ABSTRACT
Introduction: Cervical cancer is the fourth most common form of cancer among women. Smoking tobacco seems to be a risk factor for the development of cervical intra-epithelial neoplasia (CIN) and cervical cancer, but the exact role of smoking in the process of cervical carcinogenesis is not known. The aim of this study is to investigate the relationship between smoking and the development of CIN and cervical cancer. Areas covered: We searched Embase, Medline, Cochrane Central, Web of Science, and Google Scholar for studies on smoking and CIN and cervical cancer, published between 2009 and 2018. The following were the outcomes: CIN3 alone, CIN2 and CIN3 combined, CIN2+, CIN3+, and cervical cancer alone. We included 49 studies in our review and 45 in our meta-analyses. Expert opinion: Based on the available evidence it can be – cautiously – concluded that smoking increases the risk of cervical abnormalities. However, the high risk of bias indicates that for future studies, it will be important to adjust for relevant predictors, to separate CIN from cervical cancer as outcome measures, and to report research methods in detail.
1. Introduction
Cervical cancer is the fourth most common form of cancer among women worldwide. Every year, half a million women are diagnosed with this disease [Citation1]. Cervical cancer is preceded by a premalignant stage of cervical intra-epithelial neoplasia (CIN). This premalignant stage makes it possible to detect a cervical abnormality by screening before it develops into cervical cancer [Citation2]. A high-risk human papillomavirus (hrHPV) cervical infection underlies the development of cervical abnormalities [Citation3]. Only if an infection persists, can it lead to the development of a premalignant CIN lesion [Citation4,Citation5], and the progression to cancer can take up to 10 to 15 years.
It is unclear why some hrHPV infections lead to the development of CIN and ultimately cancer, while other hrHPV infections are cleared by the immune system and CIN lesions show regression. Smoking tobacco is assumed to be a significant behavioral risk factor for the persistence of hrHPV infections and the development of CIN and cervical cancer [Citation6]. Other risk factors are, for example, immunocompromised status and hormonal contraception [Citation6]. There are several explanatory hypotheses about the association between smoking and cervical cancer, including a direct oncogenic effect on chemical carcinogenesis, or a carcinogenic effect due to the suppression of cell-mediated immunity [Citation7]. However, the exact role of smoking in cervical carcinogenesis is not known [Citation8].
The aim of this study is to investigate the association between smoking and the development of CIN and cervical cancer, by means of a systematic review and meta-analysis. Previous reviews have looked at the relationship between smoking, CIN, and cervical cancer, but have not examined what the exact risk is and have not looked at different stages of CIN [Citation9–11]. The research questions are: (1) Is smoking an independent risk factor for developing (different stages of) CIN, and if so, what is the risk? (2) Is smoking an independent risk factor for developing cervical cancer, and if so, what is the risk?
2. Method
2.1. Registration
This review was registered with NARCIS under number OND1365476.
2.2. Search strategy
We performed a systematic search using a keyword-based search strategy in five databases on 7 January 2019. We searched in Embase, Medline, Cochrane Central, Web of Science, and Google Scholar. For the search strategy, we used search terms related to smoking, HPV, CIN, and cervical cancer. See supplement 1 for the full search strategy.
2.3. Selection of studies
We used the following inclusion criteria: (1) articles published from 2009 onwards; (2) articles written in English; (3) participants in the studies are between 18 and 65 years old; (4) the independent variable smoking refers to active smoking of tobacco; (5) the studies concern the development or exacerbation of CIN and/or the development of cervical cancer in combination with tobacco smoking; and (6) the studies use original data. We used the following exclusion criteria: (1) 20% or more of the study population have HIV; and (2) 20% or more of the study population are pregnant or in the postnatal period.
The articles found were initially independently assessed by two coders for suitability on the basis of the title and abstract. In the case of non-matching codes, a third coder was decisive. The full text of the selected articles was subsequently assessed for suitability by two independent coders. In the case of non-matching codes, a third coder was decisive. To ensure rapid completion of the selection process, five coders worked on the selection process simultaneously (BvS, OvdH, TM, GN, and GJM).
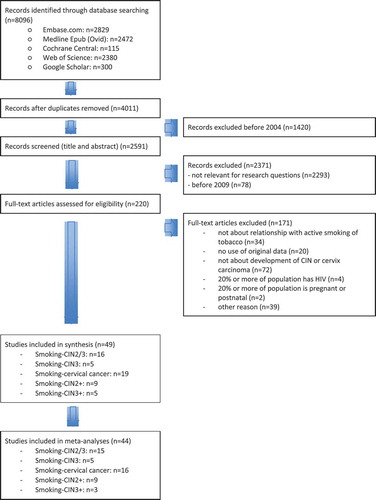
shows a flow chart of the selection process. The search yielded 4,011 articles after duplicates were removed. We screened 220 full-text articles, of which 171 were excluded. Finally, 49 articles met all inclusion criteria. Twenty-one studies were about smoking as a risk factor for CIN, 19 studies examined the question whether smoking is an independent risk factor for developing cervical cancer, and 14 studies combined CIN and cervical cancer as outcome.
