1. Introduction
Glycosylation is an important post-translational modification of cellular glycoprotein by the stepwise addition of sugar residues to form a complex glycan structure. Glycans play essential roles in many biological processes; such as cell-cell communication, cell adhesion, ligand–receptor interaction, self and nonself recognition, etc. Alteration of glycosylation has been considered a hallmark of cancer as it plays important role in tumor development and progression [Citation1]. The aberrant glycosylation is possibly triggered by the overproduction of nucleotide-sugar donors and/or altered expression of glycosyltransferase and glycosidase enzymes [Citation2]. Not only aberrant glycosylation, but the over-expression of carrier-proteins is also an important factor to promote tumor growth and metastasis. These aberrations are possibly the targets for cancer immunotherapy or chemo-sensitization. Also, the cancer-associated glycans and glycoconjugates can possibly be the biomarkers for diagnosis, monitoring, and prognostic prediction of the disease. Collective data in cholangiocarcinoma (CCA) revealed that glycosylation is altered during tumorigenesis and progression of CCA. The CCA-associated glycans were aberrantly expressed and play many essential roles in tumor metastasis and drug resistance, which were possibly used as the targets for CCA treatment. Moreover, the CCA-associated glycans that are elevated in patients’ sera are beneficial as biomarkers for diagnosis and prognosis of CCA.
2. Glycans and CCA
2.1. Roles of glycans in CCA metastasis and chemoresistance
Glycomics and glycoproteomics approach on CCA tissues revealed the aberrant glycosylation and consequent elevation of CCA-associated glycans in cancer cells compared with hepatocytes and normal biliary cells. A high level of expression of these glycans was associated with poor prognosis and shorter survival of CCA patients. This information agrees with the results from functional analyses of CCA-associated glycans which play important roles in the progression of CCA ().
Table 1. Applications of CCA-associated glycans and specific lectins in CCA
2.1.1. GalNAc glycans
N-acetyl galactosamine (GalNAc)-containing glycans were drastically increased in CCA compared with the normal bile ducts in adjacent tissues [Citation3–5]. The O-linked GalNAc glycan was found to regulate CCA metastasis via Akt/Erk signaling pathway. Modulation of GalNAc modification by knockdown or overexpression of GalNAc-transferase (GalNT)-5 was shown to affect the metastatic ability of CCA cells [Citation5].
2.1.2. GlcNAc glycans
O-GlcNAcylation, the reversible modification of nucleocytoplasmic proteins by N-acetyl glucosamine (GlcNAc), is controlled by O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA), and was found to promote metastasis of CCA. O-GlcNAc regulated N-glycosylation through Erk/Akt signaling pathway and phosphorylation of Forkhead box-O36. Blockade of surface N-glycan by a mannose-binding lectin from Pisum sativum resulted in significant suppression of the metastatic abilities of CCA cells [Citation6].
2.1.3. Fucosylated glycans
Peripheral glycans, including fucosylated- and sialylated-glycans, were also demonstrated to promote the progression of CCA. Fucosylated-glycans, such as a(1,2)-fucose-modified glycan and Lewis-associated glycans were found to facilitate the migration and invasion of CCA cells [Citation7–9]. Fucosyltransferase (FUT)-1, an enzyme response for a(1,2)-fucose modification, was found to regulate the EGF/EGFR activation in CCA cells [Citation9]. Metastatic abilities of CCA cells were significantly suppressed by specific siRNA against FUT1 and FUT3. Neutralization of surface Lewis-associated antigens using a specific monoclonal antibodies against CA-S27 or sialyl-Lewis A (sLeA) could significantly reduce the metastatic abilities of CCA cells. The metastatic suppression effect was also observed after treatment with Ulex europaeus agglutinin-I (UEA-I, a(1,2)-fucose binding lectin).
2.1.4. Sialylated glycans
Different from other glycosylation processes, the terminal sialic acid was found to be involved in the chemoresistance of CCA. Suppression of sialic acid modification by a sialyltransferase inhibitor, 3Fax-peracetyl-Neu5Ac, could enhance the sensitivity of CCA cells to 5-fluorouracil (5-FU), a general chemotherapeutic drug used for cancer treatment [Citation10].
These collective pieces of evidence provide the imperative information about the glycans in promoting CCA metastasis and chemoresistance, suggesting CCA-associated glycans as the potential targets for CCA treatment.
2.2. Glycan as a marker for diagnosis and prognostic prediction of CCA
Several CCA-associated glycans are highly expressed in patients’ tissues and sera, which can be used as the potential markers for diagnosis and prognostic prediction of CCA.
