ABSTRACT
Introduction
Efficacy of lenalidomide plus rituximab (R-LEN) compared to rituximab monotherapy (R-mono) for patients with previously treated follicular lymphoma (FL) was investigated in AUGMENT (NCT01938001). Our aim was to evaluate the cost-effectiveness of R-LEN versus R-mono in this setting from a Dutch perspective.
Areas covered
Cost-effectiveness was assessed through a partitioned survival model from three perspectives (i.e. societal, healthcare, and societal, including future non-medical costs). Patient-level data from AUGMENT informed effectiveness parameters (i.e. long-term survival) and health state utilities. Resource use and prices were based on AUGMENT and the literature. Clinical experts validated efficacy input parameters and results. Uncertainty was explored through sensitivity and scenario analyses.
Expert opinion
R-LEN resulted in 1.7 incremental discounted quality-adjusted life years (QALYs). Total incremental discounted costs were 67,161 EUR from a societal perspective. In conclusion, R-LEN was cost-effective at a willingness-to-pay (WTP) threshold of 50,000 EUR/QALY in the base-case analyses(incremental cost-effectiveness ratio = 40,493 EUR/QALY). Scenario and sensitivity analyses indicated some level of uncertainty regarding this conclusion, depending on the chosen WTP-threshold and perspective.
1. Background
Follicular lymphoma (FL) is the second most common non-Hodgkin lymphoma (NHL) in Western Europe and the United States, comprising over 35% of all NHLs and 70% of indolent lymphomas [Citation1]. In the Netherlands, the age-adjusted incidence rate for FL was 2.5 per 100,000 in 2016 [Citation2].
Currently, several treatment options are available for patients who are refractory or relapsed from first-line therapy, none of which are considered curative [Citation3]. For these patients, rituximab, interferon alfa-2b as an add-on to anticancer treatment, ibritumomab tiuxetan, obinutuzumab in combination with bendamustine, and idelalisib were the only approved treatment options by the European Medicines Agency (EMA) until recently [Citation4, Citation5, Citation6–8]. In December 2019, the EMA approved an extension of indication for the immunomodulatory drug lenalidomide [Citation9]. Lenalidomide in combination with rituximab (R-LEN) is since then authorized for adult patients with previously treated FL (Grade 1–3a) in Europe.
The EMA’s decision was based on clinical evidence from the recently published phase III, multicenter, international, randomized study AUGMENT, comparing R-LEN to rituximab plus placebo (R-mono) in previously treated patients with indolent FL or marginal zone lymphoma (MZL) [Citation10]. In AUGMENT, R-LEN demonstrated statistically significant superiority in overall survival (OS) and progression-free survival (PFS) over the comparator regimen in the FL subgroup. OS probability at two years, assessed by the independent review committee (IRC) was 95% (95% CI, 90 to 98) for R-LEN and 86% (95% CI, 79 to 91) for R-mono [Citation10]. In addition, R-LEN was found to increase median PFS by approximately 25 months when compared to R-mono (hazard ratio, 0.40; 95% CI, 0.29 to 0.56; P < .001) [Citation10]. The study also collected health-related quality of life (HRQoL) using the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire-Core 30 (QLQ-C30) and the 3-level classification system of the EQ-5D questionnaire (EQ-5D-3 L) [Citation11,Citation12]. Preliminary results of these data suggest no negative impact of R-LEN on patients’ HRQoL when compared to R-mono [Citation13].
While lenalidomide underwent central approval by the EMA, each European member state handles its own reimbursement procedure. In addition to considerations on the safety and efficacy of a new treatment, its cost-effectiveness is a key aspect when making both treatment and reimbursement decisions [Citation14]. Especially when drug prices are relatively high, economic evaluations can provide the necessary information to make informed decisions. Such assessments combine several sources of evidence (i.e. efficacy and costs) to derive conclusions on the cost-effectiveness of a treatment [Citation15,Citation16]. Analyses that also consider patients’ HRQoL are commonly referred to as cost-utility analyses (CUAs). Currently, there is no evidence available on the cost-utility of R-LEN from a societal perspective in a European country.