2.4. Data extraction and assessment
A standardized data extraction form was used for data extraction. The data extraction from the selected articles was carried out by one researcher per article (BvS, OvdH, TM, GN, and GJM) and was checked by another researcher when performing the risk of bias assessment. The form was used to collect data on the study method, the characteristics of the participants in the study, the outcomes, the results and the mentioned conflicts of interest and the funder of the study, if applicable.
For the assessment of the risk of bias, a standardized form was used based on the risk of bias form by the Cochrane Collaboration. Two independent coders assessed the risk of bias and in the case of non-matching assessments, a third coder was decisive (OvdH, TM, and GN). In each case, the coders indicated whether the risk of bias was high, low or unclear, the justification for this assessment, and the source of this justification in the text of the article. To assess the risk of bias, the following aspects were examined: (1) bias due to a non-representative or ill-defined sample of patients; (2) bias due to insufficiently long, or incomplete follow-up, or differences in follow-up between treatment groups; (3) bias due to ill-defined or inadequately measured outcome; and (4) bias due to inadequate adjustment for important prognostic factors.
For each question, two independent coders (OvdH and GN) carried out an assessment using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. In the case of non-matching codes, a third coder was decisive (TM). Evidence based on RCTs receive a quality rating ‘High’, observational studies a quality rating ‘Low’, and other studies (e.g. case reports) a quality rating ‘Very low’. There are five factors that can downgrade the quality of evidence, namely risk of bias, inconsistency, indirectness, imprecision and publication bias. There are three factors that can upgrade the quality: high effect, dose–response relationship, and plausible confounding. For each factor, the quality can be reduced by one or two levels. If the factor is a serious limitation, the quality goes down by one level; if the factor is a very serious limitation, the quality goes down by two levels.
Studies were divided according to the studied stages of CIN or cervical cancer. Before data collection, we planned to divide the studies into four groups according to the outcomes studied: CIN1, CIN2, CIN3, and cervical cancer. After data collection, we found that few studies examined CIN1 and many studies combined CIN and cervical cancer as outcomes. Therefore, the subgroups included in the study are: CIN3 alone, CIN2 and CIN3 combined, CIN2+ (which includes CIN2, CIN3 and cervical cancer), CIN3+ (which includes CIN3 and cervical cancer), and cervical cancer alone.
2.5. Meta-analysis
After data extraction, the results of each included article concerning the relationship between smoking and cervical abnormalities were converted to odds ratio (OR) if the original article was reported in other effect measures (as far as the available data allowed it). This allowed us to better compare the effects of the individual studies. If available, data from adjusted analyses were used. Otherwise data from crude analyses were used. Where possible, results from ‘never’ compared to ‘current’ smokers were included in the analyses. If data from current smokers were unavailable, results from ‘never’ compared to ‘ever’ smokers were included. Some studies were unclear about whether they used current or ever smoking (they just called it ‘smoking’), these studies were also included in the meta-analysis. The calculations were performed using the online tool MedCalc.
Meta-analyses were performed in Review Manager 5.3 (RevMan). ORs were calculated with the inverse variance method. When more than five eligible studies were included in the analyses, a random-effects model was used [Citation12,Citation13]. A random-effects model was also used when a meta-analysis consisting of five or less studies showed moderate or substantial heterogeneity according to the I2 test (<40% = no heterogeneity; 40% to 70% = moderate heterogeneity and ≥70% = substantial heterogeneity) [Citation13–15]. A fixed-effects model was used when a meta-analysis consisting of less than six studies showed no substantial heterogeneity.
Publication bias was checked with funnel plots using the Egger test [Citation16] when 10 or more studies were eligible for a meta-analysis. We performed sensitivity analyses in which we only pooled studies with a low risk of bias, i.e. studies which scored a low risk of bias on at least three of the four domains or a high risk of bias on a maximum of one of the four domains.
3. Results
3.1. Smoking as a risk factor for CIN
3.1.1. Study characteristics
The study characteristics are shown in . Of the 21 studies about smoking as a risk factor for CIN, nine were cross-sectional studies that included between 266 and 12,048 respondents [Citation17–25]. Three cross-sectional studies had a different study objective than examining the relationship between smoking and CIN [Citation23–25]. Nine case–control studies included between 186 and 4,522 respondents [Citation26–34]. Two longitudinal studies with 150 and 1,485 respondents [Citation35,Citation36] and a large prospective cohort study with 308,036 respondents were included [Citation37].
