivity in chemosensation across species, shows remarkable variability regarding cold and mechanical sensitivity.
Localization of TRPA1 in a large variety of cells and tissues suggests pleiotropic roles. Since its identification in C-fibers [Citation11] more than 10 years ago, the TRPA1 channel has been deeply scrutinized as a pain transduction mechanism. Robust evidence implicates TRPA1 in pain processing at different anatomical sites of nociceptors, from peripheral terminals and DRG somata, to central terminals in the spinal cord. Sensory neuron TRPA1, gated by an unprecedented variety of exogenous stimuli and endogenous mediators, is primarily activated by oxidative stress byproducts, which are abundantly generated at sites of tissue injury and inflammation. Because of this wide range of agonists, TRPA1 has been proposed to markedly contribute to acute nociception [Citation63,Citation71,Citation124-131–Citation132]. In addition, due to its prominent localization to peptidergic primary sensory neurons [Citation11] TRPA1 activation results in the release of SP and CGRP from peripheral terminals, thus mediating neurogenic inflammatory responses [Citation166,Citation184]. The release of CGRP from terminals of peptidergic nociceptors is now recognized as a major mechanism of migraine pain [Citation175]. Thus, TRPA1 represents an excellent candidate for analgesic drug design to treat acute pain of different origins and migraine headaches.
However, recent findings in murine models of neuropathic pain have shifted the focus from neuronal to glial TRPA1. TRPA1 expressed in Schwann cells has been found to dynamically communicate with the ensheathed nerve fibers and with surrounding inflammatory cells (expanded peripheral nerve resident macrophages or invading hematogenic macrophages) via oxidative stress generation. The feed-forward mechanism that encompasses invading macrophages, oxidative stress, and Schwann cell TRPA1 was found to be essential to sustain mechanical allodynia in mouse models of neuropathic pain [Citation56], and of complex regional pain syndrome type I [Citation185]. In addition, Schwann cell TRPA1 has been reported to generate, in a macrophage-independent manner, oxidative stress, which sustains mechanical allodynia in a chronic murine model of alcoholic neuropathy [Citation57]. Thus, the possibility to selectively target the channel in Schwann cells, may provide a unique opportunity to selectively switch off chronic hypersensitivity.
With the caveat that most relevant models are reproduced in rodents, TRPA1 contribution to inflammatory and neuropathic pain is robustly emerging. The pronounced sensitivity of TRPA1 to oxidative stress and its byproducts emphasizes the channel’s role in mediating responses evoked by such mediators at sites of tissues injury and inflammation. During the last five years, significant progress has been made toward the discovery of new TRPA1 antagonists, although drug development programs are still limited, and initial promising results with TRPA1 antagonists, which are under current clinical scrutiny, require further confirmation before they can enter the therapeutic armamentarium.
Article highlights
TRP channels belong to a large family of ion channels with pleiotropic roles in a variety of cells and systems.
The TRPA1 channel is widely studied in the mechanism/process of inflammatory, neuropathic, cancer, and migraine pain.
In addition to the peripheral nervous system, the TRPA1 channel is localized in several non-neuronal cell types.
Endogenous molecules produced by various pathological conditions, such as reactive oxygen (ROS), nitrative (RNS), and carbonylic (RCS) species, activate TRPA1 to sustain chronic pain.
TRPA1 activation leads to pain responses.
This box summarizes key points contained in the article.
Declarations of interest
F De Logu, P Geppetti and R.Nassini are founding scientists of FloNext Srl. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Nilius B, Appendino G, Owsianik G. The transient receptor potential channel TRPA1: from gene to pathophysiology. Pflugers Arch. 2012;464:425–458.
- Clapham DE, Runnels LW, Strübing C. The TRP ion channel family. Nat Rev Neurosci. 2001;2:387–396.
- Wu L-J, Sweet T-B, Clapham DE. International union of basic and clinical pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev. 2010;62:381–404.
- Palmer CP, Zhou XL, Lin J, et al. A TRP homolog in Saccharomyces cerevisiae forms an intracellular Ca2+-permeable channel in the yeast vacuolar membrane. Proc Natl Acad Sci U S A. 2001;98:7801–7805.
- Logashina YA, Korolkova YV, Kozlov SA, et al. TRPA1 channel as a regulator of neurogenic inflammation and pain: structure, function, role in pathophysiology, and therapeutic potential of ligands. Biochem. 2019;84:101–118. Pleiades Publishing.
- Nilius B, Owsianik G, Voets T, et al. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217.
- Cheng W, Sun C, Zheng J. Heteromerization of TRP channel subunits: extending functional diversity. Protein Cell. 2010;1:802–810. Higher Education Press.