The information obtained through glycomic analysis using lectin-based approaches showed that many GalNAc-binding lectins; including Wisteria floribunda agglutinin (WFA) [Citation4], Sophora japonica agglutinin (SJA) [Citation3], and Vicia villosa lectin (VVL) [Citation5]; exhibited a strong reactivity with preneoplastic bile ducts and CCA, but not normal bile ducts, suggesting their specificity to bile duct pathology. The glycans comprised in mucin glycoproteins are possibly been shredded and secreted into serum and bile of CCA patients. The CCA-associated glycoforms of mucin glycoproteins, WFA-bound MUC1 [Citation4], and soybean agglutinin (SBA)-bound MUC5AC [Citation11], were highly detected in serum and/or bile of CCA patients and applicable for the diagnosis of CCA. Lewis-associated glycans, including carbohydrate antigen 19–9 (CA19-9, sialyl-Lewis A) and carbohydrate antigen-S27 (CA-S27, Lewis-A-associated glycan), were highly detected in the sera of CCA patients compared with non-CCA controls [Citation7,Citation12]. A high level of these serum glycans was found to associate with poor prognosis and the short survival of CCA patients, suggesting their ability to be a poor prognostic marker for CCA.
Moreover, a high level of O-GlcNAc-modified proteins, MAL-II binding a(2,3)-sialylated glycan, and a(1,2)-fucose modified glycan in tumor tissues was associated with the unfavorable fate of CCA patients, suggesting their application as a potential poor prognostic indicator for CCA.
3. Expert opinion
Glycans are the potential targets for the treatment of CCA as well as biomarkers for diagnosis and prognostic prediction of the disease (). As mentioned as above, most of the CCA-associated glycans; N-glycans, O-GlcNAc, O-GalNAc, and the terminal fucose and Lewis antigens; were found to play important roles in tumor metastasis. Thus, these CCA-associated glycans are probably promising targets for the development of anti-metastatic agents. Furthermore, sialic acid-modified glycan was found to be involved in the chemoresistance of CCA. Therefore, it is possible to target the sialic acid modification for chemo-sensitization in CCA treatment. The combination of glycosylation inhibitors and the standard chemotherapeutic drugs, such as 5-FU, gemcitabine, and cisplatin, is a fascinating approach to be considered to improve the chemotherapy of CCA.
Figure 1. Schematic diagram for biosynthesis of CCA-associated glycans
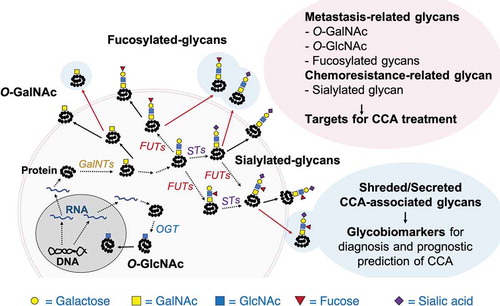
Several approaches can possibly be used to target the CCA-associated glycans for CCA treatment [Citation13]. Inhibition of glycan biosynthesis by suppression of glycosyltransferases such as OGT, GalNT, FUTs, and sialyltransferases using specific inhibitors or RNAi is one of the promising strategies for CCA treatment. Several glycosylation inhibitors have been synthesized and studied on many types of cancer () [Citation14,Citation15]. Further, comprehensive in vivo and clinical studies are still needed on the efficiency of these inhibitors for CCA treatments. The application of RNAi-based treatment for cancer patients are recently facing with many obstacles regarding the efficiency and safety of RNAi delivery. The success of the development of RNAi-based treatment for CCA may lead to the success of the targeted-therapy of CCA in the future.
Table 2. Examples of glycosylation inhibitors
Targeted immunotherapy is also a great challenge for CCA treatment. The use of specific monoclonal antibodies or the development of the chimeric antigen receptor (CAR)-T cells that recognizes CCA-associated glycans are the anticipative strategies to improve the immunotherapy of CCA.
Using lectins that exhibit high affinity and specificity to bind the target CCA-associated glycans are one of the key strategies to be considered. Many lectins were demonstrated to exhibit strong anti-cancer activity and potentially be used for cancer treatment [Citation16]. The clinical trial of a lectin from mistletoe has been performed on many types of cancer and it seems to be a promising anti-cancer drug without serious side effects to the patients [Citation16]. Based on the in vitro studies, Pisum sativum agglutinin (PSA) and UEA1 could significantly suppress the metastatic ability of CCA cells and are considered potential anti-metastatic agents for CCA. However, to assure the clinical effectiveness of these lectins as an anti-metastatic agent for CCA, further in vivo and clinical studies are needed. The chimeric antibodies or lectins against CCA-associated glycans are also possible potential anti-cancer agents as they were presumed to provide the high therapeutic efficiency with lower side effects, comparing with the full-length molecules.
Although CCA-associated glycan seems to be a promising target for CCA treatment, it may not applicable for all CCA cases as a particular glycan is expressed only in some, but not all CCA cases. To achieve the most effective way of targeting the CCA-associated glycans for CCA treatment, their expressions in the resected- or biopsied-tissues or serum samples should be investigated prior to the treatment. Inclusion of the determination of CCA-associated glycans as one of the criteria of CCA management may facilitate the clinicians to select the most effective-targeted-therapy.
