ABSTRACT
Introduction: Pediatric relapsed acute myeloid leukemia (AML) remains lethal in the majority of cases, despite intensive therapy. Randomized trials are largely lacking, and the main issues of optimal therapy and prognostic factors remain unclear.
Area covered: This systematic review includes all literature evaluating treatment outcome after first relapse. We searched databases PubMed and Embase.com. Twelve out of six thousand articles were ultimately included, based on age of the population (<21 years), relapsed AML, and information on clinical outcome (second complete remission (CR2), disease-free survival (DFS), event-free survival (EFS) and overall survival (OS)). There was only one randomized clinical trial reported. This review shows that there is no standard treatment for relapsed AML in children, and that outcome varies for CR2 and (2- to 10-year) OS rates, mean 64% (range, 50–75%), and 31% (16–43%), respectively. Children treated with chemotherapy only in first complete remission (CR1) tend to have better outcome after relapse than children receiving allo-SCT in CR1. Allo-SCT seems to be the most effective consolidation therapy in children achieving CR2, after relapse. Duration of CR1 was the most frequently reported statistically significant prognostic factor. Through randomized clinical trials, better knowledge of prognostic factors enabling risk-stratified treatment, and of more effective and less toxic therapies, should contribute to better clinical outcome for children with relapsed AML.
Expert opinion: Outcome of pediatric relapsed AML has improved to OS rates up to 40%. However, there is a lack of knowledge on (independent) prognostic factors, optimal reinduction chemotherapy, timing of allo-SCT, and late effects. International collaboration should enable large, randomized clinical trials addressing these issues.
1. Introduction
Leukemia accounts for approximately 30% of all pediatric malignancies, of which 15–20% comprises Acute Myeloid Leukemia (AML). Over the past decades, due to international collaboration and improvements in treatment protocols, the prognosis of AML has improved significantly. Long-term survival rates of AML are currently 70% or even higher, in comparison with long-term survival rates of approximately 50% around the year 1990 [Citation1–3]. However, in 24–40% of the children the AML relapses, with a probability of long-term survival of approximately 30% [Citation4,Citation5]. These poor outcomes indicate the need for improved treatment, including innovative drugs and treatment modalities [Citation6,Citation7]. Nevertheless, due to a relatively small patient population studies on pediatric relapsed AML mostly consist of case reports and only a few large cohort-based studies have been performed. Consequently, until now a systematic review on pediatric relapsed AML is lacking.
This study aims at providing an overview of the efficacy of currently used treatments for children with relapsed AML. We were especially interested in treatment outcomes in relation to patient characteristics and other potential prognostic factors. Hopefully, this will ultimately lead to a better perspective for these young patients.
2. Methods
2.1. Search strategy
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [Citation8]. A comprehensive search was performed in the bibliographic databases PubMed and Embase.com from inception to April 9th, 2019, in collaboration with a medical librarian (LS). Search terms included controlled terms (MesH in PubMed and Emtree in Embase.com) as well as free-text terms. The following terms were used (including synonyms and closely related words) as index terms or free-text words: ‘acute myeloid leukemia’ and ‘recurrence’ and ‘children.’ The search was performed without date or language restrictions. Duplicate articles and abstracts only were excluded. Reference lists of the included articles were also screened (snowball search).
2.2. Study selection
Studies were eligible for inclusion if they reported treatment outcome of a first relapse of AML in children and/or adolescents less than 21 years of age. Outcomes of interest included second complete remission (CR2), disease-free survival (DFS), event-free survival (EFS), and overall survival (OS). Studies that only included children with acute promyelocytic leukemia, secondary AML, or Down syndrome were excluded, considering their unique biology and treatment. In addition, case reports and non-English articles were disqualified for inclusion. One author (AH) screened the search results for potential inclusion based on title and abstract using the Rayyan web application [Citation9]. After reading the selected full-text articles, final inclusion was determined by agreement with an additional author (GK).