- Moran MM, McAlexander MA, Bíró T, et al. Transient receptor potential channels as therapeutic targets. Nat Rev Drug Discov. 2011;10:601–620.
- Nassini R, Materazzi S, Benemei S, et al. The TRPA1 channel in inflammatory and neuropathic pain and migraine. Rev Physiol Biochem Pharmacol. 2014;167:1–43.
- Jaquemar D, Schenker T, Trueb B. An ankyrin-like protein with transmembrane domains is specifically lost after oncogenic transformation of human fibroblasts. J Biol Chem. 1999;274:7325–7333.
- Story GM, Peier AM, Reeve AJ, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829.
- Talavera K, Startek JB, Alvarez-Collazo J, et al. Mammalian Transient Receptor Potential TRPA1 channels: from structure to disease. Physiol Rev. 2020;100:725–803.
- Zygmunt PM, Högestätt ED. TRPA1. Handb Exp Pharmacol. 2014;222:583–630.
- Bandell M, Story GM, Hwang SW, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857.
- Kwan KY, Allchorne AJ, Vollrath MA, et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289.
- Viana F. TRPA1 channels: molecular sentinels of cellular stress and tissue damage. J Physiol. 2016;594:4151–4169.
- Paulsen CE, Armache J-P, Gao Y, et al. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature. 2015;520:511–517.
- Samad A, Sura L, Benedikt J, et al. The C-terminal basic residues contribute to the chemical- and voltage-dependent activation of TRPA1. Biochem J. 2011;433:197–204.
- Cvetkov TL, Huynh KW, Cohen MR, et al. Molecular architecture and subunit organization of TRPA1 ion channel revealed by electron microscopy. J Biol Chem. 2011;286:38168–38176.
- Gaudet R. A primer on ankyrin repeat function in TRP channels and beyond. Mol Biosyst. 2008;4:372.
- Nilius B, Prenen J, Owsianik G. Irritating channels: the case of TRPA1. J Physiol. 2011;589:1543–1549.
- Eberhardt MJ, Filipovic MR, Leffler A, et al. Methylglyoxal activates nociceptors through transient receptor potential channel A1 (TRPA1): a possible mechanism of metabolic neuropathies. J Biol Chem. 2012;287:28291–28306.
- Kádková A, Synytsya V, Krusek J, et al. Molecular basis of TRPA1 regulation in nociceptive neurons. Rev Physiol Res. 2017;66:425–439. Czech Academy of Sciences.
- Bautista DM, Movahed P, Hinman A, et al. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci U S A. 2005;102:12248–12252.
- Ryskamp DA, Witkovsky P, Barabas P, et al. The polymodal ion channel transient receptor potential vanilloid 4 modulates calcium flux, spiking rate, and apoptosis of mouse retinal ganglion cells. J Neurosci. 2011;31:7089–7101.
- Kim YS, Son JY, Kim TH, et al. Expression of transient receptor potential ankyrin 1 (TRPA1) in the rat trigeminal sensory afferents and spinal dorsal horn. J Comp Neurol. 2010;518:687–698.
- Kim HY, Chung G, Jo HJ, et al. Characterization of dental nociceptive neurons. J Dent Res. 2011;90:771–776.
- Kobayashi K, Fukuoka T, Obata K, et al. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with Aδ/C-fibers and colocalization with Trk receptors. J Comp Neurol. 2005;493:596–606.
- Fischer MJM, Balasuriya D, Jeggle P, et al. Direct evidence for functional TRPV1/TRPA1 heteromers. Pflugers Arch Eur J Physiol. 2014;466:2229–2241.
- Vennekens R, Menigoz A, Nilius B. TRPs in the brain. Rev Physiol Biochem Pharmacol. 2012;163:27–64.
- Kheradpezhouh E, Choy JMC, Daria VR, et al. TRPA1 expression and its functional activation in rodent cortex. Open Biol. 2017;7:160314.
- Koch M, Kreutz S, Böttger C, et al. The cannabinoid WIN 55,212-2-mediated protection of dentate gyrus granule cells is driven by CB1 receptors and modulated by TRPA1 and Cav 2.2 channels. Hippocampus. 2011;21:554–564.
- Kunert-Keil C, Bisping F, Krüger J, et al. Tissue-specific expression of TRP channel genes in the mouse and its variation in three different mouse strains. BMC Genomics. 2006;7:159.
- Sagalajev B, Wei H, Chen Z, et al. Oxidative stress in the amygdala contributes to neuropathic pain. Neuroscience. 2018;387:92–103.
- Shigetomi E, Tong X, Kwan KY, et al. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat Neurosci. 2011;15:70–80.
