KEYWORDS:
1. Introduction
Ulcerative colitis (UC) is a chronic gastrointestinal inflammatory condition characterized by inflammation of the intestinal mucosa, which can extend from the rectum to the entire length of the colon. Together with Crohn’s Disease, it represents the most frequent manifestation of Inflammatory Bowel Disease (IBD) and its most frequent symptoms include abdominal pain and diarrhea with presence of blood. Although the precise etiology of UC is poorly understood, many factors contribute to this condition including genetic background, microbiome dysbiosis, dietary and environmental factors, and dysregulated mucosal inflammation[Citation1]. The incidence of UC is increasing among both adult and pediatric patients in developed countries, and the prevalence of disease has been estimated to range from 2.4 [Citation2] to 294 [Citation3] cases per 100,000 persons in Europe [Citation4]. Furthermore, the total direct costs for UC annually are estimated at between 12.5 and 29.1 billion euros in Europe with costs associated with hospitalizations and surgery increasing with disease severity [Citation5]. While historically, therapeutic approaches have been aimed at alleviating symptoms of disease, more recently the focus has shifted toward inhibiting specific pathways associated with dysregulated inflammation and promoting mucosal healing as a means of more effectively treating patients. Despite significant new and emerging advances in this regard, it is estimated that up to 30–40% of patients are unresponsive to currently available therapeutic options. This underlines an important need to improve and individualize treatment approaches.
2. Current therapeutic strategies for UC
Arguably the most significant advance in UC treatment in recent decades has coincided with the development of biotherapeutics aimed at targeting specific inflammatory cytokines. Prior to this, the use of agents such as sulfonamides, aminosalicylates, corticosteroids, and antibiotics, although successful, were beset with issues including high rates of relapse and deleterious side effects [Citation6–13].
Toward the end of the last century, disease modifying immunosuppressant agents such as cyclosporine and tacrolimus emerged as new therapeutic approaches, underscoring the validation of targeting aberrant inflammation as an effective strategy for UC [Citation14]. This was quickly followed by the emergence of monoclonal antibodies (mAbs) aimed at neutralizing pro-inflammatory cytokines, initially focused on treating patients for whom traditional approaches had failed [Citation15]. The first monoclonal antibody licensed for treatment of IBD was infliximab, an anti-TNF antibody [Citation16]. Subsequently, further anti-TNF agents such as adalimumab and golimumab have also entered the clinic [Citation17,Citation18]. A second successful cytokine-directed approach has been to target the shared p40 subunit of IL-12 and IL-23 which are key instructive cytokines in driving proinflammatory T helper 1/17 responses. Ustekinumab, which directly targets the p40 subunit, has been approved for moderate to severe UC [Citation19]. Further mAbs, which specifically target the p19 subunit of IL-23 including brazikumab, risankizumab, guselkumab, and mirikizumab, are also currently being evaluated as potential therapeutics in IBD [Citation20].
In addition to anti-cytokine therapies, new biotherapeutics have also been developed with the aim of restricting pathogenic immune cell infiltration to the gut. These include vedolizumab, which binds the α4β7 integrin and is suggested to inhibit leukocyte migration toward the intestinal mucosa [Citation21,Citation22]. Interestingly, Feagan et al. demonstrated that vedolizumab was efficacious as both an induction and maintenance therapy for UC [Citation23]. These findings have been supported by subsequent studies which have also reported a favorable safety profile [Citation24,Citation25] .
Another biotherapeutic that limits the migration of lymphocytes to the gut is the sphingosine 1-phosphate (S1P) receptor modulator, ozanimod. The results of phase II and III clinical trials have shown that ozanimod enhances clinical remission in patients with moderate to severe UC and has a satisfactory long-term safety profile [Citation26,Citation27]. Similar approaches, currently under clinical development include anti-adhesion molecules such as AJM347 (α4β7-integrin inhibitor), AJM300 (anti-α4-integrin antibody), ontalizumab (anti-MadCAM1 antibody), and etrolizumab (anti-β7-integrin antibody) [Citation28]. The efficacy and safety of etrolizumab as a treatment for UC has been evaluated in phase 3 clinical trials enrolling over 3,000 patients [Citation25]. Recently reported findings indicate that while treatment with etrolizumab can provide benefit to patients during the induction phase, such improvements were less evident in maintenance studies [Citation26,Citation27,Citation29–31].