Therefore, the aim of this study was to evaluate the cost-effectiveness of R-LEN when compared to R-mono in patients with previously treated FL in the Netherlands from a societal perspective. As suggested in the literature, we also present results from a healthcare perspective [Citation17]. While future non-medical costs should be considered when performing CUAs from a societal perspective, not all pharmacoeconomic guidelines explicitly mention their inclusion yet [Citation18–20]. Consequently, the impact of these costs on the results of CUAs remains understudied [Citation20]. For this study, future non-medical costs were considered for a third perspective to fill this gap [Citation21–23].
2. Methods
Patient-level data to inform model efficacy parameters were based on the AUGMENT FL subgroup. A detailed description of the methods can be found in Appendix 1.
We modeled a hypothetical cohort of patients by means of a partitioned survival model [Citation24]. This methodology is commonly used in the decision modeling of new interventions in advanced cancers [Citation24]. Our analysis follows the recommendations of the Dutch guideline for conducting economic evaluations in health care (Dutch EE guideline) [Citation25].
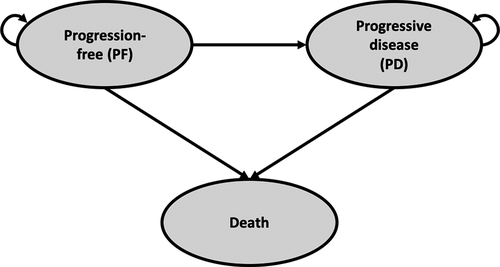
The model comprised three states: progression free (PF), progressive disease (PD), and death. The simulation starts with administering either treatment option (R-LEN or R-mono) in the PF state. Simulated patients responding to treatment remained in the PF state and follow-up resource use was considered. In case patients relapsed or were refractory to R-LEN or R-mono, they were assumed to be in the PD state. Modeled patients could die at all times. schematically represents how patients can move through the model over time.
To determine the proportion of patients in each model state, we independently fit a range of parametric models to both PFS and OS in AUGMENT [Citation26]. Consequently, short-term empirical data were extrapolated to a lifetime horizon. The model was adjusted for general population mortality (i.e. extrapolated survival could not exceed this mortality) and a finite treatment effect for R-LEN was assumed (i.e. five years, based on clinical expert opinion). Effects were discounted with 1.5% [Citation25].
The societal perspective of the base-case analysis included health-care costs for drugs (acquisition and administration), adverse events (AEs), subsequent treatment, and future medical costs. Prices for drug acquisition were taken from the Dutch drug database (April 2020), and drug administration costs were based on a micro-costing study on intravenous rituximab administration in the Netherlands [Citation27, Citation28]. The model considered grade 3–4 AEs with more than 5% occurrence as observed in AUGMENT [Citation10]. AE costs and disutilities were based on the literature [Citation29–31]. Subsequent treatment during PD and resource use during all follow-up visits were based on the Dutch clinical practice guideline (CPG) for FL [Citation32]. Prices for these items were taken from the Dutch costing manual [Citation25].
Additionally, societal costs for travel, productivity losses, and informal care were considered in the model base-case (see Appendix 1 for more detail). Future non-medical costs were included using the iMTA PAID tool (version 3.0) [Citation21–23].
All costs are expressed in 2019 Euros and both prices and costs of earlier years were indexed to 2019 with the pertinent consumer price index [Citation33]. Costs were discounted with 4% [Citation25].
Effect outcomes include total average life years (LYs) as well as quality-adjusted life years (QALYs) per patient and were validated by clinical experts (i.e. clinical coauthors of this study: MJK, MH, EFMP, and WBCS).
Furthermore, we report total incremental costs and effects as well as the incremental cost-effectiveness ratio (ICER) of R-LEN when compared to R-mono. To determine whether R-LEN is cost-effective the applicable willingness-to-pay (WTP) threshold was estimated using the Burden-of-Disease calculator [Citation34].
