ABSTRACT
Introduction: Activation of the MET pathway through MET amplifications or mutations is present in 3–4% of stage IV non-squamous non-small cell lung cancers (NSCLC). High MET amplifications and exon 14 skipping mutations are associated with poor prognosis: new treatments are needed for these patients. Capmatinib is a highly selective, potent small-molecule MET inhibitor with antitumor activity in NSCLC in vitro and in vivo.
Areas covered: This article provides an overview of the capmatinib clinical development program in NSCLC, both as monotherapy in NSCLC with a dysregulated MET pathway, and in combination with epidermal growth factor receptor (EGFR) inhibitor therapy in EGFR-mutant NSCLC with MET-based acquired resistance to previous EGFR inhibition.
Expert opinion: In the GEOMETRY Mono-1 study, treatment with capmatinib resulted in high response rates in stage IV NSCLC with MET exon 14 skipping mutations, particularly in first line, supporting testing for this biomarker at the time of diagnosis. Durable responses have been reported and results in MET-amplified NSCLC are eagerly anticipated. In EGFR-mutant NSCLC, notable responses have been observed in combination with an EGFR-tyrosine kinase inhibitor (TKI) in case of acquired resistance to EGFR-TKIs based on high MET amplification.
1. Introduction
Lung cancer is the most frequently diagnosed type of cancer in men, and the estimated incidence in both men and women in 2018 was 2 million cases worldwide [Citation1]. Non-small cell lung cancer (NSCLC) accounts for about 85% of all lung cancer cases and it is often diagnosed at an advanced stage [Citation2–Citation4]. Several factors may contribute to the late diagnosis of lung cancer; improving diagnostic pathways (e.g. screening by low-dose computerized tomography scan), and increasing symptom awareness may create a shift towards earlier diagnosis of NSCLC [Citation5–Citation9].
The most common histological subtypes of NSCLC are adenocarcinoma, large-cell carcinoma, squamous cell carcinoma, and NSCLC not otherwise specified [Citation10]. Patients with advanced lung cancer with non-squamous histology or squamous histology with the presence of clinical features that indicate a higher probability of a driver mutation should be routinely tested for EGFR mutations, ALK translocations, ROS1 rearrangements, and BRAF mutations, because valid and approved targeted therapies are available for these patients [Citation4,Citation10].
A more recently documented oncogenic driver is MET, a tyrosine kinase receptor expressed mostly in epithelial cells, whose natural ligand is the hepatocyte growth factor (HGF). MET signaling is involved in cell proliferation and cell survival, including the processes of embryonic development, wound healing, and tissue regeneration [Citation11]. Genomic alterations in MET include MET exon 14 skipping mutations, MET gene copy number (GCN) gain or amplification, and MET protein overexpression. These alterations occur in NSCLC mostly independently of other oncogenes, such as EGFR, KRAS, HER2, and BRAF mutations, or ALK and ROS1 translocations, and are mutually exclusive with these molecular drivers [Citation12–Citation15], although MET exon 14 skipping mutations and MET amplification may occur concomitantly in NSCLC [Citation13,Citation16,Citation17]. Nonetheless, MET molecular testing is not currently indicated as a routine assay [Citation4,Citation18]. Several MET-targeted therapies were or are under development, including monoclonal antibodies against MET or HGF, and small-molecule MET inhibitors.
So far, anti-MET antibodies have failed to achieve satisfactory results in clinical trials. Combination treatment of erlotinib and the monoclonal anti-MET antibody, onartuzumab, produced unsatisfactory results in a phase III trial in patients with NSCLC and MET overexpression as determined by immunohistochemistry (IHC) analysis [Citation19]. Moreover, a phase III trial with an anti-HGF monoclonal antibody, rilotumumab, in gastric cancer also failed to meet the primary endpoints [Citation20].
Small-molecule MET inhibitors are now being explored. These tyrosine kinase inhibitors (TKIs) are divided into types I (subtype Ia and Ib), II, and III. Type I inhibitors block ATP binding, thereby preventing phosphorylation/activation of the receptor; type Ib inhibitors (e.g. capmatinib, tepotinib, savolitinib, AMG 337) are more specific for MET than type Ia inhibitors (e.g. crizotinib). Type II inhibitors (e.g. cabozantinib, glesatinib, merestinib) are also ATP competitive, binding to a hydrophobic pocket adjacent to the ATP binding site, whereas type III (e.g. tivantinib) inhibitors bind to allosteric sites rather than the ATP-binding site [Citation21]. MET-targeted therapies currently in clinical development include type I and type II inhibitors [Citation21], whether they aim to target MET exon 14 skipping mutations, MET amplification (e.g. SAR125844 and AMG 337) [Citation22,Citation23], or both MET exon 14 skipping mutations and MET amplification (e.g. crizotinib, savolitinib, tepotinib, and capmatinib) [Citation24–Citation29]. Of note, clinical trials investigating the small-molecule MET inhibitor tivantinib were terminated early due to futility [Citation30,Citation31], which could partly be due to poor selection of patients into the trials [Citation32]. However, the toxicity observed with tivantinib treatment might have been a result of its microtubule disruption activity [Citation33].
Capmatinib (INC280) is an orally bioavailable, potent, and highly selective small-molecule MET inhibitor, which has been shown to effectively inhibit the MET pathway both in vitro and in vivo [Citation34,Citation35], and is being investigated in a clinical trial program. In this review, we will discuss the data available for capmatinib in NSCLC, including the results presented at the 2018 European Society for Medical Oncology (ESMO) and 2019 American Society of Clinical Oncology (ASCO) congresses.
2. MET as a primary driver oncogene
A definition of MET positivity has yet to be established and validated. Several studies have evaluated MET status based on IHC analysis [Citation19,Citation20,Citation36], which has led to conflicting results about the role of MET as a predictive biomarker. Commonly, MET positivity was defined as IHC 2+ and 3+ but, as mentioned above, these studies failed to show improved outcomes, probably because the correlation between MET protein overexpression and MET gene amplification is poor [Citation19,Citation37]. Since then, the focus of MET biomarkers has been on MET GCN amplification, and the MET exon 14 skipping mutation.
2.1. MET exon 14 skipping mutation
The presence of MET exon 14 skipping mutations is, at present, the best-defined predictive biomarker for the use of MET-TKIs [Citation32,Citation38] and the clearest proven target for capmatinib activity. MET exon 14 skipping mutations lead to decreased ubiquitination and subsequent degradation of the receptor, thus prolonging its activity [Citation39]. Several alterations of the MET exon 14 that can result in splicing have been identified, including point mutations and small deletions that may occur at different positions and disrupt the branch point of intron 13, the 3ʹ splice site of intron 13 or the 5ʹ splice site of intron 14 [Citation13,Citation38,Citation40]. This constitutes a challenge for molecular testing, because the method used should be able to identify the different types of alterations in multiple gene locations. MET mutations occur in 3–4% of adenocarcinomas and 1–2% of other histological subsets of NSCLC [Citation38,Citation41]. Several studies have reported a higher incidence of MET exon 14 skipping mutations in pulmonary sarcomatoid carcinomas, ranging from 7.7% to 31.8% [Citation13,Citation16,Citation42].
A recent meta-analysis of 11 studies with a total of 18,464 patients with NSCLC investigated the clinicopathologic and prognostic features of MET exon 14 skipping mutations in NSCLC [Citation43]. MET exon 14 skipping mutations were more frequent in women than men (P = 0.02), were associated with a significantly older age (P < 0.0001), and were less likely to be associated with a history of smoking (P = 0.008). Furthermore, MET exon 14 skipping mutations were associated with poor prognosis (pooled data from two studies), and were not associated with an increased risk for stage IV disease (data from three studies).
