1. Introduction
1.1. Pancreatic epithelial tuft cells
Tuft cells (TCs) are an isolated chemosensory cell type found in the gastrointestinal and respiratory tract epithelium, which can be recognized by their tufted/teardrop appearance, large microvilli, and fine thread-like actin cytoskeleton. TCs express specific secretory and chemosensory proteins which allow them to respond dynamically to signals in the extracellular environment [Citation1–4]. The function of TCs is best described in the small intestinal epithelium where they have been shown to regulate viral infection, the immune response to pathogens, and epithelial barrier integrity [Citation3,Citation4]. TCs have also been identified in the pancreatic ductal epithelium [Citation1,Citation2,Citation5] where their role in pancreatic inflammation and tumorigenesis is a subject of considerable interest.
1.2. Pancreatitis and associated risk of pancreatic caner
Pancreatitis is the third major cause of hospitalization related to the gastrointestinal tract and is generally thought to occur due to damage to acinar cells leading to ER stress, over-production of digestive enzymes, and initiation of associated inflammatory processes. Pancreatitis manifests in two forms, acute or chronic. Acute pancreatitis has a sudden onset and can lead to multiple organ failure and death. In contrast, chronic pancreatitis has a slow onset and is often asymptomatic, remaining undiagnosed until complications occur. Importantly, chronic pancreatitis is a known risk factor for the development of pancreatic ductal adenocarcinoma (PDAC) [Citation1,Citation2], which ranks among the most aggressive cancer types with extreme morbidity and mortality. Alarmingly, the anticipated burden for PDAC is projected to increase globally, with worldwide cases and deaths both expected to exceed 0.5 million per year within 10 years [Citation6]. Consequently, new therapeutic targets that can regulate pancreatitis and inhibit the development and progression of PDAC are desperately needed.
1.3. Acinar-ductal metaplasia, tuft cells, and pancreatic cancer
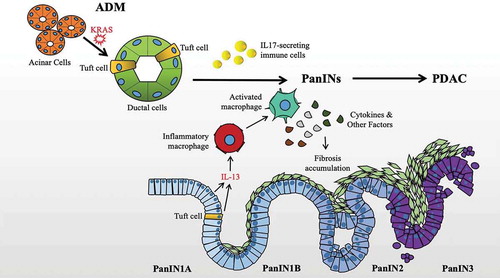
The healthy pancreas is mainly composed of acinar cells that secrete digestive enzymes, and ductal cells such as TCs, thought to be involved in bicarbonate secretion and the transport of digestive enzymes to the duodenum. A variety of studies have shown that the formation of acinar cells is a normal part of pancreatic injury, and that these cells undergo acinar-ductal metaplasia (ADM) to replace damaged ductal epithelial cells including TCs [Citation1]. The resulting cells are involved in repair of the damaged pancreas and essential to recovery. Although this reparative process is reversible, post-repair dedifferentiation can be prevented by the introduction of KRAS mutation and repeated instances of inflammation, leading to the formation of pancreatic intraepithelial neoplasia (PanIN), a precursor condition to PDAC. Moreover, the concurrent loss of a tumor suppressor such as p53 can lead directly to PDAC initiation [Citation5,Citation7]. Comparable to colon cancer, evidence suggests the possibility that PDACs may arise from mutated TCs [Citation8]. Therefore, the question of whether TCs might serve as a suitable target for pancreatitis and/or PDAC is complicated due to the double-edged nature of inflammatory repair and cancer initiation. Finally, the means by which TCs may be targeted including functional markers, secreted proteins, and various signaling receptors is also an emerging topic of therapeutic importance. The origin, localization, and functional characteristics of ADM-associated TCs are illustrated in .
Figure 1. Relationship of Tuft Cells, ADM, PanIN formation, and PDAC. Tuft cells are expanded during KRAS-associated ADM, serve an immunomodulatory role with implications for PanIN development and fibrosis via cytokine signaling, and generally disappear with advanced disease
2. Tuft cells as a therapeutic target for pancreatitis and PDAC
Tuft cells in the normal pancreas can be potentially identified by a variety of structural and functional markers. Structural markers include actin-related cross-linking proteins such as advillin and fibrin, tubulin network and ankyrin adaptor proteins, and doublecortin-like kinase 1 (DCLK1). Functional markers include secreted and receptor proteins such as taste receptors (e.g. TRPM5, α-Gustducin) and phospholipase enzymes (e.g. PLCG2) [Citation9], with likely roles in sensing activities in the pancreatic ducts and signaling to the immune system and other cell types. During pancreatitis, acinar cells replace damaged TCs with new TCs which are programmed to secrete IL-25, a cytokine with a tumor-suppressing effect in a variety of cancers [Citation1]. Moreover, several studies have shown that TCs also release acetylcholine to signal to surrounding cell types. These cholinergic TCs are selectively distributed in interlobular ducts in the middle of the pancreas and may play an important role in the immune processes of infection and cancer [Citation10].