Several studies distinguished between all children and the subgroup of children who were treated with a curative intent. As this last group tends to reach higher CR2- and OS-rates, while more population-based data are relevant as well, both groups are presented separately in our figures.
3. Results
Searching the databases resulted in over 6000 articles, including three articles retrieved through snowball search. After screening titles and abstracts, 220 articles were selected for full-text reading. Twelve studies were qualified for inclusion in this systematic review. shows an overview of the selection process.
Figure 1. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Flow Diagram
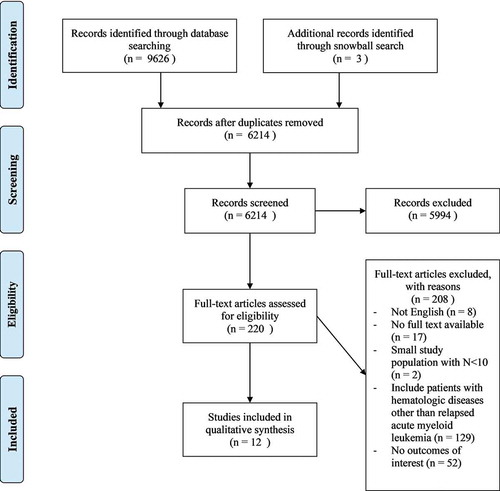
Overall, 1928 children of 20 years of age or younger were included in 12 studies between 1980 and 2014. The average of median follow-up and duration of first remission (CR1) is 4.6 years (range 1–9.5) and 10.9 months (range 9–13.2), respectively.
Further study characteristics and outcomes are summarized in .
Table 1. Study and patient characteristics of the included studies on pediatric relapsed AML sorted by last year of study inclusion
3.1. Second complete remission and survival
All studies reported CR2 rates and 2- to 10-year OS from relapse, mean 64% (range, 50–75%), and 31% (16–43%), respectively. Only five studies included DFS and three studies EFS as survival outcome parameters. The development of the CR2 and OS rates over time is shown in .
Figure 2. (a) CR2-rate and (b) 5-year OS-rate* over time of relapsed AML children sorted by last year of study inclusion. *Only studies reporting a 3-year OS-rate or longer were included in this figure. Wells et al. [Citation12] was excluded. **3-year OS-rate. ***4-year OS-rate
![Figure 2. (a) CR2-rate and (b) 5-year OS-rate* over time of relapsed AML children sorted by last year of study inclusion. *Only studies reporting a 3-year OS-rate or longer were included in this figure. Wells et al. [Citation12] was excluded. **3-year OS-rate. ***4-year OS-rate](/cms/asset/968520fa-a2e5-4092-be2e-feeaa86556e6/iery_a_1841640_f0002_oc.jpg)
As illustrated in , there was no clear correlation between the time-period of treatment and outcome for all patients or patients treated with curative intent only (0.03< R2 < 0.08 (CR2); 0.07< R2 < 0.59 (OS)).
One study compared CR1- to CR2-rates. Goemans et al. [Citation10] noted that 72% of the children who achieved CR1 on first-line treatment also attained CR2, whereas none of the children who did not achieve first remission on first-line treatment, reached CR2 (n = 11, χ2 p < 0.01).
3.2. Outcome related to type of therapy in CR1
In CR1, relapsed AML children were treated with chemotherapy only (n = 659, 79%), auto-SCT (n = 45, 5%), allo-SCT (n = 97, 12%) or with an undefined type of SCT (n = 35, 4%). Stahnke et al. [Citation11], Goemans et al. [Citation10], Wells et al. [Citation12], Gorman et al. [Citation13], and Kaspers et al. [Citation14] were left out of this analysis because of lacking detailed data on therapy in CR1.
CR2-rates after treatment in CR1 with SCT or chemotherapy only in comparison to CR2-rates of the entire study population are set out in .