Table 1. Characteristics and results of the included studies
3.1.2. Risk of bias
Of the 21 studies on smoking as a risk factor for CIN, 11 studies showed a high risk of bias and 11 an unclear risk of bias on at least one of the examined areas (supplement 2). Only five studies showed a low risk of bias on all relevant examined areas. The funnel plot is largely symmetrical (supplement 8A).
3.1.3. Results on smoking as a risk factor for CIN
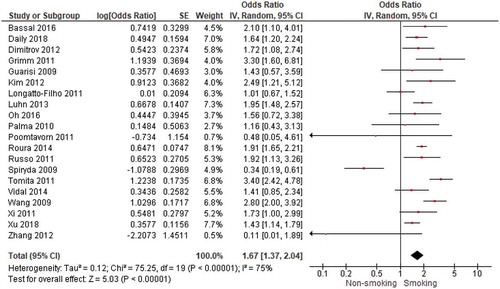
The results of 20 studies could be pooled to examine the association between smoking and CIN2/3 or CIN3 (). We found a significant positive association with an OR of 1.67 (95% CI = 1.37–2.04) between smoking and CIN2/3 or CIN3. The heterogeneity index I2 was substantial with 75%. The study by Spiryda et al. [Citation25] was included in the pooled analysis but used respondents with CIN1 as comparison group. The study by Collins et al. [Citation36] could not be included (because no OR was reported) and found no significant increased risk of CIN2/3 among women who smoked (HR = 1.33 (95% CI 0.77–2.30). When examining the 15 studies about smoking and CIN2/3 separately from the 5 studies about smoking and CIN3, we found an OR of 1.40 for CIN2/3 (supplement 3) and an OR of 2.46 for CIN3 (supplement 4). When only including studies with a low risk of bias (14 studies), we still found a significant association between smoking and CIN2/3 or CIN3 with an OR of 1.88 (95% CI = 1.56–2.27).
3.2. Smoking as a risk factor for cervical cancer
3.2.1. Study characteristics
A total of 19 studies examined the question whether smoking is an independent risk factor for developing cervical cancer. Among these 19 studies, 12 studies had a different research objective but also showed results about smoking in relation to cervical cancer. These studies generally involved a selected patient population. For the most part, these were observational case–control studies in which a certain risk factor was studied and shown in relation to smoking. In addition, there were seven studies that looked at smoking as a possible risk factor for developing cervical cancer as a primary study objective. Seventeen studies were case–control studies with between 80 and 1,259 respondents [Citation27–30,Citation38–50]. One study was a cross-sectional study with 2,231 respondents [Citation51]. And one study was a prospective cohort study with 308,036 respondents [Citation37].
3.2.2. Risk of bias
Of the 19 studies on smoking as a risk factor for cervical cancer, 16 showed a high risk of bias and 7 an unclear risk of bias on at least one of the examined areas (supplement 2). Only two studies showed a low risk of bias on all relevant examined areas. The funnel plot is largely symmetrical (supplement 8B).
3.2.3. Results on smoking as a risk factor for cervical cancer
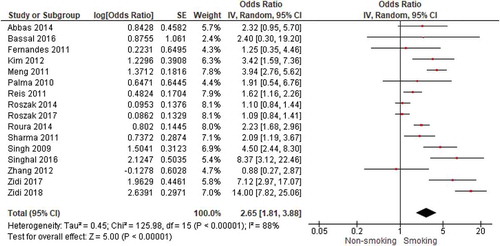
Sixteen studies could be pooled to examine the association between smoking and cervical cancer (). We found a significant positive association with an OR of 2.65 (95% CI = 1.81–3.88). Heterogeneity was substantial (I2 = 88%). No pooling was possible for the studies by Gutman et al. [Citation40] and Currin et al. [Citation51] (because no OR was reported) and Zhang et al. [Citation49] (because it was unsure whether ‘hardly smoking’ was the same as no smoking). Gutman et al. and Currin et al. showed positive associations between smoking and cervical cancer. Zhang et al. found no association. When only including studies with a low risk of bias (nine studies), we still found a significant association between smoking and cervical cancer with an OR of 3.05 (95% CI = 1.73–5.38).
3.3. Smoking as a risk factor for CIN and cervical cancer (combined)
3.3.1. Study characteristics
Fourteen studies combined CIN and cervical cancer as outcome measure. Nine of these studies examined the relationship between smoking and CIN2+ (i.e. CIN2, CIN3, and cervical cancer) and five studies examined the relationship between smoking and the development of CIN3+ (i.e. CIN3 and cervical cancer). Three studies were cross-sectional studies with between 291 and 1,315 respondents [Citation52–54]. Six studies were case–control studies with between 200 and 2,736 respondents [Citation55–60]. Four studies were cohort studies with between 1,236 and 12,076 respondents [Citation61–64] and one study was a longitudinal study with 1,628 respondents [Citation65].