MET exon 14 skipping mutations and high gene amplification overlap in a subset of patients with NSCLC [Citation16,Citation17] and are associated with poorer prognosis [Citation13,Citation41,Citation44–Citation47], while being mutually exclusive with other oncogenes [Citation13,Citation14]. In addition, MET exon 14 skipping mutations seem to be associated with a lower tumor mutational burden compared with unselected NSCLC [Citation16,Citation48], making patients with MET alterations less likely to be good candidates for checkpoint-inhibition immunotherapy.
MET exon 14 skipping mutations can be detected by genome profiling techniques such as next-generation sequencing (NGS) and quantitative reverse transcriptase-polymerase chain reaction. The diversity of alterations that can result in exon 14 skipping mutations (e.g. base substitutions and deletions) is extensive, making it challenging to find a molecular test capable of detecting all of them [Citation39]. An unmet need exists for broader, more comprehensive platforms to increase detection sensitivity, thereby improving the identification of patients with MET mutations.
2.2. MET GCN changes and amplification
Changes in MET GCN is another possible predictive biomarker for MET-TKI activity but, at present, is less well defined than the exon 14 skipping mutation [Citation32,Citation37].
Analyses of MET amplification by fluorescence in-situ hybridization (FISH) are usually expressed as GCN per cell or as a MET/CEP7 ratio. When measuring MET amplification as a MET/CEP7 ratio, an increase in copy number due to focal amplification of the MET gene can be identified, whereas measuring the GCN can include chromosome polysomy instead of MET amplification specifically [Citation46]. Generally, a MET/CEP7 ratio ≥ 1.8 to 2.2 is considered low amplification, a MET/CEP7 ratio > 2.2 to < 5 is intermediate, and a MET/CEP7 ratio ≥ 5 is a high-level amplification [Citation46,Citation49]. By GCN/cell, the cut-off values were generally defined as low: ≥ 5 to < 6 copies, intermediate: ≥ 6 to < 7 copies, or high: ≥ 7 copies [Citation46]. Validated cut-off values for MET amplification would be important to standardize methods and to optimize patient stratification in both clinical trials and clinical practice. High amplification occurs in 2–5% of newly diagnosed NSCLC cases [Citation49,Citation50].
3. MET as a mechanism of acquired resistance to EGFR-TKI
MET amplification is observed as an acquired mechanism of resistance to first-, second-, and third-generation EGFR-TKIs in EGFR-mutated NSCLC [Citation51–Citation58]. In these patients, the combination of an EGFR-TKI with a MET-TKI such as capmatinib has the potential to improve outcomes, both when used following treatment with earlier-generation TKIs such as gefitinib, erlotinib, or afatinib, or with third-generation TKIs such as osimertinib. The dominant mechanism of resistance after treatment with a first- or second-generation TKI is the secondary gatekeeper mutation T790M in the exon 20 of the EGFR gene, responsible for at least 50% of the cases [Citation51–Citation58]. MET amplification is a far less common mechanism, and it can be found concurrently with other resistance mechanisms, such as T790M [Citation51,Citation52].
Because osimertinib selectively inhibits EGFR-TKI-sensitizing and EGFR T790M resistance mutations, its use as first-line therapy in NSCLC does not lead to T790M mutations, but other mechanisms of resistance have been identified [Citation59]. A large-scale report, based on NGS analysis of circulating cell-free tumor DNA (ctDNA) was presented at the 2018 ESMO congress [Citation60]. Overall, two main types of resistance were documented: those related to the EGFR pathway itself, such as the C797S mutation in the EGFR exon 20; and the activation of alternative pathways, such as HER-2 or MET amplification, and mutations in the genes of the MAPK/PI3K or cyclin-dependent kinase pathways. Of note, the proportion of MET amplification as a resistance mechanism was much higher than after first- or second-generation EGFR-TKIs, with 15% of tumors found to have this mechanism of resistance. The frequency may be even higher in tissue, as the technology to detect gene amplification still needs further refinement on plasma ctDNA samples.
4. Capmatinib: mechanism of action, chemistry, and pharmacokinetics
Capmatinib is an orally bioavailable, potent, and highly selective small-molecule MET inhibitor (type Ib), capable of blocking MET phosphorylation and the activation of key downstream effectors in MET-dependent tumor cell lines, as determined by biochemical and cellular assays [Citation34]. The high selectivity of capmatinib for MET was confirmed using a screening platform of more than 400 kinases [Citation35]. In biochemical in vitro assays, capmatinib was shown to be approximately 30 times more potent than crizotinib (IC50 values of 0.13 nmol/L and 4 nmol/L, respectively) [Citation34,Citation61], and also more potent than tepotinib (IC50 of ~1.7 nmol/L) [Citation29]. Additionally, preclinical data have shown that capmatinib treatment resulted in the regression of MET-dependent tumor models in vivo at well-tolerated doses [Citation34,Citation35].
Capmatinib was shown to reverse the effects of MET activation on the EGFR and HER-3 pathways [Citation34] and to restore sensitivity to the EGFR-TKI erlotinib in EGFR-mutant NSCLC cell lines with acquired EGFR-TKI resistance [Citation35,Citation62]. These results suggest that MET inhibition with capmatinib may effectively block NSCLC with MET pathway oncogenic addiction and may restore the sensitivity of tumors to EGFR-TKI treatment following resistance ().
Figure 1. Schematic representation of MET signaling blockade by capmatinib. Figure adapted from Owusu 2017 [Citation63]. Reprinted with permission. © (2017) American Society of Clinical Oncology. All rights reserved.
![Figure 1. Schematic representation of MET signaling blockade by capmatinib. Figure adapted from Owusu 2017 [Citation63]. Reprinted with permission. © (2017) American Society of Clinical Oncology. All rights reserved.](/cms/asset/06388d19-5cf8-4f6b-bb57-91ca492defdf/iery_a_1643239_f0001_oc.jpg)
5. Capmatinib for NSCLC with MET as a primary oncogene (exon 14 skipping, amplification)
5.1. Early clinical trials
A phase I study (NCT01324479) has been conducted in patients with solid tumors (including NSCLC, n = 55) with MET alterations. This study was designed to contain a dose-escalation (n = 38, no NSCLC) and dose-expansion part, with the latter including an original expansion group (n = 26 NSCLC) and an additional expansion group (n = 29 NSCLC). The primary objective of the study was to determine the maximum tolerated dose/recommended phase II dose (RP2D), and safety and tolerability of capmatinib as a single oral agent. Secondary objectives were assessment of efficacy (proportion of patients with a complete response [CR] or partial response [PR] at the RP2D) and pharmacokinetics (PKs) [Citation64–Citation66].
In the dose-escalation part and original expansion group, patients had MET alterations defined as a MET/CEP7 ratio ≥ 2.0, or GCN ≥ 5, or an IHC H-score ≥ 150. The H-score was obtained by multiplying the staining intensity (between 0 and 300) by the percentage of cells stained on IHC (between 0% and 100%); therefore, it was a value between 0 and 300 [Citation64–Citation66]. Moreover, these patients did not have documented EGFR status.
The dose-escalation part of the study enrolled 38 patients with solid tumors [Citation65] treated in seven dose cohorts of 100–600 mg twice daily (BID) capsules or 400 mg BID tablets. Dose-limiting toxicities occurred at 200 mg BID, 250 mg BID, and 450 mg BID capsules (one patient each) and were grade 3 fatigue (two patients), and grade 3 serum bilirubin increased (one patient). The most frequent drug-related adverse events (AEs) of any grade were nausea (33%), fatigue (25%), and vomiting (24%), and the most common drug-related grade 3/4 AEs were fatigue (4%) and increased lipase levels (3%). Capmatinib plasma concentration generally increased with dose, and the RP2D was established as 600 mg BID capsules, but film-coated 400 mg tablets were developed and tested to improve patient convenience and compliance. Of note, the dosing with 400 mg BID tablets had comparable tolerability, safety profile, and exposure to 600 mg BID capsules. Capmatinib used as a single agent at a dosage of 450 mg BID (capsules) has been shown to inhibit MET phosphorylation, as measured by IHC [Citation64].