Figure 3. CR2-rate of relapsed AML children sorted by treatment modality in CR1a. aGoemans et al. [Citation10], Stahnke et al. [Citation11], Wells et al. [Citation12], Gorman et al. [Citation13] and Kaspers et al. [Citation14] were omitted from this analysis because of lacking detailed data on treatment modality in CR1. bCR-rate after chemotherapy + maintenance therapy in CR1: 60%
![Figure 3. CR2-rate of relapsed AML children sorted by treatment modality in CR1a. aGoemans et al. [Citation10], Stahnke et al. [Citation11], Wells et al. [Citation12], Gorman et al. [Citation13] and Kaspers et al. [Citation14] were omitted from this analysis because of lacking detailed data on treatment modality in CR1. bCR-rate after chemotherapy + maintenance therapy in CR1: 60%](/cms/asset/8322cafc-634e-46ef-9138-7022d3be5016/iery_a_1841640_f0003_b.gif)
Looking at studies reporting outcomes after SCT in CR1, CR2-rates after auto-SCT in CR1 are worse in comparison to chemotherapy only and the average remission rate. CR2-rates of patients treated with a SCT in CR1 were found to be significantly lower than overall CR2-rates in six out of seven studies [Citation15–20]. Aladjidi et al. [Citation21] reported a remarkably high CR2-rate of 83% for patients treated with an allo-SCT in CR1, exceeding the CR2-rate of 77% for patients treated with chemotherapy only and the CR2-rate of 60% for patients treated with chemotherapy and maintenance therapy.
summarizes the OS-rates after relapse, ordered by therapy used in CR1. Children treated with an SCT in CR1 tend to have lower OS-rates after relapse, compared to children treated with chemotherapy only. In contrast, Aladjidi et al. [Citation21] report a relatively high OS-rate of 52% after relapse and allo-SCT in CR1. Rubnitz et al. [Citation16] reported an OS-rate of 45% from relapse after auto-SCT, whilst Abrahamsson et al. [Citation17] and Sander et al. [Citation18] report an OS-rate of respectively 5% and 0% for these patients.
Figure 4. OS-rate of relapsed AML children sorted by treatment modality in CR1a. aStahnke et al. [Citation11], Goemans et al. [Citation10], Wells et al. [Citation12], Gorman et al. [Citation13], Nakayama et al. [Citation19] and Kaspers et al. [Citation14] were left out of this analysis because of lacking data on treatment modality in CR1. bOS-rate after chemotherapy + maintenance therapy in CR1: 12%
![Figure 4. OS-rate of relapsed AML children sorted by treatment modality in CR1a. aStahnke et al. [Citation11], Goemans et al. [Citation10], Wells et al. [Citation12], Gorman et al. [Citation13], Nakayama et al. [Citation19] and Kaspers et al. [Citation14] were left out of this analysis because of lacking data on treatment modality in CR1. bOS-rate after chemotherapy + maintenance therapy in CR1: 12%](/cms/asset/86ca87c3-a376-4902-b0b7-3fb0a048aa39/iery_a_1841640_f0004_b.gif)
3.3. Outcome related to type of therapy in CR2
In CR2, relapsed AML children were treated with chemotherapy only (n = 461, 32%), auto-SCT (n = 131, 9%), allo-SCT (n = 652, 45%) or with an undefined type of SCT (n = 206, 14%). The average median time from start of relapse treatment to transplant, documented by seven out of twelve studies, is 87 days (range 51–117).
shows a higher OS-rate for children treated with an SCT in CR2 in comparison to the entire patient population. A significant difference in OS-rate after treatment with auto-SCT or allo-SCT was not found by Sander et al. [Citation18]. Survival rates were higher after treatment with allo-SCT in CR2 compared to chemotherapy only in three out of four studies. Wells et al. [Citation12] found a somewhat lower OS-rate for allo-SCT in CR2 compared to chemotherapy only.