Most recently, small molecule inhibitors of the Janus kinases (JAK) 1/3 signaling cascade have gained considerable attention as potential treatments for IBD [Citation32]. While tofacitinib (JAK1-3) Inhibitor is approved for UC, several other inhibitors currently under clinical evaluation include peficitinib (JAK3 inhibitor), TD-1473 (gut-selective pan-JAK inhibitor), deucravacitinib (TYK2 inhibitor), filgotinib (JAK1 inhibitor), and upadacitinib (JAK1 inhibitor). Filgotinib was shown to effectively induce and maintain clinical remission in patients with moderate to severe UC [Citation33] and has recently been approved in Europe as a treatment for UC. Furthermore, a recent meta-analysis of data from 28 clinical trials showed that upadacitinib resulted in improved induction of clinical remission of moderate to severe UC in comparison to a range of other small molecules and biologics but was also more likely to lead to adverse events [Citation30].
While the landscape of new therapeutic entities for UC continues to expand, there is an appreciation that not all patients will be responsive to specific treatment approaches. This is due to an incomplete understanding of the complex nature of disease etiology and pathogenesis coupled with the individualized nature of disease progression among patients. While combination therapeutic approaches have been proposed and are being evaluated in patients that do not respond to single modes of therapy [Citation34,Citation35], there is a pressing need to identify biomarker signatures of patient responsiveness to define which approaches are most likely to achieve improved patient outcomes and the appropriate timing of such interventions. An ever growing understanding of the pathologic pathways driving mechanisms of disease continues to reveal new targets which may be relevant in certain patients. One such emerging target is the IL-36 subfamily of cytokines which appear to play a distinct role in regulating the balance between inflammation and homeostasis in the intestine.
3. Role of IL-36 cytokine family in UC
The IL-36 cytokines are members of the broader IL-1 cytokine family. The IL-36 family comprises three agonist ligands, IL-36 alpha (IL-1F6), IL-36 beta (IL-1F8), and IL-36 gamma (IL-1F9), and two antagonist ligands IL-36 Ra (IL-1F5) and IL-38. All members bind to the IL-36 receptor, a heterodimer composed of the specific IL-36 R (IL-1Rrp2) and IL-1RAcP chains [Citation36,Citation37]. IL-36 cytokines are secreted by epithelial cells and mononuclear cells in the gut [Citation36] and are thought to be secreted in a non-active form that requires proteolytic processing for full activation. It is noteworthy that neutrophil-derived proteases, including proteinase-3, elastase, and cathepsin G, have been shown to activate IL-36 family members in vitro [Citation38,Citation39], and neutrophil infiltration is a characteristic of the inflamed gastrointestinal mucosa in UC [Citation40]. Indeed, the inhibition of gastrointestinal mucosal protease activity has been proposed as a potential therapeutic option for IBD [Citation41].
IL-36 cytokines have been identified as key pathogenic drivers of a rare autoinflammatory disease condition known as Deficiency of Interleukin-36 Receptor Antagonist (DITRA), which is associated with loss-of-function mutations in the IL36RN gene and manifests primarily as chronic generalized pustular psoriasis (GPP) [Citation42–45]. These discoveries have prompted further investigations into the role of these cytokines, not only in more common dermal diseases, but also in chronic inflammatory bowel disease.
Elevated levels of IL-36α and IL-36γ expression have been described in the inflamed colonic mucosa of UC patients as well as in several murine models of disease [Citation46–49]. Despite this, the precise role of IL-36 cytokines in this setting remains controversial. While some studies have described their role in promoting intestinal inflammation through the recruitment and activation of neutrophils, inflammatory monocytes, and pro-inflammatory CD4 + T cells [Citation50,Citation51], others have demonstrated that IL-36 promotes resolution of intestinal inflammation through enhanced mucosal healing [Citation48,Citation49,Citation52]. More recently, IL-36 has been implicated in driving fibrosis as a result of chronic intestinal inflammation by Scheibe and colleagues [Citation53], who observed high levels of IL36 cytokine expression in fibrotic tissue in the colon of patients affected with IBD. In addition, several studies have indicated that IL-36 cytokines can influence microbiome dysbiosis which likely plays a significant role in intestinal homeostasis and inflammation [Citation52,Citation54]. Although further studies are required, in order to fully elucidate the specific mechanisms through which IL-36 cytokines influence disease pathogenesis in UC, several ongoing clinical trials are evaluating whether inhibition of IL-36 R signaling can improve patient outcomes.