Patients in the additional expansion group had centrally assessed MET status as IHC 3+ and documented EGFR wild-type [wt] status [Citation65,Citation66]. In the dose-expansion part of the study, at the cut-off date for analysis of 15 March 2016, 11 of the 55 patients (20%) with advanced NSCLC achieved a PR, with an overall response rate (ORR) of 20% and a disease control rate (DCR; proportion of patients with CR or PR or stable disease) of 51%. The ORR in evaluable patients with MET IHC3+ (regardless of GCN status) was 24%, and 47% in patients with MET GCN ≥ 6 () [Citation66]. These results indicated that FISH analysis of MET status is a better predictor of response than IHC status. At that time, IHC was abandoned as a possible predictive biomarker for capmatinib activity. The most frequent AEs (all grades, regardless of causality) were nausea (47%), peripheral edema (38%), vomiting (36%), decreased appetite (33%), and fatigue (29%). The most common serious AEs were grade 3 general physical health deterioration (6%), grade 3 abdominal pain, grade 2 and 3 pneumonia, grade 3 dehydration, and grade 2 pulmonary embolism (all 4%) [Citation66].
Table 1. Phase I trial (NCT01324479): Best overall response rate (investigator assessment) by MET status (IHC0/1+, IHC2+, IHC3+, GCN < 4, GCN ≥ 4 and < 6, GCN ≥ 6) [Citation66].
5.2. GEOMETRY mono-1 study
A phase II single-arm, multicenter, international study in patients with EGFR-wt, ALK-wt, advanced NSCLC was initiated in June 2015 (GEOMETRY Mono-1; NCT02414139) [Citation27] and it is still ongoing. In this study with the recommended 400 mg BID tablet dosing, patients were enrolled into several cohorts to investigate who would benefit most from capmatinib treatment (e.g. MET exon 14 skipping mutations or MET amplification, including GCN stratification; first-line or further-line therapy), with each cohort analyzed separately (). Enrolment in the pre-treated cohorts with MET amplification < 10 GCN (cohorts 1b, 2, and 3) was stopped for futility following a pre-planned interim analysis.
Figure 2. GEOMETRY Mono-1 (NCT02414139) study design.
*Cohorts 1b, 2, and 3 are closed for futility.
ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; GCN, gene copy number; NSCLC, non-small cell lung cancer.
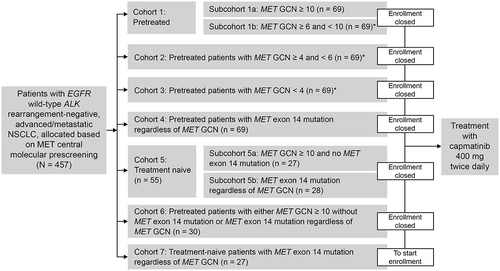
The remaining ongoing cohorts are: cohort 1a with pre-treated patients with MET GCN ≥ 10; cohort 4 with pre-treated patients with MET exon 14 mutations regardless of GCN; cohort 5 with treatment-naive patients with MET dysregulation (5a: MET GCN ≥ 10 and 5b: MET exon 14 mutations regardless of GCN); cohort 6 (expansion cohort) with pre-treated patients with either MET GCN ≥ 10 without MET exon 14 mutations or MET exon 14 mutations regardless of GCN; and cohort 7 (expansion cohort) with treatment-naive patients with MET exon 14 mutation regardless of MET GCN. At the cut-off date for this interim analysis of 8 November 2018, enrollment into MET mutated cohorts 4 (n = 69) and 5b (n = 28) had been completed [Citation67].
The primary outcome measure was the ORR, defined as the proportion of patients with a best overall response of CR or PR by blinded independent review committee (BIRC) assessment per RECIST 1.1. Secondary outcome measures included the duration of response (DOR [key secondary objective]; calculated as the time from the date of the first documented CR or PR by BIRC assessment per RECIST 1.1 to the first documented progression or death due to any cause for patients with PR or CR), ORR by investigator assessment, DCR, progression-free survival (PFS), and overall survival (OS; defined as time from first dose of capmatinib to death due to any cause). Safety outcomes were assessed and presented as the number of patients with incidence of AEs and serious AEs [Citation27].
Preliminary data are available for cohorts 4 and 5b, and these have been presented at the 2019 ASCO congress [Citation67]. By BIRC analysis, an ORR of 39.1% was achieved in patients in cohort 4 (pre-treated patients with MET exon 14 mutations regardless of GCN; second/third line [2/3 L]). The median DOR (by BIRC) was still immature at the time of this analysis, and it was reported as 9.72 months. In cohort 5b (treatment-naive patients with MET mutations regardless of GCN; first-line [1L]), the ORR was 71.4%. The median DOR (by BIRC) was 8.41 months (also immature at this cut-off date). Additionally, the median PFS was 5.42 months in cohort 4 (2/3 L) and 9.13 months in cohort 5b (1L), however the PFS results for cohort 5b were not mature at this cut-off date for analysis. The differential benefit observed between treatment-naive and pre-treated patients highlights the need for an early diagnosis for patients with MET exon 14 skipping mutation advanced NSCLC [Citation67].
Capmatinib is known to cross the blood–brain barrier and showed preliminary activity in brain metastasis [Citation67]. A case report of a patient from cohort 4 (pre-treated patients with MET mutations regardless of GCN) showed a complete resolution of brain metastasis at the first post-baseline CT scan (after 42 days of capmatinib treatment) [Citation27].
The most common AEs (≥ 25%) reported across all cohorts (n = 315), regardless of causality, included peripheral edema (49.2%), nausea (43.2%), and vomiting (28.3%) [Citation67]. In these cohorts, capmatinib was administered in fasting conditions. However, in the expansion cohorts (cohorts 6 and 7), capmatinib will be administered irrespective of the fed or fasted states.
6. Capmatinib for EGFR-mutated NSCLC with MET-driven acquired resistance to EGFR-TKI treatment
Patients with EGFR-mutant NSCLC who progress on first- or second-generation EGFR-TKIs often have acquired resistance based on a secondary T790M mutation (in about 50% of the cases) [Citation51]. MET amplification is a less common mechanism of resistance in this setting, but more common after the use of third-generation TKIs such as osimertinib [Citation51–Citation54,Citation60]. Targeting these two mechanisms simultaneously with an EGFR inhibitor plus a MET inhibitor may overcome this type of acquired resistance to EGFR-TKIs, and this approach is being investigated in clinical trials.
6.1. Capmatinib in combination with first-generation EGFR-TKIs
Capmatinib in combination with gefitinib (250 mg once daily [QD]) has been investigated in a phase Ib/II study after failure of EGFR-TKI therapy in patients with EGFR-mutated NSCLC with acquired resistance, without T790M mutation, but with MET alteration (NCT01610336) [Citation68]. Patients entering the phase Ib part had MET amplification, defined as either GCN ≥ 5 and/or MET/CEP7 ratio ≥ 2.0, or MET overexpression, defined as ≥ 50% of tumor cells with moderate or strong staining intensity. For the phase II part, patients were required to have MET GCN ≥ 5 as determined by FISH or 50% of tumor cells with IHC 2+ or 3 +. In subsequent protocol amendments, the criteria were changed to 50% of tumor cells with IHC 2+ or 3+ plus MET GCN ≥ 5 and then to 50% of tumor cells with IHC 3+ or MET GCN ≥ 4. The definition of MET activation is still evolving. At the time when this eligibility criterion was created, it was not known which method would be most effective to measure MET activation; therefore, the criterion is broad and it was subjected to protocol amendments, and both FISH and IHC were used to identify patients with MET activation.