Figure 5. OS-rate of relapsed AML children sorted by treatment modality from relapsea. aWebb et al. [Citation15], Stahnke et al. [Citation11], Aladjidi et al. [Citation21], Gorman et al. [Citation13] and Kaspers et al. [Citation14] were left out of this analysis because of lacking detailed data on therapy in CR2. b5-year pOS-rate when transplanted in CR2 vs. 5-year pOS-rate of 17% when transplanted in non-CR2
![Figure 5. OS-rate of relapsed AML children sorted by treatment modality from relapsea. aWebb et al. [Citation15], Stahnke et al. [Citation11], Aladjidi et al. [Citation21], Gorman et al. [Citation13] and Kaspers et al. [Citation14] were left out of this analysis because of lacking detailed data on therapy in CR2. b5-year pOS-rate when transplanted in CR2 vs. 5-year pOS-rate of 17% when transplanted in non-CR2](/cms/asset/87ffea9d-904f-4a36-a525-aeb1e30fad75/iery_a_1841640_f0005_b.gif)
In three studies a worse outcome was found for relapsed AML when transplanted not being in second remission, in comparison to proven CR2. Stahnke et al. [Citation11] reported that none of the six children transplanted in non-CR2 survived, while 8/15 (53%) children in proven CR2 did. Nakayama et al. [Citation19] reported significantly higher 5- and 10-year OS-rates in children who were treated with SCT in CR2 than those in non-CR2 (66 vs. 17%, P < 0.01; 65.5 vs. 9.5%, P < 0.01). In the study of Wells et al. [Citation12], 17/35 (49%) children transplanted in proven CR2 survived, but both children transplanted in non-CR2 died. Other studies focusing on pediatric relapsed AML did not describe the correlation between outcome and transplantation in non-CR2.
3.3.1. Prognostic factors
All but three studies [Citation14,Citation16,Citation19] report length of first remission to be an evident prognostic factor positively related to the CR2-rate. Regarding OS-rates, length of first remission is as well a strong positively correlated prognostic factor according to 10 out of 12 studies (reported by all but Gorman et al. [Citation13] and Kaspers et al. [Citation14]). No correlations with outcome were found regarding age at initial diagnosis [Citation13,Citation19–21], FAB-type [Citation11,Citation17,Citation19–21], WBC-count [Citation11–13,Citation21] or sex [Citation17,Citation20,Citation21]. Survival parameters disease free survival (DFS) and event free survival (EFS) were omitted from our analysis because only two studies [Citation13,Citation15] examined these outcomes in relation to prognostic factors. However, both report favorable cytogenetics as a strong favorable prognostic factor regarding DFS and EFS. Favorable cytogenetics were defined as CBF [Citation20], inv(16) and t(8;21) [Citation13–15,Citation18–20], or t(15;17) [Citation15,Citation18]. Looking at the OS-rate, three studies found a positive correlation with favorable cytogenetics [Citation14,Citation18,Citation20], whilst five studies found no correlation [Citation12,Citation13,Citation17,Citation19,Citation21]. Only five studies reported the impact of favorable cytogenetics on CR2-rates, where three studies found a positive [Citation12,Citation18,Citation20] and two studies [Citation12,Citation21] found no correlation. Other prognostic factors such as race, hepatosplenomegaly and age at relapse were reported only once or twice in total.
4. Discussion
Many and large cohort-based studies on newly diagnosed pediatric AML have been performed, but only a few focused on pediatric relapsed AML. To our knowledge, this is the first systematic review on this subject including all (more or less) cohort-based studies. Davila et al. [Citation22] published a review on current treatments of pediatric relapsed AML, but this lacked a systematic literature search and survival rates were not primary study endpoints.
Our review includes 12 studies reporting a wide diversity in treatment schedules, pointing out that there is still no consensus on the best treatment for pediatric relapsed AML. Only a few studies were of high methodological quality with only one RCT and seven prospective studies. The variability in treatment regimens and in-/and exclusion criteria makes it challenging to make comparisons between studies, and impossible to perform a meta-analysis.