3.3.2. Risk of bias
For the 14 CIN2+ and CIN3+ studies, 10 showed a high risk of bias and 6 an unclear risk of bias on at least one of the examined areas (supplement 2). Only two studies showed a low risk of bias on all relevant examined areas. The funnel plot is largely symmetrical (supplement 8 C).
3.3.3. Results on smoking as a risk factor for CIN and cervical cancer (combined)
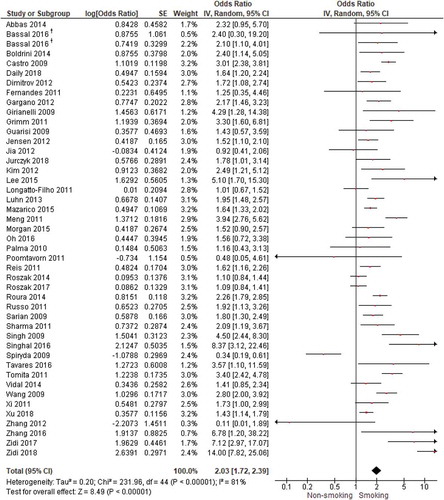
Of the 14 studies that combined CIN and cervical cancer as outcome measure, 1 study could not be included. Fang et al. [Citation63] found positive associations between smoking and CIN3+ (but did not report an OR or the numbers per group). When combining all 45 studies about CIN and cervical cancer that could be pooled (), we found a significant positive association between smoking and CIN or cervical cancer with an OR of 2.03 (95% CI = 1.72–2.39). Heterogeneity was substantial (I2 = 81%). When examining the studies with different outcomes separately, we found an OR of 1.93 for CIN2+ (supplement 5), an OR of 2.10 for CIN3+ (supplement 6), and an OR of 2.48 for CIN3, cervical cancer, and CIN3+ combined (supplement 7). When only including studies with a low risk of bias (26 studies), we still found a significant association between smoking and CIN and cervical cancer (combined) with an OR of 2.26 (95% CI = 1.83–2.79).
3.4. Quality of evidence
The quality of evidence was assessed by using the GRADE approach. For both research questions, the certainty of evidence was judged to be ‘very low’. Reasons for this were the fact that all studies were observational studies, the high risk of bias due to inadequate adjustment for important prognostic factors, insufficient description of the CIN/carcinoma determination in some studies, insufficient description of the study population in some studies (especially studies about cervical cancer), the fact that CIN and cervical cancer were often combined as outcome measures, and that different types of control groups were used in the different studies.
4. Conclusion
The majority of studies show an increased risk of cervical abnormalities among people who smoke. Our meta-analysis confirms this positive correlation between smoking and development of CIN and cervical cancer. However, given the low certainty in the effect measures in the literature found, future studies should adjust for relevant predictors, separate CIN from cervical cancer as outcome measures, and report research methods in detail.
5. Expert opinion
In this systematic review and meta-analysis, we examined the literature published between 2009 and 2018 on smoking as a possible risk factor for the development of cervical abnormalities. The majority of studies show a significant positive association between smoking and CIN2/3 and CIN2+. For CIN3 and CIN3+, the literature shows a stronger increased risk in almost all studies. The relationship between smoking and cervical cancer is also confirmed by most of the included studies. This is consistent with previous literature reviews and meta-analyses [Citation9–11]. It was not feasible for us to systematically review studies published before 2009, but an example of a case–control study published in 1998 found an increase in risk of cervical cancer with increased years of smoking and numbers of cigarettes smoked per day [Citation66]. Another case–control study published in 2003 found that long duration smoking (20 years or more) was associated with a two-fold increase in the risk of squamous cell carcinoma but not with the risk of adenocarcinoma [Citation67].
Our review focused on the relationship between smoking and the development of CIN and cervical cancer, but several studies also described dose–response relationships. Most of these studies found an increased risk of CIN(+) when the length of the period that women smoked, the number of pack-years, or the number of cigarettes per day increased [Citation20,Citation26,Citation34,Citation36,Citation37,Citation54,Citation56,Citation63,Citation64]. Only one study reported a non-significant increased risk of CIN when the number of cigarettes per day increased [Citation32]. Only one large cohort study examined a dose–response relationship for cervical cancer [Citation37]. This study used data from 10 countries and found an increased risk of cervical cancer for women who smoke 20 years or longer and who smoked 10 cigarettes per day or more. It therefore seems that the more and longer that women smoke, the higher their risk of CIN and cervical cancer.