The RP2D for capmatinib was determined at 400 mg BID tablet dosing in this study, which is similar to the monotherapy studies. The primary objective of this study was to estimate the overall clinical activity of capmatinib in combination with gefitinib in NSCLC patients with MET alterations (ORR per RECIST v1.1) and the secondary objectives were to estimate time-dependent clinical activity of capmatinib in combination with gefitinib (DOR, PFS, and OS), to determine the safety and tolerability of capmatinib in combination with gefitinib, and to characterize the PK profile of capmatinib in combination with gefitinib.
At the primary analysis cut-off date of 10 June 2016, enrollment was complete, with 61 patients enrolled in the phase Ib dose-escalation part and 100 patients in the phase II expansion part of the study. In the phase Ib part of the study, the ORR and DCR values were 23% and 57%, respectively, across all doses and regardless of MET status. In the phase II part, the ORR was 29% (29/100 patients) and the DCR was 73%. In a subgroup analysis by MET GCN category, the best observed ORR was 47% in patients with MET GCN ≥ 6 (n = 36), whereas patients with MET IHC3+ (n = 78) had an ORR of 32% (). Median PFS values in the GCN ≥ 6 and IHC3+ subgroups were 5.49 months (95% confidence interval [CI], 4.21–7.29 months) and 5.45 months (95% CI, 3.71–7.10 months), respectively [Citation68].
Figure 3. Capmatinib in combination with gefitinib (NCT01610336): Best percentage change in sum of diameters of target lesions in all phase II patients by gene copy number and immunohistochemistry subgroup (full analysis set) [Citation68].
N represents the number of patients with a baseline and ≥1 post-baseline assessment of tumor lesions (assessed by investigator).
*Percentage change in sum of diameters of target lesion contradicted by overall lesion response of progressive disease.
D, progression of disease; GCN, gene copy number; IHC, immunohistochemistry; P, partial response; S, stable disease.
![Figure 3. Capmatinib in combination with gefitinib (NCT01610336): Best percentage change in sum of diameters of target lesions in all phase II patients by gene copy number and immunohistochemistry subgroup (full analysis set) [Citation68].N represents the number of patients with a baseline and ≥1 post-baseline assessment of tumor lesions (assessed by investigator).*Percentage change in sum of diameters of target lesion contradicted by overall lesion response of progressive disease.D, progression of disease; GCN, gene copy number; IHC, immunohistochemistry; P, partial response; S, stable disease.](/cms/asset/3bec44f8-0ad0-400a-ba4e-11944678d9cc/iery_a_1643239_f0003_oc.jpg)
The most common study drugrelated all-grade AEs were nausea (28%), peripheral edema (22%), decreased appetite (21%), and rash (20%). The most frequent (> 5%) grade 3–4 AEs suspected of being related to the study drug were increased lipase/amylase (both 6%). Diarrhea (all grades) occurred in 24% of patients, which was similar to rates observed in gefitinib studies. Increased alanine aminotransferase (ALT) and aspartate aminotransferase occurred in 13% and 12% of patients, respectively, whereas these rates were 11.5% and 7.9%, respectively, in gefitinib clinical trials [Citation68,Citation69]. Serious AEs regardless of study drug relationship occurred in 33% of patients. Study drug-related serious AEs occurred in 7% of patients. The PK values of the two drugs were not significantly affected by the combination therapy [Citation68].
Another trial, GEOMETRY duo-1 (NCT02468661) is ongoing – a phase Ib/II study of capmatinib in combination with the EGFR inhibitor erlotinib versus platinum plus pemetrexed in EGFR-mutant, MET-amplified, locally advanced/metastatic NSCLC with acquired resistance to prior EGFR-TKI therapy [Citation70].
6.2. Capmatinib in combination with third-generation EGFR-TKIs
At present, there are only few and early data on the combination of third-generation EGFR-TKIs and MET inhibitors. Capmatinib in combination with the third-generation EGFR-TKI nazartinib (EGF816) has been investigated in a phase Ib/II study of patients with EGFR-mutated, previous-line resistant, NSCLC, regardless of MET status (NCT02335944) [Citation71]. Nazartinib is another irreversible EGFR-TKI that selectively inhibits T790M and EGFR-activating mutations, with antitumor activity in EGFR T790M-mutated NSCLC [Citation72]. The primary objective of this study was to determine the maximum tolerated dose/RP2D of the combination using an adaptive Bayesian logistic regression model [Citation71]. The starting doses were capmatinib 200 mg BID plus nazartinib 50 mg QD.
At the data cut-off of 1 August 2016, 33 patients were enrolled at five capmatinib BID/nazartinib QD mg dose levels. Eighteen of 33 patients (55%) discontinued treatment, mainly due to disease progression (13 patients [39%]; ) [Citation71]. Dose-limiting toxicities occurred in four patients and were: increased ALT in one patient at the 200/50 mg dose level, anaphylactic reaction in one patient at the 400/100 mg dose level and pyrexia, maculopapular rash, and allergic dermatitis in two patients at the 400/150 mg dose level. The most frequent (≥ 30%) any-grade AEs, regardless of causality, were nausea (55%), peripheral edema (45%), increased amylase (42%), increased blood creatinine (36%), decreased appetite (30%), and diarrhea (30%). The most frequent (> 10%) grade ≥ 3 AEs were maculopapular rash (18% [mainly in the 400/150 mg cohort]) and increased amylase (12%).
Table 2. Patient disposition in the phase Ib dose-escalation study of capmatinib in combination with nazartinib (NCT02335944) [Citation71].
Capmatinib and nazartinib exposure increased with dose, and the preliminary data indicate a ~ 35% increase in nazartinib exposure (area under the curve [AUC]) at steady state when co-administered with the capmatinib RP2D, compared with single-agent exposure. The investigator-assessed ORR was 42% (2/33 CR and 12/33 PR) across all dose levels and 50% (8/16 patients) at the 400/100 mg dose level (). The RP2D of the combination is now confirmed as capmatinib 400 mg BID plus nazartinib 100 mg QD. Preliminary antitumor activity was observed across dose levels, independent of T790M status.
Figure 4. Capmatinib in combination with nazartinib (NCT02335944): Best percentage change from baseline in sum of longest diameters per investigator assessment (full-analysis set) in the phase Ib/II study [Citation71].
n represents the number of patients with baseline and ≥1 post-baseline assessment of target lesions (investigator assessment). Percentage changes from baseline > 100% were set to 100%.
*Patients that discontinued treatment.
CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
![Figure 4. Capmatinib in combination with nazartinib (NCT02335944): Best percentage change from baseline in sum of longest diameters per investigator assessment (full-analysis set) in the phase Ib/II study [Citation71].n represents the number of patients with baseline and ≥1 post-baseline assessment of target lesions (investigator assessment). Percentage changes from baseline > 100% were set to 100%.*Patients that discontinued treatment.CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.](/cms/asset/98635d83-34c6-4b54-bd12-b5725cae6198/iery_a_1643239_f0004_oc.jpg)
7. Brief reflection on other MET-TKIs in development
As well as capmatinib, other TKIs targeting the MET pathway have shown antitumor activity in NSCLC with MET alteration. For reference, their single-agent activity and tolerability is summarized in .
Table 3. Summary of findings from clinical trials with MET inhibitors used as monotherapy in NSCLC.
Crizotinib was originally studied as PF-02341066, a multi-target inhibitor of MET, ROS1, and ALK kinases, in a phase I trial PROFILE 1001 of patients with heavily pretreated solid tumors [Citation74,Citation75]. However, when the ALK translocation oncogenic driver in NSCLC was first described in Japan in 2007 [Citation76], it transpired that most major responses in that phase I trial were in tumors with ALK translocation [Citation75]. Hence, crizotinib was mainly further developed in ALK translocated tumors, and later also in ROS1 translocated tumors, leading to the current US Food and Drug Administration and European Medicines Agency registration for crizotinib [Citation77,Citation78]. The most recently presented results of the ongoing PROFILE 1001 trial in patients who had tumors with MET alterations are provided in . Clinical trial data have shown that the most serious AEs in 1722 patients with advanced NSCLC (ALK-positive or ROS1-positive) were hepatotoxicity, interstitial lung disease/pneumonitis, neutropenia, and QT interval prolongation. The most common AEs (≥ 25%) were vision disorder, nausea, diarrhea, vomiting, edema, constipation, elevated transaminases, fatigue, decreased appetite, dizziness, and neuropathy [Citation78].