Acinar-ductal transdifferentiation occurs as a regenerative response to stressors and epithelial damage associated with pancreatitis. In the presence of KRAS mutation, metaplastic ducts that form during pancreatitis are associated with ADM and initiation of PDAC. KRAS mutation-induced ADM results in significant cellular heterogeneity including a dramatic expansion of TCs [Citation1], and evidence suggests that this phenomenon may occur via a SOX17-dependent mechanism and associated acinar cell-derived pancreatic progenitor populations [Citation2]. In genetically engineered mouse models, the majority of pancreatitis and ADM-associated TCs are lineage traced from acinar cells including DCLK1+ progenitors, do not express ductal marker PDX1, appear in the largest numbers during the early stages of disease, and decrease with progression [Citation2,Citation8]. These findings are potentially consistent with a cancer stem cell (CSC)-role for TCs in the development of PDAC [Citation8]. The significance of these TCs in the initiation and progression of PDAC remains to be confirmed, but ongoing studies are likely to shed further light on their role in these conditions. In order to achieve this, the identification of highly specific markers of pancreatic TCs and the development of related models (e.g. TC-marker driven inducible Cre recombinase mouse models crossed with the KrasLSL-G12D model) will be necessary.
In summary, evidence indicates that TCs do have potential as a new therapeutic target for pancreatitis and PDAC. TCs play an important role in inflammatory repair through secretion of tumor-suppressing cytokine IL-25 and release of acetylcholine. At the same time, pre-cancerous metaplastic ducts are closely associated with PDAC development and frequently contain a large number of TCs. Moreover, the possibility remains that some PDACs may arise specifically from mutated TCs.
3. Expert opinion: Therapeutic strategies for targeting tuft cells in pancreatitis and PDAC
A variety of potential TC targeting strategies exist. These include kinase inhibitors, monoclonal antibodies (mAbs), and immunotherapies. Prominent tuft cell marker DCLK1 has an active kinase domain and early studies with non-DCLK1 selective inhibitors such as LRRK2-IN-1, indicated anti-PDAC properties potentially linked to this activity [Citation11]. However, work toward the goal of understanding the selective activity of DCLK1 is complicated by its multiple functional isoforms. Promisingly, a recently developed, highly selective inhibitor, DCLK1-IN-1, with favorable properties of absorption and bioavailability should prove a powerful tool in finally determining the potential for this therapeutic modality in PDAC. Although testing showed limited efficacy for this novel inhibitor in adherent PDAC cell lines, it was able to suppress tumorigenesis in patient-derived DCLK1+ organoids [Citation12]. These disparate findings may be explained by the absence of tuft cells in adherent PDAC cell lines. Other targeting modalities that have been proposed for DCLK1 include mAbs and engineered nanoparticles targeting its extracellular domain. Finally, we note that although DCLK1 is a marker of TCs in the pancreas, it is not selective and its exact function in that cellular compartment remains a subject of investigation. Indeed, DCLK1 expression has also been reported in acinar, centroacinar, and tumor epithelial cells of the pancreas to varying degrees, and tracing experiments using a Dclk1 promoter-driven Cre recombinase mouse model demonstrate that it is a lineage marker for subsets of acinar and ductal cells [Citation8]. Moreover, the vast majority of studies focused on the anti-cancer potential of targeting DCLK1 in TC-containing organs have been directed toward its function in tumor epithelial cells and involvement in key oncogenic processes such as epithelial–mesenchymal transition, where it appears to have a strong regulatory role. Therefore, microenvironmental context especially as it concerns TCs, should be taken into careful consideration in planning future studies of DCLK1’s function and in the development of therapies based on this target.
Therapies exploiting the TC immunomodulatory role are another potential option for pancreatitis and PDAC. Despite an abundance of previous studies linking TCs to PDAC initiation and progression, recent murine studies indicate that in PanIN lesions, TCs may actually limit pancreatic tumorigenesis via secretion of prostaglandin D2 and subsequent inhibition of fibrosis [Citation13]. TCs are also capable of signaling to innate lymphoid type 2 cells (ILC2s) through IL-25 and its receptor (IL-17Rb) [Citation3], and ILC2s can infiltrate PDAC tissue and activate tissue-specific anti-tumor immunity leading to better outcomes. Additionally, PDAC-specific ILC2s express PD1 and anti-PD1 mAb enhances their anti-tumor potential [Citation14]. Furthermore, blocking IL-17Rb with a specific monoclonal antibody results in inhibition of PDAC metastasis and improved survival in in vivo models [Citation15]. Overall, immunotherapies that can repurpose TC-regulated systems, such as those responsible for type II immunity, against pancreatitis and PDAC are likely to be a potential mode of action that could be exploited in the near future, but further research will be required.