4. Potential therapies focused on IL-36 R inhibition
As evidence accumulates that current gold standard treatment approaches for UC remain ineffective among large numbers of patients [Citation55], efforts to identify new and improved targets for this cohort have continued. With the emergence of IL-36 cytokines as instructive signals in gastrointestinal inflammation several strategies aimed at their inhibition have been developed (). Spesolimab (BI655130) is a humanized monoclonal IgG1 antibody against IL-36 R that has shown therapeutic potential in a phase I proof-of-concept study in patients affected with Generalized Pustular Psoriasis (GPP) [Citation56]. More recently, a follow on phase II clinical trial (NCT03782792) also reported enhanced disease remission in GPP patients treated with spesolimab. However, it was also noted that treatment was accompanied by the development of anti-drug anitbodies and infection in some patients [Citation55], indicating that further, more expansive trials are warranted.
Figure 1. Therapeutic interventions in IL-36-mediated inflammation. Neutrophil-derived proteases process IL-36 cytokines (IL-36a, IL-36B and IL-36y) into fully active forms via proteolytic cleavage. Activation of IL-36 agonists allows their interaction with the IL-36 receptor (IL-36R) and recruitment of the interleukin-1 receptor accessory protein (IL-1-RAcP) to form an IL-36R/IL-1RAcP heterodimer. The formation of this receptor complex initiates NFkB and MAPK signalling and a subsequent IL-36-mediated inflammatory response. The IL-36 receptor antagonist (IL-36Ra) exists naturally and inhibits the pro-inflammatory response elicited by IL-36 agonists by binding to the IL-36R and preventing the association of IL-36R and IL-1RAcP. Therapeutics are also capable of inhibiting this IL-36-mediated inflammatory cascade. Spesolimab, for example, is a monoclonal antibody that binds to the IL-36R and prevents its interaction with IL-36 agonists. Additional therapeutics include Anti-IL-1RAcP, which interacts with IL-1RAcP to inhibit the formation of the IL-36R/IL-1RAcP complex, and protease inhibitors, which prevent the processing of IL-36 agonists into active forms. A-552 is a more recently discovered small molecule that exclusively antagonizes IL-36y to attenuate IL-36y-specific responses.
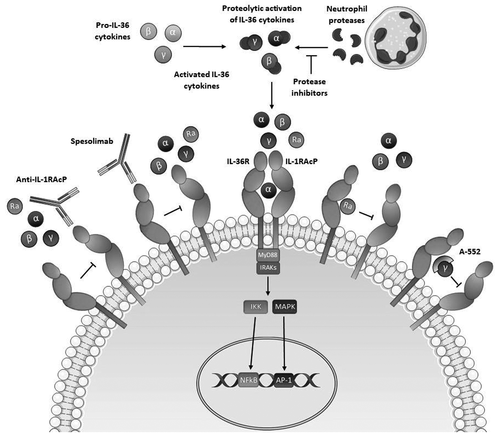
Spesolimab is also currently under clinical investigation in UC. Importantly, these trials will evaluate efficacy in non-responder UC patients (NCT03482635) [Citation56], who have demonstrated neither a clinical response during the induction period nor any improvement in symptomatology following previous treatment with TNF antagonists and/or vedolizumab. In addition, healing of the intestinal mucosa is being evaluated in patients with mild or moderate UC receiving spesolimab as a combination treatment with TNF-inhibitors (NCT03123120) [Citation57], while the safety and long-term efficacy of IL-36 R blockade with spesolimab in UC are also under evaluation (NCT03648541) [Citation58]. Although at earlier stages in development, several other approaches aimed at targeting IL-36 activity may offer therapeutic potential for UC. For example, specific protease inhibitors of cathepsin G and elastase have been shown to inhibit IL-36 pathogenic activity in the context of psoriatic disease [Citation59] and may constitute a potential therapy for other inflammatory diseases including UC. While blockade of the IL36R with spesolimab will inhibit the signaling of all IL-36 ligands, specific targeting of individual IL-36 cytokines may represent a more directed approach. This may prove more beneficial given the reported dichotomous roles of IL-36 cytokines in inflammation versus resolution. In this regard, the approach of Todorovic and colleagues who developed a specific antagonist of IL-36γ, named A-552, with validation in preclinical models of psoriasis, is of interest [Citation60]. In contrast, in the inflamed intestine a broader inhibitory approach may be more appropriate given the reported influence of other IL-1 family members in mucosal inflammation. Recently, Højen and colleagues [Citation61] developed a monoclonal antibody targeting the shared IL-1RAcP which was found to be effective in blocking inflammation driven by IL-1 family members, including IL-36 cytokines, in several mouse models of disease [Citation61].