- Stokes A, Wakano C, Koblan-Huberson M, et al. TRPA1 is a substrate for de-ubiquitination by the tumor suppressor CYLD. Cell Signal. 2006;18:1584–1594.
- Werkheiser JL, Rawls SM, Cowan A. Icilin evokes a dose- and time-dependent increase in glutamate within the dorsal striatum of rats. Amino Acids. 2006;30:307–309.
- Morelli MB, Amantini C, Liberati S, et al. TRP channels: new potential therapeutic approaches in CNS neuropathies. CNS Neurol Disord Drug Targets. 2013;12:274–293.
- Araújo DSM, Miya-Coreixas VS, Pandolfo P, et al. Cannabinoid receptors and TRPA1 on neuroprotection in a model of retinal ischemia. Exp Eye Res. 2017;154:116–125.
- Souza Monteiro de Araújo D, De Logu F, Adembri C, et al. TRPA1 mediates damage of the retina induced by ischemia and reperfusion in mice. Cell Death Dis. 2020;11:633.
- García-Añoveros J, Duggan A. TRPA1 in auditory and nociceptive organs. In: Liedtke W, Heller S, editors. TRP ion channel funct sens transduct cell signal cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. p. 163–175.
- Earley S, Gonzales AL, Crnich R. Endothelium-dependent cerebral artery dilation mediated by trpa1 and CA2+-activated K+ channels. Circ Res. 2009;104:987–994.
- Mukhopadhyay I, Gomes P, Aranake S, et al. Expression of functional TRPA1 receptor on human lung fibroblast and epithelial cells. J Recept Signal Transduct. 2011;31:350–358.
- Nassini R, Pedretti P, Moretto N, et al. Transient receptor potential ankyrin 1 channel localized to non-neuronal airway cells promotes non-neurogenic inflammation. PLoS One. 2012;7:e42454.
- Meents JE, Ciotu CI, Fischer MJM. Trpa1: A molecular view. J Neurophysiol. 2019;121:427–443. American Physiological Society.
- Andrade EL, Meotti FC, Calixto JB. TRPA1 antagonists as potential analgesic drugs. Pharmacol Ther. 2012;133:189–204.
- Baraldi PG, Preti D, Materazzi S, et al. Transient Receptor Potential Ankyrin 1 (TRPA1) channel as emerging target for novel analgesics and anti-inflammatory agents. J Med Chem. 2010;53:5085–5107.
- Bellono NW, Kammel LG, Zimmerman AL, et al. UV light phototransduction activates transient receptor potential A1 ion channels in human melanocytes. Proc Natl Acad Sci U S A. 2013;110:2383–2388.
- Bellono NW, Oancea E. UV light phototransduction depolarizes human melanocytes. Channels (Austin). 2013;7:243–248.
- Büch TRH, Schäfer EAM, Demmel M-T, et al. Functional expression of the transient receptor potential channel TRPA1, a sensor for toxic lung inhalants, in pulmonary epithelial cells. Chem Biol Interact. 2013;206:462–471.
- Earley S. TRPA1 channels in the vasculature. Br J Pharmacol. 2012;167:13–22.
- Oh M-H, Oh SY, Lu J, et al. TRPA1-dependent pruritus in IL-13-induced chronic atopic dermatitis. J Immunol. 2013;191:5371–5382.
- Prasad P, Yanagihara AA, Small-Howard AL, et al. Secretogranin III directs secretory vesicle biogenesis in mast cells in a manner dependent upon interaction with chromogranin A. J Immunol. 2008;181:5024–5034.
- Takizawa M, Harada K, Nakamura K, et al. Transient receptor potential ankyrin 1 channels are involved in spontaneous peptide hormone release from astrocytes. Biochem Biophys Res Commun. 2018;501:988–995.
- Hamilton NB, Kolodziejczyk K, Kougioumtzidou E, et al. Proton-gated Ca2+-permeable TRP channels damage myelin in conditions mimicking ischaemia. Nature. 2016;529:523–527.
- De Logu F, Nassini R, Materazzi S, et al. Schwann cell TRPA1 mediates neuroinflammation that sustains macrophage-dependent neuropathic pain in mice. Nat Commun. 2017;8:1887.
- De Logu F, Puma SL, Landini L, et al. Schwann cells expressing nociceptive channel TRPA1 orchestrate ethanol-evoked neuropathic pain in mice. J Clin Invest. 2019;129:5424–5441.
- Bernardini M, Fiorio Pla A, Prevarskaya N, et al. Human transient receptor potential (TRP) channel expression profiling in carcinogenesis. Int J Dev Biol. 2015;59:399–406.