Inclusion criteria varied. Stahnke et al. [Citation11], Aladjidi et al. [Citation21], and Wells et al. [Citation12] included children with acute promyelocytic leukemia (APL). The study population of Abrahamsson et al. [Citation17] and Wells et al. [Citation12] contained children with Down syndrome. In addition, children with refractory AML were not excluded by Gorman et al. [Citation13] and Kaspers et al. [Citation14]. Nevertheless, the studies that included some children with APL or refractory AML or Down syndrome do not seem to report clearly different outcomes in comparison to studies excluding these children.
Regarding clinical outcome, several studies reported a difference between the entire study population and the subpopulation of children who were treated with curative intent, in favor of the latter group. Not all studies distinguished between these populations or do not report their outcomes separately.
The CR2- and OS-rates sorted by study time-period showed no significant change over time (R2 of trendlines varied from 0.03 to 0.59), in contrast to what we would expect because of improvements in the treatment of pediatric AML over the past decades [Citation22]. The major fluctuation in remission rates and survival outcomes is likely because the treatment regimens varied over time and even within one study. Moreover, the wide range in patient population sizes may have biased these study outcomes. Finally, the studies did not always concern population-based cohorts, and selection bias may have occurred.
Regarding treatment modalities, 80% of relapsed children had been treated with chemotherapy only in CR1. These children tend to have higher second remission and survival rates than children treated with an SCT in CR1. However, these findings are biased by studies inconsequently reporting data on therapy in CR1, and by selection bias since higher-risk patients are more likely to be treated with an SCT in CR1.
After relapse, the majority of children was treated with an SCT. Outcome seemed better in these patients compared to chemotherapy only, with OS-rates comparable to other recent studies [Citation23,Citation24]. However, conclusions cannot easily be drawn, since SCT is mostly performed in children achieving CR2 and in relatively good state [Citation25,Citation26]. However, we suggest, in accordance with previous studies [Citation23,Citation27,Citation28], that allo-SCT in CR2 is resulting in better long-term clinical outcome than chemotherapy only, acknowledging that several studies have reported a few survivors with the latter approach. This difference may be due to the limited 2-year OS-rate reported, and thus, in comparison with 5- and 10-year OS-rates, result in biased outcome in favor of chemotherapy only. Our review also shows that allo-SCT in CR2 leads to better outcome than in case of a transplant not in CR2. However, also Leung et al. [Citation29] reported that an allo-SCT not in CR2 can lead to cure in pediatric AML, probably because of the graft-versus-leukemia effect, in addition to the antileukemic activity of the conditioning regimen itself.
Considering prognostic risk factors, length of first complete remission is strongly positively correlated with second complete remission and overall survival rates in (nearly) all studies. Favorable cytogenetics as known from studies in newly diagnosed AML, such as inv(16) and t(8;21), is associated with better clinical outcome at relapse as well in several but not all studies. White blood cell count at relapse was only studied in three studies, and shows no correlation with clinical outcome, in contrast to findings in newly diagnosed pediatric AML [Citation30]. Creutzig et al. [Citation31] reported early treatment response (<20% blasts on day 28 bone marrow after relapse) to be a strong and independent prognostic factor, based on the same cohort of pediatric relapsed AML patients as described by Kaspers et al. [Citation14]. To the best of our knowledge, no other studies have looked at the prognostic significance of early treatment response in pediatric relapsed AML, although it is a well-known prognostic factor at initial diagnosis [Citation2]. Other prognostic factors were described infrequently, thus no conclusions can be drawn regarding these factors.
There are limitations to this review. At first, we are aware of restrictions to our study methods. Although the search was performed consulting a medical librarian (LS), search results were analyzed by one researcher only. Therefore, interpretation bias may have occurred during inclusion. Second, we focused on published articles only which provokes to miss smaller studies, presented only at meetings. Publication bias was not assessed in this review, but selection bias has occurred by selecting English articles only. Third, four out of the twelve studies [Citation10,Citation12,Citation16,Citation19] lacked definitions of relapse and CR. In combination with differences in study methodology and patient populations, outcomes should be interpreted thoughtfully. Fourth, remission rates and survival outcomes regarding sub-groups were not included for each study, mostly due to lack of information. At last, the methodological quality of included studies is low, with only one randomized clinical trial included [Citation14], and with several retrospective studies as well.