Tepotinib, a MET inhibitor targeting tumors with the MET exon 14 skipping mutation, has been studied in the phase II trial VISION [Citation29]. Both treatment-naive and pre-treated patients with a tumor harboring a MET exon 14 skipping mutation were included ().
Tepotinib plus gefitinib was studied in an Asian cohort. Tumors had a MET IHC 2+ or 3+ or MET amplification (GCN ≥ 5 or MET/CEP7 ratio ≥ 2) and resistance to first-line EGFR-TKI, but were T790M-negative [Citation79]. The initial aim was for 156 patients to be randomized to either tepotinib plus gefitinib or platinum–pemetrexed chemotherapy. The enrollment was stopped due to low recruitment, with 31 patients in the combined TKI arm and 24 in the chemotherapy arm. The ORR in the TKI arm was 45.2% (14/31), 68% (13/19) in those with MET IHC 3+, and 66.7% (8/12) in tumors with MET amplification. The most frequent grade 3–4 AEs were increased lipase/amylase (four patients) and neutropenia (one patient). The serious AE rate was 16.1%, and 9.7% of the patients discontinued owing to AEs.
Savolitinib (or volitinib) monotherapy has been investigated in patients with advanced papillary renal cell cancer [Citation80]. For NSCLC, preliminary results in combination therapy are available [Citation81]. TATTON is an ongoing phase Ib trial, in which the third-generation EGFR-TKI osimertinib is combined with the MET inhibitor savolitinib in patients with NSCLC having EGFR mutation and MET amplification (GCN > 5 or MET/CEP7 ratio > 2), with or without prior treatment with a third-generation EGFR-TKI (NCT02143466) [Citation81]. At the cut-off date for analysis (February 2018), 24 of the 46 patients with EGFR-mutant, MET-amplified NSCLC after progression on prior first-/second-generation EGFR-TKI had achieved a confirmed PR (ORR = 52%), with a median DOR of 7.1 months [Citation82]. The most common (≥ 20%) all-causality AEs were nausea (37%), diarrhea (30%), fatigue (28%), decreased appetite (28%), pyrexia (26%), and vomiting (22%). Serious AEs were reported in 37% of patients. Treatment-related AEs were reported in 91% of patients, of which 43% were grade ≥ 3. Two patients died due to an AE (n = 1 acute kidney injury possibly related to savolitinib; n = 1 pneumonia considered unrelated to study medication). In the group of patients with EGFR-mutated, MET-amplified NSCLC after progression on prior third-generation EGFR-TKI (n = 48) the most common (≥ 20%) all-causality AEs were nausea (52%), vomiting (38%), diarrhea (27%), fatigue (25%), decreased appetite (23%), and pyrexia (21%) [Citation83]. Serious AEs were reported in 29% of patients. Treatment-related AEs were reported in 90% of patients, of which 23% were grade ≥ 3. Two patients died but neither death was considered related to study medication. Of the 43 patients eligible for efficacy analysis, 12 patients achieved a confirmed PR (ORR = 28%), with a median duration of response of 9.7 months.
An open-label, single-arm, multicenter phase II trial with savolitinib is ongoing in patients with lung sarcomatoid carcinoma and other NSCLC with MET exon 14 skipping mutation (NCT02897479). As of December 2018, 34 patients with MET exon 14 mutations were treated with savolitinib (17 patients were treatment-naive). At this cut-off date for analysis, in the 31 patients with responses assessed by the investigator, 12 patients had a confirmed PR, 4 patients had a yet-to-be-confirmed PR, 10 patients had stable disease, 2 patients had disease progression and 3 patients were non-evaluable due to treatment discontinuation [Citation84]. The most common (≥ 20%) treatment-related AEs in the 34 patients treated with savolitinib were nausea (41%), peripheral edema (38%), increased ALT (32%), increased aspartate aminotransferase (29%), and vomiting (21%). Twelve patients (35%) had grade ≥ 3 treatment-related AEs and 5 (15%) discontinued treatment due to treatment-related AEs, with drug-induced liver toxicity as the most common cause for this discontinuation (6%). Five patients died, with 3 unrelated or unlikely related to AEs, 1 probably related to treatment (tumor lysis syndrome), and 1 primary cause unknown.
Safety data are still scarce for most of the MET inhibitors but gastrointestinal intolerance and peripheral edema seem to have emerged as AEs that occur with these inhibitors.
8. Conclusion
Activation of the MET pathway through MET mutation or amplification is an important oncogenic mechanism in NSCLC. MET exon 14 skipping mutations and high MET amplifications are associated with poor prognosis in patients with NSCLC, and the availability of new treatment options are an unmet need for these patients. Capmatinib is a specific and potent small-molecule MET inhibitor with in vivo and in vitro antitumor activity in NSCLC. The primary results of the phase II GEOMETRY Mono-1 trial in EGFR-wt advanced-stage NSCLC patients show clinically significant activity in patients with MET exon 14 skipping mutations. Treatment-naive patients have an especially high response to the therapy, supporting the upfront testing for this oncogene driver and the use of capmatinib in this molecularly defined patient subgroup. Importantly, capmatinib crosses the blood-brain barrier and some brain activity has been observed. Moreover, the trial has confirmed the tolerability and safety of the recommended dose of capmatinib 400 mg BID. The efficacy of capmatinib in MET-amplified tumors is still awaited.
In addition to its single-agent activity, capmatinib has also demonstrated activity in EGFR-mutated NSCLC with MET amplification based on acquired resistance after treatment with first-generation EGFR-TKIs. Responses have been observed in patients with high MET-amplified tumors. Importantly, at a similar dose as in the monotherapy studies, the combination of capmatinib with an EGFR-TKI seems tolerable and no significant drug–drug interactions have yet been reported. Combinations with third-generation EGFR-TKIs are now actively being studied. The use of capmatinib in combination with other kinase inhibitors may be an important therapeutic option in the future, as in vitro data on resistance mechanisms suggest that capmatinib may increase the sensitivity of cancer cells to kinase inhibitors [Citation35]. In addition, in vitro data indicate that resistance to capmatinib can occur in MET-amplified cell lines [Citation85]. This resistance to capmatinib involved EGFR activation, which reinforces the potential benefits of combination therapy. However, it will be important to obtain in vivo data to confirm these results and evaluate the effects of the combination therapy.
Overall, capmatinib is a promising novel treatment option for patients with MET-dysregulated NSCLC.
9. Expert opinion
Although in doubt for quite some time, the data discussed above establish MET exon 14 skipping mutations as primary oncogenic drivers that can effectively be targeted in NSCLC. These alterations are present in 3–4% of cases with stage IV non-squamous NSCLC, and are mutually exclusive with established driver mutations in EGFR, ALK, ROS1, and BRAF. In addition, ORRs and DCRs observed with selective small-molecule MET inhibitors are high and in the same range as those observed with approved EGFR-, ALK-, ROS1-, and BRAF-TKIs [Citation4]. Based on these data, the routine testing of MET exon 14 skipping in stage IV non-squamous NSCLC should be recommended. Moreover, the GEOMETRY Mono-1 data, which showed clearly higher response rates with capmatinib in treatment-naive than in pretreated patients, indicate that MET exon 14 skipping mutations should preferably be molecularly assessed at baseline. Testing for these alterations could be included in a broader DNA sequencing panel. However, given the various genomic locations of exon 14 skipping alterations, RNA-based approaches are now being assessed to more comprehensively capture MET exon 14 skipping events in the future [Citation86].