- Jordt S-E, Bautista DM, Chuang -H-H, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265.
- Macpherson LJ, Geierstanger BH, Viswanath V, et al. The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr Biol. 2005;15:929–934.
- Andrè E, Campi B, Materazzi S, et al. Cigarette smoke-induced neurogenic inflammation is mediated by alpha,beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest. 2008;118:2574–2582.
- Escalera J, Von Hehn CA, Bessac BF, et al. TRPA1 mediates the noxious effects of natural sesquiterpene deterrents. J Biol Chem. 2008;283:24136–24144.
- Andersson DA, Gentry C, Moss S, et al. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci. 2008;28:2485–2494.
- Bessac BF, Sivula M, Von Hehn CA, et al. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest. 2008;118:1899–1910.
- Sawada Y, Hosokawa H, Matsumura K, et al. Activation of transient receptor potential ankyrin 1 by hydrogen peroxide. Eur J Neurosci. 2008;27:1131–1142.
- Matta JA, Cornett PM, Miyares RL, et al. General anesthetics activate a nociceptive ion channel to enhance pain and inflammation. Proc Natl Acad Sci U S A. 2008;105:8784–8789.
- Leffler A, Lattrell A, Kronewald S, et al. Activation of TRPA1 by membrane permeable local anesthetics. Mol Pain. 2011;7:62.
- Fischer MJM, Leffler A, Niedermirtl F, et al. The general anesthetic propofol excites nociceptors by activating TRPV1 and TRPA1 rather than GABAA receptors. J Biol Chem. 2010;285:34781–34792.
- Fischer M, Carli G, Raboisson P, et al. The interphase of the formalin test. Pain. 2014;155:511–521.
- Macpherson LJ, Xiao B, Kwan KY, et al. An ion channel essential for sensing chemical damage. J Neurosci. 2007;27:11412–11415.
- McNamara CR, Mandel-Brehm J, Bautista DM, et al. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci. 2007;104:13525–13530.
- Miyamoto T, Dublin AE, Petrus MJ, et al. TRPV1 and TRPA1 mediate peripheral nitric oxide-induced nociception in mice. PLoS One. 2009;4:e7596.
- Andersson DA, Gentry C, Moss S, et al. Clioquinol and pyrithione activate TRPA1 by increasing intracellular Zn2+. Proc Natl Acad Sci U S A. 2009;106:8374–8379.
- Hu H, Bandell M, Petrus MJ, et al. Zinc activates damage-sensing TRPA1 ion channels. Nat Chem Biol. 2009;5:183–190.
- Andersson DA, Gentry C, Light E, et al. Methylglyoxal evokes pain by stimulating TRPA1. PLoS One. 2013;8:e77986.
- Cao D-S, Zhong L, Hsieh T, et al. Expression of Transient Receptor Potential Ankyrin 1 (TRPA1) and its role in insulin release from rat pancreatic beta cells. PLoS One. 2012;7:e38005. Obukhov AG, editor.
- Karashima Y, Damann N, Prenen J, et al. Bimodal action of menthol on the transient receptor potential channel TRPA1. J Neurosci. 2007;27:9874–9884.
- Lee SP, Buber MT, Yang Q, et al. Thymol and related alkyl phenols activate the hTRPA1 channel. Br J Pharmacol. 2008;153:1739–1749.
- Xu H, Delling M, Jun JC, et al. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci. 2006;9:628–635.
- Talavera K, Gees M, Karashima Y, et al. Nicotine activates the chemosensory cation channel TRPA1. Nat Neurosci. 2009;12:1293–1299.
- De Petrocellis L, Vellani V, Schiano-Moriello A, et al. Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8. J Pharmacol Exp Ther. 2008;325:1007–1015.
- Meseguer V, Karashima Y, Talavera K, et al. Transient receptor potential channels in sensory neurons are targets of the antimycotic agent clotrimazole. J Neurosci. 2008;28:576–586.
- Fajardo O, Meseguer V, Belmonte C, et al. TRPA1 channels: novel targets of 1,4-dihydropyridines. Channels. 2008;2:429–438.
- Hu H, Tian J, Zhu Y, et al. Activation of TRPA1 channels by fenamate nonsteroidal anti-inflammatory drugs. Pflugers Arch Eur J Physiol. 2010;459:579–592.
- De Logu F, Li Puma S, Landini L, et al. The acyl-glucuronide metabolite of ibuprofen has analgesic and anti-inflammatory effects via the TRPA1 channel. Pharmacol Res. 2019;142:127–139.
- Bessac BF, Jordt S-E. Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology (Bethesda). 2008;23:360–370.