Based on the results of our review, one could hypothesize that quitting smoking may reduce the increased risk of CIN and cervical cancer for women who smoke. Some studies have examined this and indeed found this reduction for CIN(+) [Citation20,Citation36,Citation61–63], while others found a non-significant trend toward a reduction [Citation34,Citation37,Citation64], and some other studies did not find a reduction [Citation21,Citation65]. For cervical cancer, one study examined and found a reduction in the risk of cervical cancer for women who had quit smoking [Citation37]. These mixed results preclude us from drawing definitive conclusions about the benefits of quitting smoking for the reduction in risk of CIN and cervical cancer for women who smoke. However, given the many adverse health effects of smoking, our opinion is that health professionals should always advise women who smoke to stop smoking, even without strong evidence of the impact on cervical abnormalities. Giving advice to stop smoking is not routine practice for all health professionals yet, mainly because it is felt to be too time-consuming and may jeopardize the patient-professional relationship [Citation68,Citation69]. A ‘Very Brief Advice’ may be a solution as this method is quick and non-confrontational [Citation70].
It is important to note that some of the conducted studies included in our review had important limitations, resulting in a high risk of bias and a low confidence in the reported evidence. Many studies did not (adequately) adjust the analyses for important confounders like an HPV infection, hormonal contraception, parity, and sexual (risk) behaviors. Without adjusting the analyses for these confounders, we cannot be certain that smoking is an independent risk factor for the development of cervical abnormalities. Another important limitation of many studies was an insufficient description of both the determination of smoking status and CIN/carcinoma, which makes it unfeasible to assess the risk of bias due to measurement issues. Few studies focused on the relationship between smoking and CIN1 and, therefore, we cannot draw a conclusion about this relationship. A relatively large number of studies combined CIN and cervical cancer as outcome measures, making it impossible to describe results separately. Although the funnel plots appear largely symmetrical, our review may be affected by publication bias. Non-significant results of studies investigating the association between smoking and cervical cancer probably have a lower chance of being published than studies with significant results. Finally, many studies that examined cervical cancer were additionally limited because of ill-defined samples. The meta-analyses showed substantial heterogeneity. We think it is important that future studies adjust for relevant predictors, separate CIN from cervical cancer as outcome measures, and report research methods in detail. Besides the authors themselves, editors and reviewers can play an important role by asking authors to make these adjustments in their analyses and reports, before publication in peer-reviewed journals.
Article highlights
Smoking tobacco seems to be a risk factor for development of cervical intra-epithelial neoplasia (CIN) and cervical cancer, but the exact role of smoking in the process of cervical carcinogenesis is not known.
Previous reviews have looked at the relationship between smoking, CIN, and cervical cancer, but have not examined what the exact risk is, and have not looked at different stages of CIN.
In this systematic review and meta-analysis, most studies showed an increased risk of CIN and cervical cancer among smokers.
Many studies had a high risk of bias, e.g. not adjusting for relevant predictors or not separating CIN from cervical cancer.
It can be – cautiously – concluded that smoking increases the risk of cervical abnormalities.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Contributor statement
Gera E. Nagelhout: Conceptualization, Methodology, Investigation, Writing original draft, Funding acquisition. Renée M. F. Ebisch: Conceptualization, Investigation, Writing original draft. Olga L. van der Hel: Investigation, Writing review and editing. Gert-Jan Meerkerk: Investigation, Writing review and editing. Tessa Magnée: Investigation, Writing review and editing. Thomas M. de Bruijn: Formal analysis, Writing review and editing. Barbara van Straaten: Investigation, Writing review and editing, Project administration.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (120.3 KB)Acknowledgments
We thank Gerdien B. de Jonge, Biomedical Information Specialist of Medical Library Erasmus MC, for her help with the literature search. We also thank Ammerins Moss-de Boer from Babylonia translations for translating our article from Dutch to English and Denise van den Broek for help with the tables.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012 [Article]. CA Cancer J Clin. 2015;65(2):87–108.
- Ebisch RM, Siebers AG, Bosgraaf RP, et al. Triage of high-risk HPV positive women in cervical cancer screening. Expert Rev Anticancer Ther. 2016;16(10):1073–1085. .
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19.
- Ho GYF, Bierman R, Beardsley L, et al. Natural history of cervicovaginal papillomavirus infection in young women [Article]. New Engl J Med. 1998;338(7):423–428.
- Rodríguez AC, Schiffman M, Herrero R, et al. Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J Natl Cancer Inst. 2008;100(7):513–517.
- Gadducci A, Barsotti C, Cosio S, et al. Smoking habit, immune suppression, oral contraceptive use, and hormone replacement therapy use and cervical carcinogenesis: a review of the literature [Article]. Gynecol Endocrinol. 2011;27(8):597–604.
- Bosch FX, De Sanjosé S. The epidemiology of human papillomavirus infection and cervical cancer [Review]. Dis Markers. 2007;23(4):213–227.
- Brinton LA, Schairer C, Haenszel W. Cigarette smoking and invasive cervical cancer [Article]. J Am Med Assoc. 1986;255(23):3265–3269.