5. Expert opinion
Emerging data continue to implicate IL-36 cytokines as central instructive signals in the pathogenesis of UC. While this data has provided a rationale for the evaluation of monoclonal antibodies against IL-36 R in the clinic, much remains to be resolved concerning how IL-36 family members direct intestinal inflammation and homeostasis. Outstanding issues include the relative importance of the specific IL-36 cytokine family members to the pathogenesis of UC, and whether they can elicit differential effects at different phases of the disease. While most reports are in agreement that the expression of both IL-36α and IL-36γ are elevated in the inflamed colon [Citation62], whether there is functional redundancy between both cytokines has not been fully investigated. This may be of particular relevance if their expression is evident at different temporal phases in the development of chronic intestinal inflammation as observed in UC. Indeed, we have recently demonstrated that IL-36α expression is most significantly elevated in newly diagnosed, treatment-naïve patients presenting with UC, indicating potential mechanistic significance during the earliest phases of disease pathogenesis [Citation63]. Uncovering the precise roles of individual IL-36 cytokines in orchestrating intestinal inflammation and/or resolution will also likely assist in resolving the controversy surrounding the apparent dichotomous roles of IL-36 in the intestine as described above.
Related to this is the question of identifying which specific cell subsets are responsible for mediating the effects of IL-36 R signaling in the intestine during the initiation and chronic phases of UC. Several reports have demonstrated that IL-36 R expression is detected on diverse immune and parenchymal cell subsets and consequently can play diverse roles in colonic inflammation [Citation48,Citation53,Citation63]. Recent advances in single cell transcriptomic and proteomic analysis from the inflamed intestines of UC patients will likely be very informative in this regard [Citation64]. In addition, the development of cell-specific knock-out mice, as recently reported [Citation65,Citation66], will be helpful in uncovering mechanistic insights into the distinct roles of IL-36 at different stages of disease, as has previously been described for the related IL-1 family member, IL-18 [Citation67].
The possible influence of IL-36 cytokines on the intestinal microbiome also remains an important area for investigation which remains relatively unaddressed [Citation52,Citation54]. As a deeper understanding is gained of how alterations in the intestinal microbiome influences disease pathogenesis in UC, the potential role of IL-36 cytokines as mediators of dysbiosis, and how this might influence disease progression is an area which warrants further investigation.
While the results of early clinical trials of spesolimab among patients with UC will provide critically important information in advancing the validation of the IL-36 family as a viable target, elucidating answers to basic questions surrounding their mechanism of action, such as those highlighted above, will continue to be invaluable in efforts to maximize the clinical success of IL-36 as a viable target for UC. In addition, the recent establishment of proof-of-concept for spesolimab in generalized pustular psoriasis will also likely be informative [Citation56]. As further clinical data on the use of spesolimab is reported, the possible identification of specific molecular biomarker signatures to facilitate the potential of utilizing IL-36 inhibition in an individualized fashion will be important [Citation68]. Such advances will not only assist in determining which patients might benefit most from IL-36 directed treatment but will also help identify the circumstances in which patients will likely benefit more from a combination therapeutic approach.
Declaration of interest
PT Walsh receives research funding from Takeda and Janssen. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
Additional information
Funding
References
- Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009 Nov 19; 361(21):2066–2078.
- Gheorghe C, Pascu O, Gheorghe L, et al. Epidemiology of inflammatory bowel disease in adults who refer to gastroenterology care in Romania: a multicentre study. Eur J Gastroenterol Hepatol. 2004 Nov;16(11):1153–1159.
- Jacobsen BA, Fallingborg J, Rasmussen HH, et al. Increase in incidence and prevalence of inflammatory bowel disease in northern Denmark: a population-based study, 1978-2002. Eur J Gastroenterol Hepatol. 2006 Jun;18(6):601–606.
- Burisch J, Jess T, Martinato M, et al. The burden of inflammatory bowel disease in Europe. J Crohns Colitis. 2013 May;7(4):322–337.
- Cohen RD, Yu AP, Wu EQ, et al. Systematic review: the costs of ulcerative colitis in Western countries. Aliment Pharmacol Ther. 2010 Apr;31(7):693–707.