MET exon 14 skipping mutations are, therefore, clear biomarkers of response to capmatinib. Preliminary data from the GEOMETRY Mono-1 trial showed that responses analyzed by independent review committee were noted in 39.1% of pre-treated patients and 71.4% of treatment-naive patients. These responses were durable, with a median DOR of 9.72 months in pre-treated patients and 8.41 months in treatment-naive patients; however, the DOR and PFS data were immature at the cut-off date for this analysis [Citation67]. The safety profile of capmatinib was favorable, and future data from the GEOMETRY Mono-1 trial will reveal whether the tolerability of the drug improves further when taken with food.
Research should now start to focus on the mechanisms of intrinsic and acquired resistance to further improve treatment options in this molecularly defined patient subgroup. Preliminary findings were presented at the ASCO 2019 meeting [Citation87]. For intrinsic resistance, the absence of either MET protein expression or activation of the KRAS pathway had negative predictive value. Responses to MET-TKIs were noted in 63% (7/11) of patients with MET expression and in 0% (0/5) of those without MET expression. Acquired resistance was reported in 29 patients, 9 of which had paired pre- and post-treatment samples for analysis. On-target resistance in the MET pathway was found in 2 patients (secondary MET D1128N mutation, amplification of HGF), and 5 cases were off target (1 KRAS mutation, 1 RASA1 mutation, 2 MDM2 amplification, and 1 EGFR amplification).
A change in MET GCN is another potential predictive biomarker for MET inhibitor activity, although it is currently less well characterized than exon 14 skipping alterations. The efficacy of capmatinib in MET-amplified tumors is still to be established. The experience with crizotinib in tumors with low or median MET amplification, however, suggests lower response rates and shorter survival in this setting compared with MET-mutated tumors. Although expectations for high MET amplification are higher, the most appropriate definition of this potential biomarker and associated responses to MET inhibitors need to be further clarified. Based on disappointing data, the investigation of IHC and low GCN alterations (GCN < 10) as biomarkers predictive of response to MET inhibitors has already been abandoned.
MET is now also a key target in EGFR-mutant NSCLC. In particular, MET amplifications have been established as important drivers of resistance to EGFR-TKIs, especially at relapse on first-line osimertinib. Following the availability of osimertinib as an EGFR-TKI treatment, T790M is no longer a resistance mechanism to be expected as with the upfront use of first- or second-generation TKIs, and MET amplification is reported to be present in 15% of resistant cases [Citation60]. However, this may be an underestimation of the real frequency of MET-driven resistance, as these data were obtained by liquid biopsy analysis. Indeed, the sensitivity of NGS-based assays on plasma is probably suboptimal compared with tissue analysis, due to the presence of DNA from normal tissue. Technological improvements will likely give more insights into the importance of MET at the point of acquired resistance to EGFR-TKIs. In addition, MET amplifications may coexist with activating EGFR mutations at baseline and their role in early EGFR-TKI failure should be assessed. A recent report has shown a suboptimal response to EGFR-TKIs in patients with coexisting MET amplification prior to EGFR-TKI therapy [Citation88]. Importantly, the shorter time to treatment failure described in that report was only observed when MET amplification was classified by both a GCN ≥ 5 and MET/CEP7 ratio ≥ 2 and not by GCN ≥ 5 alone. This finding again emphasizes the high need for a clear validated definition of MET amplification for patient selection. However, if confirmed, the baseline status of MET should be included in future trials to investigate whether the combined MET blockade from the start of EGFR-TKIs may delay the onset of resistance.
Article highlights
Capmatinib is a highly selective, potent, small-molecule MET inhibitor with proven in vitro and in vivo antitumor activity in NSCLC
Capmatinib is an investigational drug being explored as monotherapy in advanced NSCLC with MET exon 14 skipping mutations and high MET amplification
In the GEOMETRY Mono-1 study, treatment with capmatinib resulted in high response rates in -advanced NSCLC with MET exon 14 skipping mutations, particularly in first-line treatment
Capmatinib in the recommended dose of 400 mg tablets BID has a good tolerability and safety profile
Capmatinib is also being investigated in combination with EGFR inhibitor therapy in EGFR-mutant NSCLC with MET-based acquired resistance to previous EGFR inhibitors
Capmatinib is a promising novel treatment option for patients with MET-dysregulated NSCLC
Declaration of interest
J Vansteenkiste has received institutional research funding from MSD, provided advisory functions for AstraZeneca, Boehringer-Ingelheim, MSD, Novartis and Roche, and lectures for AstraZeneca, BMS, Boehringer-lngelheim, MSD and Roche. E Wauters has received a grant for clinical and translational oncology research of the Foundation against Cancer Belgium (2017-2022), and lectures for AstraZeneca and Boehringer-lngelheim. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors thank Dr Ana Costa from Chameleon Communications International for editorial assistance in the preparation of the manuscript, with funding from Novartis.
Additional information
Funding
References
- International Agency for Research on Cancer [Internet]. 900 world fact sheets. [cited 2018 Dec 21]. Available from: http://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf
- Walters S, Maringe C, Coleman MP, et al. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population-based study, 2004-2007. Thorax. 2013;68(6):551–564.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34.
- Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl4):iv192–iv237.
- Walter FM, Rubin G, Bankhead C, et al. Symptoms and other factors associated with time to diagnosis and stage of lung cancer: a prospective cohort study. Br J Cancer. 2015;112(Suppl 1):S6–S13.
- Koo MM, Hamilton W, Walter FM, et al. Symptom signatures and diagnostic timeliness in cancer patients: a review of current evidence. Neoplasia. 2018;20(2):165–174.
- Cassim S, Chepulis L, Keenan R, et al. Patient and carer perceived barriers to early presentation and diagnosis of lung cancer: a systematic review. BMC Cancer. 2019;19(1):25.
- Hill LLE, Collier G, Gemine RE. A patient perspective: identifying and understanding the barriers associated with the diagnostic delay of lung cancer. EMJ Respir. 2017;5(1):92–98.
- Raghu VK, Zhao W, Pu J, et al. Feasibility of lung cancer prediction from low-dose CT scan and smoking factors using causal models. Thorax. 2019;74(7):643–649.
- Ettinger DS, Wood DE, Aisner DL, et al. Non-small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15(4):504–535.
- Smyth EC, Sclafani F, Cunningham D. Emerging molecular targets in oncology: clinical potential of MET/hepatocyte growth-factor inhibitors. Onco Targets Ther. 2014;7:1001–1014.
- Onozato R, Kosaka T, Kuwano H, et al. Activation of MET by gene amplification or by splice mutations deleting the juxtamembrane domain in primary resected lung cancers. J Thorac Oncol. 2009;4(1):5–11.
- Tong JH, Yeung SF, Chan AW, et al. MET amplification and exon 14 splice site mutation define unique molecular subgroups of non-small cell lung carcinoma with poor prognosis. Clin Cancer Res. 2016;22(12):3048–3056.
- Heist RS, Shim HS, Gingipally S, et al. MET exon 14 skipping in non-small cell lung cancer. Oncologist. 2016;21(4):481–486.
- Cancer Genome Atlas Research N. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511(7511):543–550.
- Schrock AB, Frampton GM, Suh J, et al. Characterization of 298 patients with lung cancer harboring MET exon 14 skipping alterations. J Thorac Oncol. 2016;11(9):1493–1502.
- Awad MM, Oxnard GR, Jackman DM, et al. MET exon 14 mutations in non-small-cell lung cancer are associated with advanced age and stage-dependent MET genomic amplification and c-Met overexpression. J Clin Oncol. 2016;34(7):721–730.
- Lindeman NI, Cagle PT, Aisner DL, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the study of lung cancer, and the Association for Molecular Pathology. J Mol Diagn. 2018;20(2):129–159.
- Spigel DR, Edelman MJ, O’Byrne K, et al. Results from the phase III randomized trial of onartuzumab plus erlotinib versus erlotinib in previously treated stage IIIB or IV non-small-cell lung cancer: METLung. J Clin Oncol. 2017;35(4):412–420.