5. Conclusion
In conclusion, treatment outcome of children with relapsed AML remain dismal, with pOS at 3 to 5 years of about 40% in the most recent studies for children treated with curative intent. We could not identify an evident increase of remission or survival rates over the past decades in literature. Therapy including SCT in CR1 seems to reduce the chance of second remission and overall survival rates for relapsed disease. However, allo-SCT in CR2 after reinduction chemotherapy is associated with better outcome than chemotherapy only, although several studies report a modest chance of cure with chemotherapy only. Optimal treatment for pediatric relapsed AML thus is not well defined yet, regarding optimal chemotherapy, the need for allo-SCT, the timing of an allo-SCT and risk-group stratified treatment based on well-defined prognostic factors. Future controlled clinical trials on pediatric relapsed AML will hopefully lead to more effective treatment, without undue side-effects.
6. Expert opinion
This review mainly illustrates the gaps in knowledge on optimal treatment of pediatric relapsed AML. While OS has improved over the decades to 40% and above, much is unknown. Thus, there is an urgent need for large, randomized clinical trials which is feasible only in the setting of international collaborations. Since outcome from relapsed AML is worse in case of allo-SCT applied in CR1, the indications for such a procedure at initial treatment must be carefully defined. Once a child relapses, working toward an allo-SCT is rule. However, it is possible that some patients do not have a relapse, but a second AML, emerging from a preleukemic stem cell that survived initial therapy. Such patients may not need an allo-SCT for cure, and since SCT causes significant late effects, this issue must be investigated. In addition, we need more knowledge on prognostic factors that can be used for risk-group stratified treatment, on optimal reinduction chemotherapy including the introduction of novel agents and novel treatment modalities, on timing of allo-SCT and on quality-of-life and late effects. Regarding novel agents and treatment modalities, we face an exciting time period, including drugs such as venetoclax, monoclonal antibodies, and cellular therapies. Ultimately, the best approach is to avoid relapses in children with AML. There is a wide range of possibilities to further decrease relapse rates. First, by better selection of patients that will benefit significantly from allo-SCT in CR1. Second, by selection of patients that will benefit from the addition of targeted agents in combination with chemotherapy. There is a large number of novel drugs becoming available, and especially for patients with a high likelihood of relapse and in whom the benefit of an allo-SCT in CR1 is not so clear, it makes much sense to explore the benefit of such agents already in first-line treatment. Third, dosing of drugs has not been individualized, while there is a lot of data showing differences in pharmacokinetics of anticancer agents in children. It makes sense to apply individualized dosing of antileukemic agents, as is common practice with antibiotics and other agents. In view of all developments, the future is bright for children and adolescents with AML. However, it requires much preclinical and clinical research before we will be close to 100% cure and before we can avoid all relapses.
Article Highlights
This review illustrates that there is no standard treatment for relapsed AML in children, although allogeneic stem cell transplantation (allo-SCT) is routinely used in second complete remission (CR2).
Outcome varies for CR2 and (2- to 10-year) OS rates, mean 64% (range, 50-75%) and 31% (16-43%), respectively.
Children treated with chemotherapy only in CR1 tend to have better outcome after relapse than children receiving allo-SCT in CR1.
Allo-SCT indeed seems to be a more effective consolidation therapy in children achieving CR2, after relapse, than chemotherapy.
Duration of CR1 was the most frequently reported statistically significant prognostic factor.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors would like to thank all colleagues for performing and contributing to research on (relapsed) AML in children.
Additional information
Funding
References
- Rasche M, Zimmermann M, Borschel L, et al. Successes and challenges in the treatment of pediatric acute myeloid leukemia: a retrospective analysis of the AML-BFM trials from 1987 to 2012. Leukemia. 2018 Oct;32(10):2167–2177. PubMed PMID: 29550834; PubMed Central PMCID: PMCPMC6170392. eng.