- Kirsner JB. Historical aspects of inflammatory bowel disease. J Clin Gastroenterol. 1988 Jun;10(3):286–297.
- Actis GC, Pellicano R, Fagoonee S, et al. History of inflammatory bowel diseases. J Clin Med. 2019 Nov 14; 8(11):1970.
- Watkinson G. Sulphasalazine: a review of 40 years’ experience. Drugs. 1986;32(1):1–11.
- KIRSNER JB, Palmer WL, Klotz AP. ACTH and cortisone in chronic ulcerative colitis: a comparison of clinical effects. J Lab Clin Med. 1950 Nov;36(5):846.
- Dulai PS, Jairath V. Acute severe ulcerative colitis: latest evidence and therapeutic implications. Ther Adv Chronic Dis. 2018 Feb;9(2):65–72.
- Actis GC, Pellicano R, Ribaldone DG. A concise history of thiopurines for inflammatory bowel disease: from anecdotal reporting to treat-to-target algorithms. Rev Recent Clin Trials. 2019;14(1):4–9.
- Bean RH. The treatment of chronic ulcerative colitis with 6-mercaptopurine. Med J Aust. 1962 Oct 13; 49(2):592–593.
- Hauso Ø, Martinsen TC, Waldum H. 5-Aminosalicylic acid, a specific drug for ulcerative colitis. Scand J Gastroenterol. 2015 Aug;50(8):933–941.
- Actis GC, Pellicano R. Cyclosporine for severe steroid-refractory ulcerative colitis: commenting the comment. Minerva Gastroenterol Dietol. 2018 09;64(3):190–192.
- Guan Q, Zhang J. Recent advances: the imbalance of cytokines in the pathogenesis of inflammatory bowel disease. Mediators Inflamm. 2017;2017:4810258.
- Papamichael K, Rakowsky S, Rivera C, et al. Infliximab trough concentrations during maintenance therapy are associated with endoscopic and histologic healing in ulcerative colitis. Aliment Pharmacol Ther. 2018 02;47(4):478–484.
- Reinisch W, Sandborn WJ, Hommes DW, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011 Jun;60(6):780–787.
- Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014 Jan;1461:85–95. quiz e14-5.
- Sabino J, Verstockt B, Vermeire S, et al. New biologics and small molecules in inflammatory bowel disease: an update. Therap Adv Gastroenterol. 2019;12:1756284819853208.
- Hanžel J, D’Haens GR. Anti-interleukin-23 agents for the treatment of ulcerative colitis. Expert Opin Biol Ther. 2020 04;20(4):399–406.
- Fischer A, Zundler S, Atreya R, et al. Differential effects of α4β7 and GPR15 on homing of effector and regulatory T cells from patients with UC to the inflamed gut in vivo. Gut. 2016 10;65(10):1642–1664.
- Luzentales-Simpson M, Pang YCF, Zhang A, et al. Vedolizumab: potential mechanisms of action for reducing pathological inflammation in inflammatory bowel diseases. Front Cell Dev Biol. 2021;9:612830.
- Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013 Aug 22;369(8):699–710.
- Motoya S, Watanabe K, Ogata H, et al. Vedolizumab in Japanese patients with ulcerative colitis: a phase 3, randomized, double-blind, placebo-controlled study. PLoS One. 2019;14(2):e0212989.
- Takatsu N, Hisabe T, Higashi D, et al. Vedolizumab in the treatment of ulcerative colitis: an evidence-based review of safety, efficacy, and place of therapy. Core Evid. 2020;15:7–20.
- Peyrin-Biroulet L, Hart A, Bossuyt P, et al. Etrolizumab as induction and maintenance therapy for ulcerative colitis in patients previously treated with tumour necrosis factor inhibitors (HICKORY): a phase 3, randomised, controlled trial. Lancet Gastroenterol Hepatol. 2022 02;7(2):128–140.
- Vermeire S, Lakatos PL, Ritter T, et al. Etrolizumab for maintenance therapy in patients with moderately to severely active ulcerative colitis (LAUREL): a randomised, placebo-controlled, double-blind, phase 3 study. Lancet Gastroenterol Hepatol. 2022 01;7(1):28–37.
- Kobayashi T, Siegmund B, Le Berre C, et al. Ulcerative colitis. Nat Rev Dis Primers. 2020;6(1):74.