Besides the target for CCA treatment, glycans are also applicable as the biomarkers for diagnosis and prognostic prediction of the disease. CCA-associated glycans, especially GalNAc-containing glycans, were highly detected in tissue and/or serum of the CCA patients. The expression pattern of these glycans are variable among CCA patients [Citation12]. Combined analysis of these CCA-associated glycans can improve the diagnostic and prognostic power of glycans [Citation12]. To improve the diagnosis and prognostic prediction of CCA, the development of the promising multi-glycobiomarker panel is an important and urgent task to be considered.
As glycoproteins are composed of peptide and glycans, the lectin-based approaches are commonly used for the analysis of CCA-associated glycans. Development of diagnostic kit using lectin-lectin or lectin-antibody sandwich approaches may improve their efficiency to detect CCA-associated glycobiomarkers as it combines the preferential ability of monoclonal antibodies to proteins and of lectins to glycans.
4. Conclusion
The cumulative information showed that CCA cells exhibited the altered glycosylation, which leads to the aberrant expression of CCA-associated glycans. These tumor-associated glycans can be a potential biomarker for diagnosis and prognostic prediction of CCA as well as the targets for treatment.
Declaration of interests
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Acknowledgments
The author would like to thank Prof. Yukifumi Nawa for the English editing via KKU Publication Clinic.
Additional information
Funding
References
- Vajaria BN, Patel PS. Glycosylation: a hallmark of cancer? Glycoconj J. 2017 Apr;34(2):147–156.
- Silsirivanit A. Glycosylation markers in cancer. Adv Clin Chem. 2019;89:189–213.
- Saentaweesuk W, Silsirivanit A, Vaeteewoottacharn K, et al. Clinical significance of GalNAcylated glycans in cholangiocarcinoma: values for diagnosis and prognosis. Clin Chim Acta. 2018;477:66–71.
- Matsuda A, Kuno A, Kawamoto T, et al. Wisteria floribunda agglutinin-positive mucin 1 is a sensitive biliary marker for human cholangiocarcinoma. Hepatology. 2010 Jul;52(1):174–182.
- Detarya M, Sawanyawisuth K, Aphivatanasiri C, et al. The O-GalNAcylating enzyme GALNT5 mediates carcinogenesis and progression of cholangiocarcinoma via activation of AKT/ERK signaling. Glycobiology. 2020 Apr 20;30(5):312–324.
- Phoomak C, Silsirivanit A, Park D, et al. O-GlcNAcylation mediates metastasis of cholangiocarcinoma through FOXO3 and MAN1A1. Oncogene. 2018 Oct;37(42):5648–5665.
- Silsirivanit A, Araki N, Wongkham C, et al. CA-S27: a novel Lewis a associated carbohydrate epitope is diagnostic and prognostic for cholangiocarcinoma. Cancer Sci. 2013 Oct;104(10):1278–1284.
- Juntavee A, Sripa B, Pugkhem A, et al. Expression of sialyl Lewis(a) relates to poor prognosis in cholangiocarcinoma. World J Gastroenterol. 2005 Jan 14;11(2):249–254.
- Indramanee S, Sawanyawisuth K, Silsirivanit A, et al. Terminal fucose mediates progression of human cholangiocarcinoma through EGF/EGFR activation and the Akt/Erk signaling pathway. Sci Rep. 2019 Nov 21;9(1):17266.
- Wattanavises S, Silsirivanit A, Sawanyawisuth K, et al. Increase of MAL-II binding alpha2,3-sialylated glycan is associated with 5-FU resistance and short survival of cholangiocarcinoma patients. Medicina (Kaunas). 2019 Nov 28;55(12):1–10.
- Bamrungphon W, Prempracha N, Bunchu N, et al. A new mucin antibody/enzyme-linked lectin-sandwich assay of serum MUC5AC mucin for the diagnosis of cholangiocarcinoma. Cancer Lett. 2007 Mar 18;247(2):301–308.
- Silsirivanit A, Matsuda A, Kuno A, et al. Multi-serum glycobiomarkers improves the diagnosis and prognostic prediction of cholangiocarcinoma. Clin Chim Acta. 2020 Jul 11;510:142–149.
- Costa AF, Campos D, Reis CA, et al. Targeting glycosylation: a new road for cancer drug discovery. Trends Cancer. 2020 Sep 6;6(9):757–766.
- Esko JD, Bertozzi C, Schnaar RL, et al. Chemical Tools for Inhibiting Glycosylation. In: Varki A, Cummings RD, Esko JD, et al., editors Essentials of Glycobiology. (NY): Cold Spring Harbor; 2015. p. 701–712. rd.
- Mereiter S, Balmana M, Campos D, et al. Glycosylation in the era of cancer-targeted therapy: where are we heading? Cancer Cell. 2019 Jul 8;36(1):6–16.
- Yau T, Dan X, Ng CC, et al. Lectins with potential for anti-cancer therapy. Molecules. 2015 Feb 26;20(3):3791–3810.