- Cavanaugh EJ, Simkin D, Kim D. Activation of transient receptor potential A1 channels by mustard oil, tetrahydrocannabinol and Ca2+ reveals different functional channel states. Neuroscience. 2008;154:1467–1476.
- Doerner JF, Gisselmann G, Hatt H, et al. Transient receptor potential channel A1 is directly gated by calcium ions. J Biol Chem. 2007;282:13180–13189.
- Nagata K, Duggan A, Kumar G, et al. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci. 2005;25:4052–4061.
- Wang YY, Chang RB, Waters HN, et al. The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J Biol Chem. 2008;283:32691–32703.
- Zurborg S, Yurgionas B, Jira JA, et al. Direct activation of the ion channel TRPA1 by Ca2+. Nat Neurosci. 2007;10:277–279.
- Schmidt M, Dubin AE, Petrus MJ, et al. Nociceptive signals induce trafficking of TRPA1 to the plasma membrane. Neuron. 2009;64:498–509.
- Benemei S, De Logu F, Li Puma S, et al. The anti-migraine component of butterbur extracts, isopetasin, desensitizes peptidergic nociceptors by acting on TRPA1 cation channel. Br J Pharmacol. 2017;174:2897–2911.
- Wei H, Karimaa M, Korjamo T, et al. Transient receptor potential ankyrin 1 ion channel contributes to guarding pain and mechanical hypersensitivity in a rat model of postoperative pain. Anesthesiology. 2012;117:137–148.
- Koivisto A, Hukkanen M, Saarnilehto M, et al. Inhibiting TRPA1 ion channel reduces loss of cutaneous nerve fiber function in diabetic animals: sustained activation of the TRPA1 channel contributes to the pathogenesis of peripheral diabetic neuropathy. Pharmacol Res. 2012;65:149–158.
- Chen J, Joshi SK, Didomenico S, et al. Selective blockade of TRPA1 channel attenuates pathological pain without altering noxious cold sensation or body temperature regulation. Pain. 2011;152:1165–1172.
- Klionsky L, Tamir R, Gao BX, et al. Species-specific pharmacology of trichloro(sulfanyl)ethyl benzamides as transient receptor potential ankyrin 1 (TRPA1) antagonists. Mol Pain. 2007;3:39.
- Mukhopadhyay I, Kulkarni A, Aranake S, et al. Transient receptor potential ankyrin 1 receptor activation in vitro and in vivo by pro-tussive agents: GRC 17536 as a promising anti-tussive therapeutic. PLoS One. 2014;9:e97005.
- Janssen Pharmaceutica NV. Heterocyclic amides as modulators of TRPA1. WO2010141805. 2010.
- Merck Sharp & Dohme Corp. Novel TRPA1 antagonists. WO2011043954. 2011.
- Orion Corporation. Phenyl- sulfonyl derivatives as mediators of TRPA1 receptor activity for the treatment of pain. WO2012152983. 2012.
- EA Pharma Co Ltd. Heterocyclic amide derivative and pharmaceutical product containing same. EP2805718. 2013.
- Copeland KW, Boezio AA, Cheung E, et al. Development of novel azabenzofuran TRPA1 antagonists as in vivo tools. Bioorganic Med Chem Lett. 2014;24:3464–3468.
- Rooney L, Vidal A, D’Souza AM, et al. Discovery, optimization, and biological evaluation of 5-(2- (trifluoromethyl)phenyl)indazoles as a novel class of transient receptor potential A1 (TRPA1) antagonists. J Med Chem. 2014;57:5129–5140.
- Glenmark Pharmaceuticals Ltd. A clinical trial to study the effects GRC 17536 in patients with painful diabetic peripheral neuropathy (Painful extremities due to peripheral nerve damage in diabetic patients). Full Text View - ClinicalTrials.gov.
- Cubist pharmaceuticals and hydra biosciences announce plans to begin Phase 1 clinical trial for novel TRPA1 modulator to treat acute. Available at: http://hydrabiosciences.com/pdf/press_releases/2012_01_10.pdf [Last accessed 3 August 2020]
- Hydra biosciences receives approval from health canada to begin Phase 1 trial for HX-100. Available at: http://hydrabiosciences.com/pdf/press_releases/2015_04_14.pdf [Last accessed 3 August 2020]
- Orion Corporation. Safety, tolerability, pharmacokinetic and pharmacodynamic effects of ODM-108: in healthy male volunteers. Full Text View - ClinicalTrials.gov.
- Genentech IA. Study of the safety, tolerability, pharmacokinetics and pharmacodynamic effects of single and multiple ascending doses of GDC-0334 and the effect of food on the pharmacokinetics of GDC-0334 in healthy adult participants. Full Text View - ClinicalTrials.