- Klein K, de Haas V, Kaspers GJL. Clinical challenges in de novo pediatric acute myeloid leukemia. Expert Rev Anticancer Ther. 2018 Mar;18(3):277–293. . PubMed PMID: 29338495; eng
- Kaspers GJ. Pediatric acute myeloid leukemia. Expert Rev Anticancer Ther. 2012 Mar;12(3):405–413. . PubMed PMID: 22369331; eng
- Mustafa O, Abdalla K, AlAzmi AA, et al. FLAG/FLAG-IDA regimen for children with relapsed/refractory acute leukemia in the era of targeted novel therapies. J Oncol Pharm Pract. 2019 Dec;25(8):1831–1838. PubMed PMID: 30518307; eng.
- Kaspers G. How I treat paediatric relapsed acute myeloid leukaemia. Br J Haematol. 2014 Sep;166(5):636–645. . PubMed PMID: 24837715; eng
- Foster JB, Maude SL. New developments in immunotherapy for pediatric leukemia. Curr Opin Pediatr. 2018 Feb;30(1):25–29. . PubMed PMID: 29176353; eng
- Yu MG, Zheng HY. Acute Myeloid Leukemia: advancements in diagnosis and treatment. Chin Med J (Engl). 2017 Jan 20;130(2):211–218. . PubMed PMID: 28091414; PubMed Central PMCID: PMCPMC5282679. eng.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009 Jul 21;6(7):e1000097. PubMed PMID: 19621072; PubMed Central PMCID: PMCPMC2707599. eng.
- Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016 Dec 5;5(1):210. PubMed PMID: 27919275; PubMed Central PMCID: PMCPMC5139140. eng.
- Goemans BF, Tamminga RY, Corbijn CM, et al. Outcome for children with relapsed acute myeloid leukemia in the Netherlands following initial treatment between 1980 and 1998: survival after chemotherapy only? Haematologica. 2008 Sep;93(9):1418–1420. PubMed PMID: 18757852; eng
- Stahnke K, Boos J, Bender-Gotze C, et al. Duration of first remission predicts remission rates and long-term survival in children with relapsed acute myelogenous leukemia. Leukemia. 1998 Oct;12(10):1534–1538. PubMed PMID: 9766496; eng
- Wells RJ, Adams MT, Alonzo TA, et al. Mitoxantrone and cytarabine induction, high-dose cytarabine, and etoposide intensification for pediatric patients with relapsed or refractory acute myeloid leukemia: children’s cancer group study 2951. J Clin Oncol. 2003 Aug 1;21(15):2940–2947. PubMed PMID: 12885813; eng
- Gorman MF, Ji L, Ko RH, et al. Outcome for children treated for relapsed or refractory acute myelogenous leukemia (rAML): a therapeutic advances in childhood leukemia (TACL) consortium study. Pediatr Blood Cancer. 2010 Sep;55(3):421–429. PubMed PMID: 20658611; eng
- Kaspers GJ, Zimmermann M, Reinhardt D, et al. Improved outcome in pediatric relapsed acute myeloid leukemia: results of a randomized trial on liposomal daunorubicin by the International BFM study group. J Clin Oncol. 2013 Feb 10;31(5):599–607. PubMed PMID: 23319696; eng
- Webb DK, Wheatley K, Harrison G, et al. Outcome for children with relapsed acute myeloid leukaemia following initial therapy in the Medical research council (MRC) AML 10 trial. MRC childhood leukaemia working party. Leukemia. 1999 Jan;13(1):25–31. PubMed PMID: 10049056; eng
- Rubnitz JE, Razzouk BI, Lensing S, et al. Prognostic factors and outcome of recurrence in childhood acute myeloid leukemia. Cancer. 2007 Jan 1;109(1):157–163. PubMed PMID: 17133407; eng.