- Agrawal M, Verstockt B. Etrolizumab for ulcerative colitis: beyond what meets the eye. Lancet Gastroenterol Hepatol. 2022 01;7(1):2–4.
- Rubin DT, Dotan I, DuVall A, et al. Etrolizumab versus adalimumab or placebo as induction therapy for moderately to severely active ulcerative colitis (HIBISCUS): two phase 3 randomised, controlled trials. Lancet Gastroenterol Hepatol. 2022 01;7(1):17–27.
- Danese S, Colombel JF, Lukas M, et al. Etrolizumab versus infliximab for the treatment of moderately to severely active ulcerative colitis (GARDENIA): a randomised, double-blind, double-dummy, phase 3 study. Lancet Gastroenterol Hepatol. 2022 02;7(2):118–127.
- Danese S. New therapies for inflammatory bowel disease: from the bench to the bedside. Gut. 2012 Jun;61(6):918–932.
- Feagan BG, Danese S, Loftus EV, et al. Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): a phase 2b/3 double-blind, randomised, placebo-controlled trial. Lancet. 2021 Jun 19;397(10292):2372–2384.
- Ribaldone DG, Pellicano R, Vernero M, et al. Dual biological therapy with anti-TNF, vedolizumab or ustekinumab in inflammatory bowel disease: a systematic review with pool analysis. Scand J Gastroenterol. 2019 Apr;54(4):407–413.
- Ahmed W, Galati J, Kumar A, et al. Dual biologic or small molecule therapy for treatment of inflammatory bowel disease: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2021 Mar 31;20(3):e361–e379.
- Walsh PT, Fallon PG. The emergence of the IL-36 cytokine family as novel targets for inflammatory diseases. Ann N Y Acad Sci. 2018 Apr;1417(1):23–34.
- Towne JE, Renshaw BR, Douangpanya J, et al. Interleukin-36 (IL-36) ligands require processing for full agonist (IL-36alpha, IL-36beta, and IL-36gamma) or antagonist (IL-36Ra) activity. J Biol Chem. 2011 Dec 09;286(49):42594–42602.
- Clancy DM, Sullivan GP, Moran HBT, et al. Extracellular neutrophil proteases are efficient regulators of IL-1, IL-33, and IL-36 cytokine activity but poor effectors of microbial killing. Cell Rep. 2018;22(11):2937–2950.
- Henry CM, Sullivan GP, Clancy DM, et al. Neutrophil-derived proteases escalate inflammation through activation of IL-36 family cytokines. Cell Rep. 2016 Feb 02; 14(4):708–722.
- Bressenot A, Salleron J, Bastien C, et al. Comparing histological activity indexes in UC. Gut. 2015 Sep;64(9):1412–1418.
- Vergnolle N. Protease inhibition as new therapeutic strategy for GI diseases. Gut. 2016 07;65(7):1215–1224.
- Cordoro KM, Ucmak D, Hitraya-Low M, et al. Response to Interleukin (IL)-17 inhibition in an adolescent with severe manifestations of IL-36 receptor antagonist deficiency (DITRA). JAMA Dermatol. 2017 Jan 01; 153(1):106–108.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011 Aug;365(7):620–628.
- Onoufriadis A, Simpson MA, Pink AE, et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am J Hum Genet. 2011 Sep;89(3):432–437.
- Sugiura K, Takemoto A, Yamaguchi M, et al. The majority of generalized pustular psoriasis without psoriasis vulgaris is caused by deficiency of interleukin-36 receptor antagonist. J Invest Dermatol. 2013 Nov;133(11):2514–2521.
- Russell SE, Horan RM, Stefanska AM, et al. IL-36α expression is elevated in ulcerative colitis and promotes colonic inflammation. Mucosal Immunol. 2016 09;9(5):1193–1204.
- Boutet MA, Bart G, Penhoat M, et al. Distinct expression of interleukin (IL)-36alpha, beta and gamma, their antagonist IL-36Ra and IL-38 in psoriasis, rheumatoid arthritis and crohn’s disease. Clin Exp Immunol. 2016 May;184(2):159–173.
- Scheibe K, Backert I, Wirtz S, et al. IL-36R signalling activates intestinal epithelial cells and fibroblasts and promotes mucosal healing in vivo. Gut. 2017 05;66(5):823–838.