- Cunningham D, Tebbutt NC, Davidenko I, et al. Phase III, randomized, double-blind, multicenter, placebo (P)-controlled trial of rilotumumab (R) plus epirubicin, cisplatin and capecitabine (ECX) as first-line therapy in patients (pts) with advanced MET-positive (pos) gastric or gastroesophageal junction (G/GEJ) cancer: RILOMET-1 study [abstract]. J Clin Oncol. 2015;15(Suppl):Abstract 4000.
- Reungwetwattana T, Liang Y, Zhu V, et al. The race to target MET exon 14 skipping alterations in non-small cell lung cancer: the why, the how, the who, the unknown, and the inevitable. Lung Cancer. 2017;103:27–37.
- Angevin E, Spitaleri G, Rodon J, et al. A first-in-human phase I study of SAR125844, a selective MET tyrosine kinase inhibitor, in patients with advanced solid tumours with MET amplification. Eur J Cancer. 2017;87:131–139.
- Hughes PE, Rex K, Caenepeel S, et al. In vitro and in vivo activity of AMG 337, a potent and selective MET kinase inhibitor, in MET-dependent cancer models. Mol Cancer Ther. 2016;15(7):1568–1579.
- Drilon A, Ou S-H, Clark J, et al. Antitumor activity and safety of crizotinib in patients with MET exon 14-altered advanced non-small cell lung cancer [abstract]. J Thorac Oncol. 2017;12(1 Suppl):S438–S439.Abstract MA16.09.
- Camidge DR, Otterson GA, Clark JW, et al. Crizotinib in patients (pts) with MET-amplified non-small cell lung cancer (NSCLC): updated safety and efficacy findings from a phase 1 trial [abstract]. J Clin Oncol. 2018;36(15Suppl):Abstract 9062.
- Caparica R, Yen CT, Coudry R, et al. Responses to crizotinib can occur in high-level MET-amplified non-small cell lung cancer independent of MET exon 14 alterations. J Thorac Oncol. 2017;12(1):141–144.
- Wolf J, Seto T, Han J, et al. Results of the GEOMETRY mono-1 phase II study for evaluation of the MET inhibitor capmatinib (INC280) in patients (pts) with METΔex14 mutated advanced non-small cell lung cancer (NSCLC). Presented at: European Society for Medical Oncology; 2018 Oct19–23; Munich, Germany.
- Cheng Y, Zhou J, Lu S, et al. Phase II study of tepotinib 1 gefitinib (TEP1GEF) in MET-positive (MET1)/epidermal growth factor receptor (EGFR)-mutant (MT) nonsmall cell lung cancer (NSCLC). Ann Oncol. 2018;29(Supplement8):viii493–viii547.Abstract 13770.
- Paik PK, Veillon R, Cortot AB, et al. Phase II study of tepotinib in NSCLC patients with METex14 mutations [abstract]. J Clin Oncol. 2019;37(15Suppl):Abstract 9005.
- Azuma K, Yoshioka H, Yamamoto N, et al. Tivantinib plus erlotinib versus placebo plus erlotinib in Asian patients with previously treated nonsquamous NSCLC with wild-type EGFR: first report of a phase III ATTENTION trial [abstract]. J Clin Oncol. 2014;32(15Suppl):Abstract 8044.
- Scagliotti G, von Pawel J, Novello S, et al. Phase III multinational, randomized, double-blind, placebo-controlled study of tivantinib (ARQ 197) plus erlotinib versus erlotinib alone in previously treated patients with locally advanced or metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol. 2015;33(24):2667–2674.
- Kim JH, Kim HS, Kim BJ. MET inhibitors in advanced non-small-cell lung cancer: a meta-analysis and review. Oncotarget. 2017;8(43):75500–75508.
- Katayama R, Aoyama A, Yamori T, et al. Cytotoxic activity of tivantinib (ARQ 197) is not due solely to c-MET inhibition. Cancer Res. 2013;73(10):3087–3096.
- Liu X, Wang Q, Yang G, et al. A novel kinase inhibitor, INCB28060, blocks c-MET-dependent signaling, neoplastic activities, and cross-talk with EGFR and HER-3. Clin Cancer Res. 2011;17(22):7127–7138.
- Baltschukat S, Schacher Engstler B, Huang A, et al. Capmatinib (INC280) is active against models of non-small cell lung cancer and other cancer types with defined mechanisms of MET activation. Clin Cancer Res. 2019;25(10):3164–3175.
- Hirsch FR, Govindan R, Zvirbule Z, et al. Efficacy and safety results from a phase ii, placebo-controlled study of onartuzumab plus first-line platinum-doublet chemotherapy for advanced squamous cell non-small-cell lung cancer. Clin Lung Cancer. 2017;18(1):43–49.
- Sterlacci W, Fiegl M, Gugger M, et al. MET overexpression and gene amplification: prevalence, clinico-pathological characteristics and prognostic significance in a large cohort of patients with surgically resected NSCLC. Virchows Arch. 2017;471(1):49–55.
- Frampton GM, Ali SM, Rosenzweig M, et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov. 2015;5(8):850–859.
- Drilon A. MET exon 14 alterations in lung cancer: exon skipping extends half-life. Clin Cancer Res. 2016;22(12):2832–2834.
- Drilon A, Somwar R, Wagner JP, et al. A novel crizotinib-resistant solvent-front mutation responsive to cabozantinib therapy in a patient with ROS1-rearranged lung cancer. Clin Cancer Res. 2016;22(10):2351–2358.
- Paik PK, Drilon A, Fan PD, et al. Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discov. 2015;5(8):842–849.
- Liu X, Jia Y, Stoopler MB, et al. Next-generation sequencing of pulmonary sarcomatoid carcinoma reveals high frequency of actionable MET gene mutations. J Clin Oncol. 2016;34(8):794–802.
- Vuong HG, Ho ATN, Altibi AMA, et al. Clinicopathological implications of MET exon 14 mutations in non-small cell lung cancer - A systematic review and meta-analysis. Lung Cancer. 2018;123:76–82.
- Guo B, Cen H, Tan X, et al. Prognostic value of MET gene copy number and protein expression in patients with surgically resected non-small cell lung cancer: a meta-analysis of published literatures. PLoS One. 2014;9(6):e99399.
- Dimou A, Non L, Chae YK, et al. MET gene copy number predicts worse overall survival in patients with non-small cell lung cancer (NSCLC); a systematic review and meta-analysis. PLoS One. 2014;9(9):e107677.
- Noonan SA, Berry L, Lu X, et al. Identifying the appropriate FISH criteria for defining MET copy number-driven lung adenocarcinoma through oncogene overlap analysis. J Thorac Oncol. 2016;11(8):1293–1304.
- Okuda K, Sasaki H, Yukiue H, et al. Met gene copy number predicts the prognosis for completely resected non-small cell lung cancer. Cancer Sci. 2008;99(11):2280–2285.
- Sabari JK, Leonardi GC, Shu CA, et al. PD-L1 expression, tumor mutational burden, and response to immunotherapy in patients with MET exon 14 altered lung cancers. Ann Oncol. 2018;29(10):2085–2091.
- Kawakami H, Okamoto I, Okamoto W, et al. Targeting MET amplification as a new oncogenic driver. Cancers (Basel). 2014;6(3):1540–1552.
- Cappuzzo F, Marchetti A, Skokan M, et al. Increased MET gene copy number negatively affects survival of surgically resected non-small-cell lung cancer patients. J Clin Oncol. 2009;27(10):1667–1674.
- Westover D, Zugazagoitia J, Cho BC, et al. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol. 2018;29(Suppl 1):i10–i19.
- Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci USA. 2007;104(52):20932–20937.
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3(75):75ra26.
- Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316(5827):1039–1043.