- Abd-Elsayed A, Deer TR. Different types of pain. Pain. Springer, Cham, 2019;15–16.
- Haanpää M, Attal N, Backonja M, et al. NeuPSIG guidelines on neuropathic pain assessment. Pain. 2011;152:14–27.
- Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32.
- Woolf CJ. What is this thing called pain? J Clin Invest. 2010;120:3742–3744.
- Porter J, Turk DC. Management of pain: best of times, worst of times? Clin J Pain. 2001;17:107–109.
- Sikandar S, Dickenson AH. Visceral pain: the ins and outs, the ups and downs. Curr Opin Support Palliat Care. 2012;6:17–26.
- Nicholas M, Vlaeyen JWS, Rief W, et al. The IASP classification of chronic pain for ICD-11: chronic primary pain. Pain. 2019;160:28–37. Lippincott Williams and Wilkins.
- Dubin AE, Patapoutian A. Nociceptors: the sensors of the pain pathway. J Clin Invest. 2010;120:3760–3772.
- Vandewauw I, De Clercq K, Mulier M, et al. A TRP channel trio mediates acute noxious heat sensing. Nature. 2018;555:662–666.
- Karashima Y, Talavera K, Everaerts W, et al. TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natl Acad Sci U S A. 2009;106:1273–1278.
- Del Camino D, Murphy S, Heiry M, et al. TRPA1 contributes to cold hypersensitivity. J Neurosci. 2010;30:15165–15174.
- Corey DP, Garcia-Añoveros J, Holt JR, et al. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature. 2004;432:723–730.
- Moparthi L, Zygmunt PM. Human TRPA1 is an inherently mechanosensitive bilayer-gated ion channel. Cell Calcium. 2020;91:102255.
- Bautista DM, Jordt S-E, Nikai T, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282.
- Petrus M, Peier AM, Bandell M, et al. A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol Pain. 2007;3:40.
- McGaraughty S, Chu KL, Perner RJ, et al. TRPA1 modulation of spontaneous and mechanically evoked firing of spinal neurons in uninjured, osteoarthritic, and inflamed rats. Mol Pain. 2010;6:6–14.
- da Costa DSM, Meotti FC, Andrade EL, et al. The involvement of the transient receptor potential A1 (TRPA1) in the maintenance of mechanical and cold hyperalgesia in persistent inflammation. Pain. 2010;148:431–437.
- Trevisan G, Materazzi S, Fusi C, et al. Novel therapeutic strategy to prevent chemotherapy-induced persistent sensory neuropathy by TRPA1 blockade. Cancer Res. 2013;73:3120–3131.
- Moilanen LJ, Laavola M, Kukkonen M, et al. TRPA1 contributes to the acute inflammatory response and mediates carrageenan-induced paw edema in the mouse. Sci Rep. 2012;2:380.
- Bonet IJM, Fischer L, Parada CA, et al. The role of transient receptor potential A 1 (TRPA1) in the development and maintenance of carrageenan-induced hyperalgesia. Neuropharmacology. 2013;65:206–212.
- Okun A, Liu P, Davis P, et al. Afferent drive elicits ongoing pain in a model of advanced osteoarthritis. Pain. 2012;153:924–933.
- Chen J, Zhang XF, Kort ME, et al. Molecular determinants of species-specific activation or blockade of TRPA1 channels. J Neurosci. 2008;28:5063–5071.
- Fernandes ES, Russell FA, Spina D, et al. A distinct role for transient receptor potential ankyrin 1, in addition to transient receptor potential vanilloid 1, in tumor necrosis factor α-induced inflammatory hyperalgesia and Freund’s complete adjuvant-induced monarthritis. Arthritis Rheum. 2011;63:819–829.
- Colloca L, Ludman T, Bouhassira D, et al. Neuropathic pain. Nat Rev Dis Prim. 2017;3:17002.
- Stavros K, Simpson DM. Understanding the etiology and management of HIV-associated peripheral neuropathy. Curr HIV/AIDS Rep. 2014;11:195–201.
- Thakur S, Dworkin RH, Haroun OMO, et al. Acute and chronic pain associated with leprosy. Pain. 2015;156:998–1002. Lippincott Williams and Wilkins.
- Haroutounian S, Nikolajsen L, Bendtsen TF, et al. Primary afferent input critical for maintaining spontaneous pain in peripheral neuropathy. Pain. 2014;155:1272–1279.
- Siddall PJ, McClelland JM, Rutkowski SB, et al. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003;103:249–257.