- P Bv A. Berrington de González A, Colin D, Franceschi S, Goodill A, Green J, Peto J, Plummer M, Sweetland S. Carcinoma of the cervix and tobacco smoking: collaborative reanalysis of individual data on 13,541 women with carcinoma of the cervix and 23,017 women without carcinoma of the cervix from 23 epidemiological studies. Int J Cancer. 2006;118(6):1481–1495.
- Gandini S, Botteri E, Iodice S, et al. Tobacco smoking and cancer: a meta‐analysis. Int J Cancer. 2008;122(1):155–164.
- Secretan B, Straif K, Baan R, et al. A review of human carcinogens–Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10(11):1033.
- Nikolakopoulou A, Mavridis D, Salanti G. Demystifying fixed and random effects meta-analysis. Royal College of Psychiatrists. Evidence-Based Mental Health. 2014;17:53-57.
- Tufanaru C, Munn Z, Stephenson M, et al. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int J Evid Based Healthc. 2015;13(3):196–207.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21(11):1539–1558.
- Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Vol. 4. Chichester: John Wiley & Sons; 2011.
- Simmonds M. Quantifying the risk of error when interpreting funnel plots. Syst Rev. 2015;4(1):24.
- Poomtavorn Y, Suwannarurk K, Thaweekul Y, et al. Risk Factors for high-grade cervical intraepithelial neoplasia in patients with atypical squamous cells of undetermined significance (ASC-US) papanicolaou smears [Article]. Asian Pac J Cancer Preven. 2011;12(1):235–238.
- Russo E, Kupek E, Zanine RM. Vaginal delivery and low immunity are strongly associated with high-grade cervical intraepithelial neoplasia in a high-risk population [Article]. J Lower Genital Tract Dis. 2011;15(3):195–199.
- Daily LR, Erickson BK, Pasko DN, et al. High rates of high-grade cervical dysplasia in high-risk young women with low-grade cervical cytology [article]. J Lower Genital Tract Dis. 2018;22(3):207–211.
- Wang SS, Zuna RE, Wentzensen N, et al. Human papillomavirus cofactors by disease progression and human papillomavirus types in the study to understand cervical cancer early endpoints and determinants [Article]. Cancer Epidemiol Biomarkers Prev. 2009;18(1):113–120.
- Luhn P, Walker J, Schiffman M, et al. The role of co-factors in the progression from human papillomavirus infection to cervical cancer [Article]. Gynecol Oncol. 2013;128(2):265–270.
- Longatto-Filho A, Hammes LS, Sarian LO, et al., Hormonal contraceptives and the length of their use are not independent risk factors for high-risk HPV infections or high-grade CIN [Article]. Gynecol Obstet Invest. 71(2): 93–103. 2011.
- Vidal AC, Smith JS, Valea F, et al. HPV genotypes and cervical intraepithelial neoplasia in a multiethnic cohort in the southeastern USA [Article]. Cancer Causes Control. 2014;25(8):1055–1062.
- Xi LF, Jiang M, Shen Z, et al. Inverse association between methylation of human papillomavirus type 16 DNA and risk of cervical intraepithelial neoplasia grades 2 or 3 [Article]. PLoS ONE. 2011;6(8):8.
- Spiryda LB, Brown M, Creek KE, et al. HSIL pap test and risk factors predicting acquisition of CIN 2/3 on colposcopy-directed biopsies [Article]. J S C Med Assoc. 2009;105(7):281–286.
- Dimitrov G, Džikova E, Dimitrov G, et al. The influence of HPV16, smoking and coitarche in the development of cervical dysplasia in the stage where conization is the treatment of choice [Article]. Acta Fac Med Naissensis. 2012;29(4):181–186.
- Palma S, Novelli F, Padua L, et al. Interaction between glutathione-S-transferase polymorphisms, smoking habit, and HPV infection in cervical cancer risk [Article]. J Cancer Res Clin Oncol. 2010;136(7):1101–1109.
- Kim J, Kim BK, Lee CH, et al. Human papillomavirus genotypes and cofactors causing cervical intraepithelial neoplasia and cervical cancer in Korean women [Article]. Int J Gynecol Cancer. 2012;22(9):1570–1576.
- Bassal R, Schejter E, Bachar R, et al. Risk factors for cervical cancer and CIN3 in Jewish women in Israel - two case control studies [Article]. Asian Pac J Cancer Prev. 2016;17(4):2067–2073.
- Zhang L, Ruan Z, Hong Q, et al. Single nucleotide polymorphisms in DNA repair genes and risk of cervical cancer: a case-control study [Article]. Oncol Lett. 2012;3(2):351–362.