- Wang Y, Li L, Han R, et al. Clinical analysis by next-generation sequencing for NSCLC patients with MET amplification resistant to osimertinib. Lung Cancer. 2018;118:105–110.
- Tan CS, Kumarakulasinghe NB, Huang YQ, et al. Third generation EGFR TKIs: current data and future directions. Mol Cancer. 2018;17(1):29.
- Ninomiya K, Ohashi K, Makimoto G, et al. MET or NRAS amplification is an acquired resistance mechanism to the third-generation EGFR inhibitor naquotinib. Sci Rep. 2018;8(1):1955.
- Piotrowska Z, Thress K, Mooradian M, et al. MET amplification (amp) as a resistance mechanism to osimertinib [abstract]. J Clin Oncol. 2017;35(15):Abstract 9020.
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378(2):113–125.
- Ramalingam SS, Cheng Y, Zhou C, et al. Mechanisms of acquired resistance to first-line osimertinib: preliminary data from the Phase III FLAURA study [abstract]. Ann Oncol. 2018;29(Suppl):LBA50.
- Zou HY, Li Q, Lee JH, et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res. 2007;67(9):4408–4417.
- Lara MS, Holland WS, Chinn D, et al. preclinical evaluation of MET inhibitor INC-280 with or without the epidermal growth factor receptor inhibitor erlotinib in non-small-cell lung cancer. Clin Lung Cancer. 2017;18(3):281–285.
- Owusu BY, Galemmo R, Janetka J, et al. Hepatocyte growth factor, a key tumor-promoting factor in the tumor microenvironment. Cancers (Basel). 2017;9(4):e35.
- Bang YJ, Su WC, Nam DH, et al. Phase I study the safety and efficacy of INC280 in patients with advanced c-MET-dependent solid tumors. Presented at: American Society of Clinical Oncology; 2014 May 30–Jun 3; Chicago, USA.
- Ma B, Bang YJ, Lim WT, et al. Phase I dose escalation and expansion study to evaluate safety and efficacy of INC280 in patients with advanced MET-dependent solid tumors [abstract]. Ann Oncol. 2015;26(Suppl2):ii16–ii19. Abstract P1.04.
- Schuler MH, Berardi R, Lim WT, et al. Phase (Ph) I study of the safety and efficacy of the cMET inhibitor capmatinib (INC280) in patients (pts) with advanced cMET+ non-small cell lung cancer (NSCLC). Presented at: American Society of Clinical Oncology; 2016 Jun 3–7; Chicago, USA.
- Wolf J, Seto T, Han J-Y, et al. Capmatinib in METΔex14-mutated advanced non-small cell lung cancer (NSCLC): efficacy data from the phase II GEOMETRY mono-1 study [abstract]. J Clin Oncol. 2019;37(15Suppl):Abstract 9004.
- Wu YL, Zhang L, Kim DW, et al. Phase Ib/II study of capmatinib (INC280) plus gefitinib after failure of epidermal growth factor receptor (EGFR) inhibitor therapy in patients with EGFR-mutated, MET factor-dysregulated non-small-cell lung cancer. J Clin Oncol. 2018;36(31):3101–3109.
- AstraZeneca Pharmaceuticals LP. Iressa (Gefitinib) prescribing information. Wilmington: USA; 2019 May [cited 2019 May 31]. Available from: https://www.iressa-usahcp.com/sitemap.html
- Novartis Pharmaceuticals [Internet]. A safety and efficacy study of INC280 alone, and in combination with erlotinib, compared to chemotherapy, in advanced/metastatic non-small cell lung cancer patients with EGFR mutation and cMET amplification. [cited 2018 Dec 21]. Available from: https://clinicaltrials.gov/ct2/show/NCT02468661
- Tan D, Lee DH, Soo R, et al. Phase Ib results from a study of capmatinib (INC280) + EGF816 in patients with EGFR-mutant non-small cell lung cancer (NSCLC) [abstract]. Presented at: International Association for the Study of Lung Cancer; 2016 Dec 4–7; Vienna, Austria. 2017.
- Masuzawa K, Yasuda H, Hamamoto J, et al. Characterization of the efficacies of osimertinib and nazartinib against cells expressing clinically relevant epidermal growth factor receptor mutations. Oncotarget. 2017;8(62):105479–105491.
- Drilon A, Clark J, Weiss J, et al. Updated antitumor activity of crizotinib in patients with MET exon 14-altered advanced non-small cell lung cancer [abstract]. J Thorac Oncol. 2018;13(10(Suppl)):S348. Abstract OA12.02.
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693–1703.
- Ou SH, Bartlett CH, Mino-Kenudson M, et al. Crizotinib for the treatment of ALK-rearranged non-small cell lung cancer: a success story to usher in the second decade of molecular targeted therapy in oncology. Oncologist. 2012;17(11):1351–1375.
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–566.
- Pfizer Labs. Xalkori (crizotinib) Prescribing Information. New York: USA; 2019 Jan [cited 2019 May 29]. Available from: http://labeling.pfizer.com/showlabeling.aspx?id=676
- Pfizer Europe MA EEIG. XALKORI (crizotinib) summary of product characteristics. Brussels: Belgium; 2019 Mar [cited 2019 May 29]. Available from: https://www.medicines.org.uk/emc/product/7983/smpc/print
- Takigawa N, Ochi N, Yamane H. Blocking both epidermal growth factor receptor and mesenchymal-to-epithelial transition pathways in EGFR-mutated lung cancer. Transl Lung Cancer Res. 2018;7(Suppl 4):S352–S355.
- Choueiri TK, Plimack E, Arkenau HT, et al. Biomarker-based phase II trial of savolitinib in patients with advanced papillary renal cell cancer. J Clin Oncol. 2017;35(26):2993–3001.
- Ahn M, Han J, Sequist L, et al. TATTON Ph Ib expansion cohort: osimertinib plus savolitinib for pts with EGFR-mutant MET-amplified NSCLC after progression on prior EGFR-TKI. J Thorac Oncol. 2017;12:S1768.
- Yu H, Ahn MJ, Kim SW, et al. TATTON phase Ib expansion cohort: osimertinib plus savolitinib for patients (pts) with EGFR-mutant, MET-amplified NSCLC after progression on prior first/second-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI). Presented at: American Association for Cancer Research; 2019 Mar 29–Apr 03; Atlanta, USA.
- Sequist LV, Lee JS, Han JY, et al. TATTON phase Ib expansion cohort: osimertinib plus savolitinib for patients (pts) with EGFR-mutant, MET-amplified NSCLC after progression on prior third-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI). Presented at: American Association for Cancer Research; 2019 Mar 29–Apr 03; Atlanta, USA.
- Lu S, Fang J, Cao L, et al. Preliminary efficacy and safety results of savolitinib treating patients with pulmonary sarcomatoid carcinoma (PSC) and other types of non-small cell lung cancer(NSCLC) harboring MET exon 14 skipping mutations. Presented at: American Association for Cancer Research; 2019 Mar 29–Apr 3; Atlanta, USA.
- Kim S, Kim TM, Kim DW, et al. Acquired resistance of MET-amplified non-small cell lung cancer cells to the MET inhibitor capmatinib. Cancer Res Treat. 2018. DOI:10.4143/crt.2018.052
- Davies KD, Lomboy A, Lawrence CA, et al. Brief Report: DNA-based versus RNA-based detection of MET exon 14 skipping events in lung cancer. J Thorac Oncol. 2019;14(4):737–741.
- Guo R, Offin M, Brannon AR, et al. MET inhibitor resistance in patients with MET exon 14-altered lung cancers. Presented at: American Society of Clinical Oncology; 2019 May 31–Jun 4; Chicago, USA.
- Lai GGY, Lim TH, Lim J, et al. Clonal MET amplification as a determinant of tyrosine kinase inhibitor resistance in epidermal growth factor receptor-mutant non-small-cell lung cancer. J Clin Oncol. 2019;37(11):876–884.