- Abrahamsson J, Clausen N, Gustafsson G, et al. Improved outcome after relapse in children with acute myeloid leukaemia. Br J Haematol. 2006 Jan;136(2):229–236. PubMed PMID: 17278259; eng
- Sander A, Zimmermann M, Dworzak M, et al. Consequent and intensified relapse therapy improved survival in pediatric AML: results of relapse treatment in 379 patients of three consecutive AML-BFM trials. Leukemia. 2010 Aug;24(8):1422–1428. PubMed PMID: 20535146; eng
- Nakayama H, Tabuchi K, Tawa A, et al. Outcome of children with relapsed acute myeloid leukemia following initial therapy under the AML99 protocol. Int J Hematol. 2014 Aug;100(2):171–179. PubMed PMID: 24961644; eng
- Karlsson L, Forestier E, Hasle H, et al. Outcome after intensive reinduction therapy and allogeneic stem cell transplant in paediatric relapsed acute myeloid leukaemia. Br J Haematol. 2017 Aug;178(4):592–602. PubMed PMID: 28439893; eng
- Aladjidi N, Auvrignon A, Leblanc T, et al. Outcome in children with relapsed acute myeloid leukemia after initial treatment with the French Leucemie Aique Myeloide Enfant (LAME) 89/91 protocol of the French society of pediatric hematology and immunology. J Clin Oncol. 2003 Dec 1;21(23):4377–4385. PubMed PMID: 14645428; eng
- Davila J, Slotkin E, Renaud T. Relapsed and refractory pediatric acute myeloid leukemia: current and emerging treatments. Paediatr Drugs. 2013 Apr;16(2):151–168. PubMed PMID: 24158739; eng
- Niktoreh N, Lerius B, Zimmermann M, et al. Gemtuzumab ozogamicin in children with relapsed or refractory acute myeloid leukemia: a report by Berlin-Frankfurt-Munster study group. Haematologica. 2019 Jan;104(1):120–127. PubMed PMID: 30093401; eng.
- van Eijkelenburg NKA, Rasche M, Ghazaly E, et al. Clofarabine, high-dose cytarabine and liposomal daunorubicin in pediatric relapsed/refractory acute myeloid leukemia: a phase IB study. Haematologica. 2018 Sep;103(9):1484–1492. PubMed PMID: 29773602; eng
- Bacigalupo A, Lamparelli T, Gual, et al. Allogeneic hemopoietic stem cell transplants for patients with relapsed acute leukemia: long-term outcome. Bone Marrow Transplant. 2007 Mar;39(6):341–346. PubMed PMID: 17277788; eng
- Byrne JL, Dasgupta E, Pallis M, et al. Early allogeneic transplantation for refractory or relapsed acute leukaemia following remission induction with FLAG. Leukemia. 1999 May;13(5):786–791. PubMed PMID: 10374884; eng
- Lund TC, Ahn KW, Tecca HR, et al. Outcomes after second hematopoietic cell transplantation in children and young adults with relapsed acute leukemia. Biol Blood Marrow Transplant. 2019 Feb;25(2):301–306. PubMed PMID: 30244103; eng
- Creutzig U, Ritter J, Boos J, et al. [Prognosis of children with acute myelocytic leukemia after first relapse]. Klin Padiatr. 1998 Jul-Aug;210(4):207–211. PubMed PMID: 9743954; ger
- Leung W, Pui CH, Coustan-Smith E, et al. Detectable minimal residual disease before hematopoietic cell transplantation is prognostic but does not preclude cure for children with very-high-risk leukemia. Blood. 2012 Jul 12;120(2):468–472. PubMed PMID: 22517895; PubMed Central PMCID: PMCPMC3398757. eng.
- Bals E, Ter, Kaspers GJ. Treatment of childhood acute myeloid leukemia. Expert Rev Anticancer Ther. 2005 Oct;5(5):917–929. . PubMed PMID: 16221060; eng
- Creutzig U, Zimmermann M, Dworzak MN, et al. The prognostic significance of early treatment response in pediatric relapsed acute myeloid leukemia: results of the international study relapsed AML 2001/01. Haematologica. 2014 Sep;99(9):1472–1478. PubMed PMID: 24763401; eng. ** Included in systematic review