- Grimm C, Watrowski R, Polterauer S, et al. Vascular endothelial growth factor gene polymorphisms and risk of cervical intraepithelial neoplasia [Article]. Int J Gynecol Cancer. 2011;21(4):597–601.
- Oh HY, Kim MK, Seo SS, et al. Association of combined tobacco smoking and oral contraceptive use with cervical intraepithelial neoplasia 2 or 3 in Korean women [article]. J Epidemiol. 2016;26(1):22–29.
- Tomita LY, Roteli-Martins CM, Villa LL, et al. Associations of dietary dark-green and deep-yellow vegetables and fruits with cervical intraepithelial neoplasia: modification by smoking [Article]. Br J Nutr. 2011;105(6):928–937.
- Xu H, Egger S, Sv L, et al. Hormonal contraceptive use and smoking as risk factors for high-grade cervical intraepithelial neoplasia in unvaccinated women aged 30–44 years: a case-control study in New South Wales, Australia [Article]. Cancer Epidemiol. 2018;55:162–169.
- Guarisi R, Sarian LO, Hammes LS, et al. Smoking worsens the prognosis of mild abnormalities in cervical cytology [Article]. Acta Obstet Gynecol Scand. 2009;88(5):514–520.
- Collins S, Rollason TP, Young LS, et al. Cigarette smoking is an independent risk factor for cervical intraepithelial neoplasia in young women: a longitudinal study [Article]. Eur J Cancer. 2010;46(2):405–411.
- Roura E, Castellsagué X, Pawlita M, et al., Smoking as a major risk factor for cervical cancer and pre-cancer: results from the EPIC cohort [Article]. Int J Cancer. 135(2): 453–466. 2014.
- Abbas M, Srivastava K, Imran M, et al. Association of CYP1A1 gene variants rs4646903 (T>C) and rs1048943 (A>G) with cervical cancer in a North Indian population [Article]. Eur J Obstet Gynecol Reprod Biol. 2014;176(1):68–74.
- Fernandes JV, Meer RDV, Carvalho MGF, et al. Human papillomavirus prevalence in women with normal cytology and with cervical cancer in Natal, Brazil [Article]. Mol Med Rep. 2011;4(6):1321–1326.
- Gutman G, Morad T, Peleg B, et al. CYP1A1 and CYP2D6 gene polymorphisms in Israeli Jewish women with cervical cancer [Article]. Int J Gynecol Cancer. 2009;19(8):1300–1302.
- Meng F, Song H, Luo C, et al. Correlation of LAPTM4B polymorphisms with cervical carcinoma [Article]. Cancer. 2011;117(12):2652–2658.
- Roszak A, Lianeri M, Sowinśka A, et al. CYP1A1 Ile462Val polymorphism as a risk factor in cervical cancer development in the Polish population [Article]. Mol Diagn Ther. 2014;18(4):445–450.
- Roszak A, Lutkowska A, Lianeri M, et al. Involvement of myeloperoxidase gene polymorphism 463G>A in development of cervical squamous cell carcinoma [Article]. Int J Biol Markers. 2017;31(4):e440–e445.
- Sharma A, Gupta S, Sodhani P, et al. Glutathione S-transferase M1 and T1 Polymorphisms, Cigarette Smoking and HPV Infection in Precancerous and Cancerous Lesions of the Uterine Cervix [Article]. Asian Pac J Cancer Prev. 2015;16(15):6429–6438.
- Singh H, Jain M, Mittal B. Role of TGF-β1 (−509C>T) promoter polymorphism in susceptibility to cervical cancer [Article]. Oncol Res. 2009;18(1):41–45.
- Singhal P, Sharma U, Hussain S, et al. Identification of genetic variants in TNF receptor 2 which are associated with the development of cervical carcinoma [Article]. Biomarkers. 2016;21(7):665–672.
- Zidi S, Stayoussef M, Alsaleh BL, et al. Relationships between common and novel interleukin-6 gene polymorphisms and risk of cervical cancer: a case-control study [Article]. Pathol Oncol Res. 2017;23(2):385–392.
- Reis N, Beji NK, Kilic D. Risk factors for cervical cancer: results from a hospital-based case-control study [Article]. Uhod-Uluslararasi Hematoloji-Onkoloji Dergisi. 2011;21(3):153–159.
- Zhang B, Zhou AF, Zhu CC, et al. Risk factors for cervical cancer in rural areas of Wuhan china: a matched case-control study [Article]. Asian Pac J Cancer Preven. 2013;14(12):7595–7600.
- Zidi S, Sahli M, Mezlini A, et al. Association of combined tobacco smoking, hormonal contraceptive use and status matrimonial with cervical cancer evolution in Tunisian women [article in press]. Pathol Oncol Res. 2018;24(1):1–6.
- Currin LG, Jack RH, Linklater KM, et al. Inequalities in the incidence of cervical cancer in South East England 2001-2005: an investigation of population risk factors [Article]. In: BMC Public Health. 2009;9. article number 62.