- Davies M, Brophy S, Williams R, et al. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care. 2006;29:1518–1522.
- Klit H, Finnerup NB, Andersen G, et al. Central poststroke pain: A population-based study. Pain. 2011;152:818–824.
- Bennett MI, Rayment C, Hjermstad M, et al. Prevalence and aetiology of neuropathic pain in cancer patients: A systematic review. Pain. 2012;153:359–365.
- Borsook D. Neurological diseases and pain. Brain. 2011;135:320–344.
- Watson JC, Sandroni P. Central neuropathic pain syndromes. Mayo Clin Proc. 2016;91:372–385. Elsevier Ltd.
- Obata K, Katsura H, Mizushima T, et al. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest. 2005;115:2393–2401.
- Katsura H, Obata K, Mizushima T, et al. Antisense knock down of TRPA1, but not TRPM8, alleviates cold hyperalgesia after spinal nerve ligation in rats. Exp Neurol. 2006;200:112–123.
- Caspani O, Zurborg S, Labuz D, et al. The contribution of TRPM8 and TRPA1 channels to cold allodynia and neuropathic pain. PLoS One. 2009;4:e7383.
- Staaf S, Oerther S, Lucas G, et al. Differential regulation of TRP channels in a rat model of neuropathic pain. Pain. 2009;144:187–199.
- Eid SR, Crown ED, Moore EL, et al. HC-030031, a TRPA1 selective antagonist, attenuates inflammatory- and neuropathy-induced mechanical hypersensitivity. Mol Pain. 2008;4:48.
- Wei H, Koivisto A, Saarnilehto M, et al. Spinal transient receptor potential ankyrin 1 channel contributes to central pain hypersensitivity in various pathophysiological conditions in the rat. Pain. 2011;152:582–591.
- Kwan KY, Glazer JM, Corey DP, et al. TRPA1 modulates mechanotransduction in cutaneous sensory neurons. J Neurosci. 2009;29:4808–4819.
- Kerstein PC, Del Camino D, Moran MM, et al. Pharmacological blockade of TRPA1 inhibits mechanical firing in nociceptors. Mol Pain. 2009;5:19.
- Zappia KJ, O’Hara CL, Moehring F, et al. Sensory neuron-specific deletion of TRPA1 results in mechanical cutaneous sensory deficits. eNeuro. 2017;4:ENEURO.0069-16.2017.
- Horváth Á, Tékus V, Boros M, et al. Transient receptor potential ankyrin 1 (TRPA1) receptor is involved in chronic arthritis: in vivo study using TRPA1-deficient mice. Arthritis Res Ther. 2016;18:6.
- Wei H, Hämäläinen MM, Saarnilehto M, et al. Attenuation of mechanical hypersensitivity by an antagonist of the TRPA1 ion channel in diabetic animals. Anesthesiology. 2009;111:147–154.
- Trevisani M, Siemens J, Materazzi S, et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci U S A. 2007;104:13519–13524.
- Viisanen H, Chapman H, Wei H, et al. Pronociceptive effects induced by cutaneous application of a Transient Receptor Potential Ankyrin 1 (TRPA1) channel agonist methylglyoxal in diabetic animals: comparison with tunicamycin-induced endoplastic reticulum stress - PubMed. J Physiol Pharmacol. 2016;67:587–594.
- Staff NP, Grisold A, Grisold W, et al. Chemotherapy-induced peripheral neuropathy: A current review. Ann Neurol. 2017;81:772–781. John Wiley and Sons Inc.
- Chen Y, Yang C, Wang ZJ. Proteinase-activated receptor 2 sensitizes transient receptor potential vanilloid 1, transient receptor potential vanilloid 4, and transient receptor potential ankyrin 1 in paclitaxel-induced neuropathic pain. Neuroscience. 2011;193:440–451.
- Nassini R, Gees M, Harrison S, et al. Oxaliplatin elicits mechanical and cold allodynia in rodents via TRPA1 receptor stimulation. Pain. 2011;152:1621–1631.
- Breivik H, Cherny N, Collett B, et al. Cancer-related pain: a pan-European survey of prevalence, treatment, and patient attitudes. Ann Oncol. 2009;20:1420–1433.
- Falk S, Bannister K, Dickenson AH. Cancer pain physiology. Br J Pain. 2014;8:154–162.
- Caraceni A, Portenoy RK. An international survey of cancer pain characteristics and syndromes. Pain. 1999;82:263–274.
- Mantyh WG, Jimenez-Andrade JM, Stake JI, et al. Blockade of nerve sprouting and neuroma formation markedly attenuates the development of late stage cancer pain. Neuroscience. 2010;171:588–598.