In conclusion, before TC-specific therapeutic strategies can be implemented, it will be key to clarify the underlying biology of TCs in both pancreatitis and PDAC. Notably, in the intestine, TCs have been definitively identified as both key regulators of the immune and neuronal response to infection and inflammation, as well as cells of origin for cancer. Multiple recent studies using single-cell RNA sequencing (scRNA-Seq) have demonstrated the existence of two unique classes of TCs with apparent variation in function. The field of pancreatic TC research has not yet reached this stage, but granular detail will be necessary to safely target TCs and their associated processes to alleviate pancreatitis and reduce PDAC burden. Taken together, findings representing an apparent contradiction in terms of TC function demand further study. It is possible that the need for TCs to balance dual roles in their sentinel/inflammatory signaling capacity and potential cell-of-origin capacity may lead to evolutionary selection of tumor suppressor functions that allow them to expand to counter threats from non-cancerous disease while limiting their potential to initiate PDAC. If this is the case, a unified view of TCs is likely to emerge that may be conceptually likened to the discovery in immunology of roles for M2 macrophages in both essential anti-inflammatory and potentially pro-tumorigenic processes of the tissue microenvironment.
Declaration of Interest
N Weygant and D Qu are Co-inventors on United States Patent US9663585 describing a DCLK1 monoclonal antibody therapy. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
Additional information
Funding
References
- DelGiorno KE, Naeem RF, Fang L, et al. Tuft cell formation reflects epithelial plasticity in pancreatic injury: implications for modeling human pancreatitis. Front Physiol. 2020;11:88.
- Delgiorno KE, Hall JC, Takeuchi KK, et al. Identification and manipulation of biliary metaplasia in pancreatic tumors. Gastroenterology. 2014 Jan;146(1):233–44 e5.
- von Moltke J, Ji M, Liang HE, et al. 25 regulates an intestinal ILC2-epithelial response circuit. Nature. 2016 Jan 14; 529(7585):221–225.
- Gerbe F, Sidot E, Smyth DJ, et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature. 2016 Jan 14;529(7585):226–230.
- Liou GY, Bastea L, Fleming A, et al. The presence of interleukin-13 at pancreatic ADM/PanIN lesions alters macrophage populations and mediates pancreatic tumorigenesis. Cell Rep. 2017 May 16;19(7):1322–1333.
- Are C, Chowdhury S, Ahmad H, et al. Predictive global trends in the incidence and mortality of pancreatic cancer based on geographic location, socio-economic status, and demographic shift. J Surg Oncol. 2016 Nov;114(6):736–742.
- Stopa KB, Kusiak AA, Szopa MD, et al. Pancreatic cancer and its microenvironment-recent advances and current controversies. Int J Mol Sci. 2020 May 1;21(9):3218.
- Westphalen CB, Takemoto Y, Tanaka T, et al. Dclk1 defines quiescent pancreatic progenitors that promote injury-induced regeneration and tumorigenesis. Cell Stem Cell. 2016 Apr 7;18(4):441–455.
- Gerbe F, Legraverend C, Jay P. The intestinal epithelium tuft cells: specification and function. Cell Mol Life Sci. 2012;69(17):2907–2917.
- Schütz B, Ruppert A-L, Strobel O, et al. Distribution pattern and molecular signature of cholinergic tuft cells in human gastro-intestinal and pancreatic-biliary tract. Sci Rep. 2019;9(1). DOI:10.1038/s41598-019-53997-3
- Weygant N, Qu D, Berry WL, et al. Small molecule kinase inhibitor LRRK2-IN-1 demonstrates potent activity against colorectal and pancreatic cancer through inhibition of doublecortin-like kinase 1. Mol Cancer. 2014 May 6;13:103.
- Ferguson FM, Nabet B, Raghavan S, et al. Discovery of a selective inhibitor of doublecortin like kinase 1. Nat Chem Biol. 2020 Jun;16(6):635–643.
- DelGiorno KE, Chung CY, Vavinskaya V, et al. Tuft cells inhibit pancreatic tumorigenesis in mice by producing prostaglandin D2. Gastroenterology. 2020 Jul 24;S0016-5085(20)34999-4.
- Moral JA, Leung J, Rojas LA, et al. ILC2s amplify PD-1 blockade by activating tissue-specific cancer immunity. Nature. 2020 Mar;579(7797):130–135.
- Wu HH, Hwang-Verslues WW, Lee WH, et al. Targeting IL-17B-IL-17RB signaling with an anti-IL-17RB antibody blocks pancreatic cancer metastasis by silencing multiple chemokines. J Exp Med. 2015 Mar 9;212(3):333–349.