- Medina-Contreras O, Harusato A, Nishio H, et al. Cutting edge: IL-36 receptor promotes resolution of intestinal damage. J Immunol. 2016 Jan 01;196(1):34–38.
- Harusato A, Abo H, Ngo VL, et al. IL-36gamma signaling controls the induced regulatory T cell-Th9 cell balance via NFkappaB activation and STAT transcription factors. Mucosal Immunol. 2017 Mar 22;10(6):1455–1467.
- Hovhannisyan Z, Liu N, Khalil-Aguero S, et al. Enhanced IL-36R signaling promotes barrier impairment and inflammation in skin and intestine. Sci Immunol. 2020;5(54). doi:https://doi.org/10.1126/sciimmunol.aax1686.
- Ngo VL, Abo H, Maxim E, et al. A cytokine network involving IL-36γ, IL-23, and IL-22 promotes antimicrobial defense and recovery from intestinal barrier damage. Proc Natl Acad Sci U S A. 2018 May;115(22):E5076–E85.
- Scheibe K, Kersten C, Schmied A, et al. Inhibiting interleukin 36 receptor signaling reduces fibrosis in mice with chronic intestinal inflammation. Gastroenterology. 2019 Nov;156(4):1082–1097.
- Giannoudaki E, Hernandez-Santana YE, Mulfaul K, et al. Interleukin-36 cytokines alter the intestinal microbiome and can protect against obesity and metabolic dysfunction. Nat Commun. 2019 Sep;10(1):4003.
- Ungar B, Kopylov U. Advances in the development of new biologics in inflammatory bowel disease. Ann Gastroenterol. 2016;29(3):243–248.
- Bachelez H, Choon SE, Marrakchi S, et al. Inhibition of the Interleukin-36 pathway for the treatment of generalized pustular psoriasis. N Engl J Med. 2019 03;380(10):981–983.
- Ingelheim B. NCT03123120: a study in patients with mild or moderate ulcerative colitis who take a TNF inhibitor. The Study Investigates Whether Bowel Inflammation Improves When Patients Take BI 655130 in Addition to Their Current Therapy. 2017.
- Ingelheim B. NCT03648541: BI 655130 Long-term treatment in patients with moderate-to severe ulcerative colitis. Vet Rec. 2018;182(21). doi:https://doi.org/10.1136/vr.k2260
- Sullivan GP, Henry CM, Clancy DM, et al. Suppressing IL-36-driven inflammation using peptide pseudosubstrates for neutrophil proteases. Cell Death Dis. 2018 Mar;9(3):378.
- Todorović V, Su Z, Putman CB, et al. Small molecule IL-36γ antagonist as a novel therapeutic approach for plaque psoriasis. Sci Rep. 2019;9(1):9089.
- Højen JF, Kristensen MLV, McKee AS, et al. IL-1R3 blockade broadly attenuates the functions of six members of the IL-1 family, revealing their contribution to models of disease. Nat Immunol. 2019 09;20(9):1138–1149.
- Leon G, Hussey S, Walsh PT. The diverse roles of the IL-36 family in gastrointestinal inflammation and resolution. Inflamm Bowel Dis. 2021;27(3):440–450.
- Leon G, Hernandez Santana YE, Irwin N, et al. IIL-36 cytokines imprint a colitogenic phenotype on CD4+ T helper cells. Mucosal Immunol 2022; In Press. 2022 Jan 18th;15(3):491–503.
- Smillie CS, Biton M, Ordovas-Montanes J, et al. Intra- and inter-cellular rewiring of the human colon during ulcerative colitis. Cell. 2019;178(3):714–30.e22.
- Hernández-Santana YE, Leon G, St Leger D, et al. Keratinocyte interleukin-36 receptor expression orchestrates psoriasiform inflammation in mice. Life Sci Alliance. 2020 04;3(4):e201900586.
- Goldstein JD, Bassoy EY, Caruso A, et al. IL-36 signaling in keratinocytes controls early IL-23 production in psoriasis-like dermatitis. Life Sci Alliance. 2020 06;3(6):e202000688.
- Nowarski R, Jackson R, Gagliani N, et al. Epithelial IL-18 equilibrium controls barrier function in colitis. Cell. 2015 Dec 3;163(6):1444–1456.
- Baum P, Visvanathan S, Garcet S, et al. Pustular psoriasis: molecular pathways and effects of spesolimab in generalized pustular psoriasis. J Allergy Clin Immunol. 2022 Apr;149(4):1402–1412.