- Jänig W, Baron R. Complex regional pain syndrome: mystery explained? Lancet Neurol. 2003;2:687–697. Lancet Publishing Group.
- Antoniazzi CTDD, Nassini R, Rigo FK, et al. Transient receptor potential ankyrin 1 (TRPA1) plays a critical role in a mouse model of cancer pain. Int J Cancer. 2019;144:355–365.
- Nagakura Y. Challenges in drug discovery for overcoming dysfunctional pain: an emerging category of chronic pain. Expert Opin Drug Discov. 2015;10:1043–1045. Taylor and Francis Ltd.
- Marone IM, De Logu F, Nassini R, et al. TRPA1/NOX in the soma of trigeminal ganglion neurons mediates migraine-related pain of glyceryl trinitrate in mice. Brain. 2018;141:2312–2328.
- Wang S, Brigoli B, Lim J, et al. Roles of TRPV1 and TRPA1 in spontaneous pain from inflamed masseter muscle. Neuroscience. 2018;384:290–299.
- Liu CC, Zhang XS, Ruan YT, et al. Accumulation of methylglyoxal increases the advanced glycation end-product levels in DRG and contributes to lumbar disk herniation-induced persistent pain. J Neurophysiol. 2017;118:1321–1328.
- Achenbach J, Rhein M, Gombert S, et al. Childhood traumatization is associated with differences in TRPA1 promoter methylation in female patients with multisomatoform disorder with pain as the leading bodily symptom. Clin Epigenetics. 2019;11:126.
- Cattaruzza F, Johnson C, Leggit A, et al. Transient receptor potential ankyrin 1 mediates chronic pancreatitis pain in mice. Am J Physiol Gastrointest Liver Physiol. 2013;304:G1002–1012.
- Yang J, Li Y, Zuo X, et al. Transient receptor potential ankyrin-1 participates in visceral hyperalgesia following experimental colitis. Neurosci Lett. 2008;440:237–241.
- Engel MA, Leffler A, Niedermirtl F, et al. TRPA1 and substance P mediate colitis in mice. Gastroenterology. 2011;141:1346–1358.
- Jain P, Materazzi S, De Logu F, et al. Transient receptor potential ankyrin 1 contributes to somatic pain hypersensitivity in experimental colitis. Sci Rep. 2020;10:8632.
- Balemans D, Aguilera-Lizarraga J, Florens MV, et al. Histamine-mediated potentiation of transient receptor potential (TRP) ankyrin 1 and TRP vanilloid 4 signaling in submucosal neurons in patients with irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2019;316:G338–G349.
- Edvinsson L. CGRP receptor antagonists and antibodies against CGRP and its receptor in migraine treatment. Br J Clin Pharmacol. 2015;80:193–199.
- De Logu F, Landini L, Janal MN, et al. Migraine-provoking substances evoke periorbital allodynia in mice. J Headache Pain. 2019;20:18.
- Benemei S, Fusi C, Trevisan G, et al. The TRPA1 channel in migraine mechanism and treatment. BrJ Pharmacol. 2014;171:2552–2567. John Wiley and Sons Inc.
- Kunkler PE, Ballard CJ, Oxford GS, et al. TRPA1 receptors mediate environmental irritant-induced meningeal vasodilatation. Pain. 2011;152:38–44.
- Ashina M, Hansen JM, Dunga Á, et al. Human models of migraine-short-Term pain for long-Term gain. Nat Rev Neurol. 2017;13:713–724. Nature Publishing Group.
- Koroleva K, Mustafina A, Yakovlev A, et al. Receptor mechanisms mediating the pro-nociceptive action of hydrogen sulfide in rat trigeminal neurons and meningeal afferents. Front Cell Neurosci. 2017;11:226.
- Gaudet AD, Popovich PG, Ramer MS. Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation. 2011;8:110.
- Ramer MS, French GD, Bisby MA. Wallerian degeneration is required for both neuropathic pain and sympathetic sprouting into the DRG. Pain. 1997;72:71–78.
- Trevisan G, Benemei S, Materazzi S, et al. TRPA1 mediates trigeminal neuropathic pain in mice downstream of monocytes/macrophages and oxidative stress. Brain. 2016;139:1361–1377.
- Nassini R, Materazzi S, Vriens J, et al. The “headache tree” via umbellulone and TRPA1 activates the trigeminovascular system. Brain. 2012;135:376–390.
- De Logu F, De Prá SDT, de David Antoniazzi CT, et al. Macrophages and Schwann cell TRPA1 mediate chronic allodynia in a mouse model of complex regional pain syndrome type I. Brain Behav Immun. 2020;88:535–546.