- Zhang Q, Xie W, Wang F, et al. Epidemiological investigation and risk factors for cervical lesions: cervical cancer screening among women in rural areas of Henan Province China [Article]. Med Sci Monit. 2016;22:1858–1865.
- Boldrini NT, Freitas LB, Coutinho AR, et al. High-grade cervical lesions among women attending a reference clinic in Brazil: associated factors and comparison among screening Methods [Article]. PLoS ONE. 2014;9(7):7.
- Mazarico E, Gómez-Roig MD, Guirado L, et al. Relationship between smoking, HPV infection, and risk of cervical cancer [Article]. Eur J Gynaecol Oncol. 2015;36(6):677–680.
- Morgan TK, Hanifin J, Mahmood M, et al. Atopic dermatitis is associated with cervical high risk human papillomavirus infection [article]. J Lower Genital Tract Dis. 2015;19(4):345–349.
- Lee CH, Peng CY, Li RN, et al. Risk evaluation for the development of cervical intraepithelial neoplasia: development and validation of risk-scoring schemes [Article]. Int J Cancer. 2015;136(2):340–349.
- Jia Y, Hu T, Hang CY, et al. Case-control study of diet in patients with cervical cancer or precancerosis in wufeng, a high incidence region in China [Article]. Asian Pac J Cancer Preven. 2012;13(10):5299–5302.
- Tavares MC, De Lima Júnior SF, Coelho AV, et al. Tumor necrosis factor (TNF) alpha and interleukin (IL) 18 genes polymorphisms are correlated with susceptibility to HPV infection in patients with and without cervical intraepithelial lesion [Article]. Ann Hum Biol. 2016;43(3):261–268.
- Castro FA, Haimila K, Sareneva I, et al. Association of HLA-DRB1, interleukin-6 and cyclin D1 polymorphisms with cervical cancer in the Swedish population - A candidate gene approach [Article]. Int J Cancer. 2009;125(8):1851–1858.
- Jurczyk MU, Chmaj-Wierzchowska K, Kamińska M. Effects of lifestyle on the occurrence of precancerous conditions and cervical cancer [Article]. Eur J Gynaecol Oncol. 2018;39(4):609–614.
- Girianelli VR. Azevedo e Silva G, Thuler LCS. Factors associated with the risk of progression to precursor lesions or cervical cancer in women with negative cytologic findings [Article]. Int J Gynecol Obstet. 2009;107(3):228–231.
- Sarian LO, Hammes LS, Longatto-Filho A, et al. Increased risk of oncogenic human papillomavirus infections and incident high-grade cervical intraepithelial neoplasia among smokers: experience from the Latin American screening study [Article]. Sex Transm Dis. 2009;36(4):241–248.
- Fang JH, Yu XM, Zhang SH, et al. Effect of smoking on high-grade cervical cancer in women on the basis of human papillomavirus infection studies [Article]. J Cancer Res Ther. 2018;14(8):S184–S189.
- Jensen KE, Schmiedel S, Frederiksen K, et al. Risk for cervical intraepithelial neoplasia grade 3 or worse in relation to smoking among women with persistent human papillomavirus infection [Article]. Cancer Epidemiol Biomarkers Prev. 2012;21(11):1949–1955.
- Gargano JW, Nisenbaum R, Lee DR, et al. Age-group differences in human papillomavirus types and cofactors for cervical intraepithelial neoplasia 3 among women referred to colposcopy [Article]. Cancer Epidemiol Biomarkers Prev. 2012;21(1):111–121.
- Ngelange C, Munoz N, Bosch FX, et al. Causes of cervical cancer in the Philippines: a case-control study. JNCI. 1998;90(1):43–49.
- Green J. Berrington de Gonzalez A, Sweetland S, et al. Risk factors for adenocarcinoma and squamous cell carcinoma of the cervix in women aged 20–44 years: the UK National case–control study of cervical cancer. Br J Cancer. 2003 [2003 December 01];89(11):2078–2086.
- Van Eerd EAM, Bech Risør M, Spigt M, et al. Why do physicians lack engagement with smoking cessation treatment in their COPD patients? A multinational qualitative study. NPJ Prim Care Respir Med. 2017 [2017 June 23];27(1):41.
- Bar-Zeev Y, Skelton E, Bonevski B, et al. Overcoming challenges to treating tobacco use during pregnancy - a qualitative study of Australian general practitioners barriers. BMC Pregnancy Childbirth. 2019 [2019 February 07];19(1):61.
- Van Schayck OCP, Bindels L, Nijs A, et al. The experience of general practitioners with very brief advice in the treatment of tobacco addiction. NPJ Prim Care Respir Med. 2020 [2020 September 23];30(1):40.