We conducted several scenario analyses with varying efficacy, utility, and cost parameters. Also, we tested different assumptions of the treatment effect duration (see Appendix 1). Uncertainty of the model input parameters and estimates was assessed through deterministic sensitivity analyses (DSA) and probabilistic sensitivity analyses (PSA).
An overview of all parameter (mean) values, their standard error (SE), assumed distributions as well as lower and upper bounds are summarized in .
Table 1. Overview of model input parameters
3. Results
3.1. Effectiveness
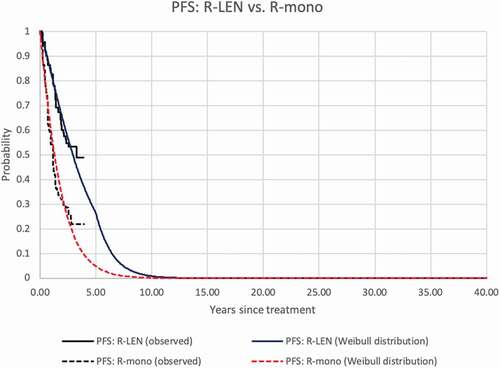
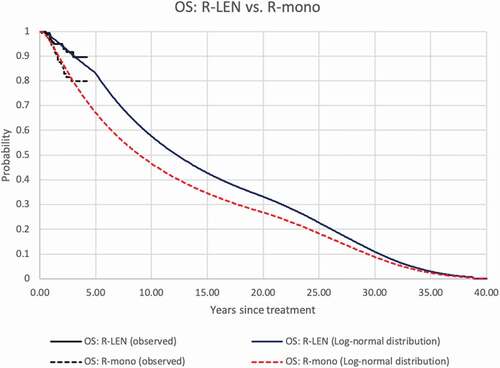
Based on the statistical goodness-of-fit criteria (see Appendix 1) and clinical expert opinion, we selected the Weibull and the log-normal distribution for the survival extrapolation of PFS and OS, respectively. After five years in the model, the hazard ratios between R-LEN and R-mono were kept constant both for PFS and OS extrapolations (see Appendix 1). For the R-LEN arm, the estimated probability of PFS at 5 and 10 years was 27% and 1%, respectively, in the R-LEN group. For patients receiving R-mono, these probabilities were 5% and 0%, meaning that all patients had progressed or died after 10 years. The estimated OS probabilities after R-LEN at 5, 10, 20, and 30 years amounted to 83%, 58%, 33%, and 11%, respectively. After R-mono, these probabilities were 67%, 47%, 27%, and 9%, respectively. Empirical and modeled PFS and OS of both groups are depicted in . The model estimated 12.9 LYs and 10.8 QALYs for R-LEN and 10.9 LYs and 9.1 QALYs for R-mono (discounted results).
3.2. Costs
The presented costs in are average, discounted, lifetime costs per patient. Undiscounted costs can be found in Appendix 2.
Table 2. Model results: costs, effects, and increments
Total costs from the base-case societal perspective were 200,355 EUR and 132,789 EUR for R-LEN and R-mono, respectively. Major cost drivers were future medical costs (R-LEN: 78,127 EUR; R-mono: 65,960 EUR), followed by drug acquisition costs for R-LEN (57,455 EUR), and informal care (R-LEN: 28,128 EUR; R-mono: 23,952 EUR. Total health-care costs were 165,547 EUR and 102,233 EUR for R-LEN and R-mono, respectively. When future non-medical costs were considered in a societal perspective, total costs were 299,943 EUR and 217,687 for R-LEN and R-mono, respectively.
3.3. Incremental cost-effectiveness ratio
Total incremental costs of R-LEN when compared to R-mono were 67,566 EUR from a societal perspective. Incremental costs were highest for drug acquisition (50,483 EUR), followed by incremental future medical costs (12,167 EUR). Only total costs for subsequent treatment regimens were lower for R-LEN when compared to R-mono. This was because modeled patients receiving R-LEN progressed later when compared to patients with R-mono. Consequently, subsequent treatment is administered later and hence the effect of discounting these costs leads to lower costs.
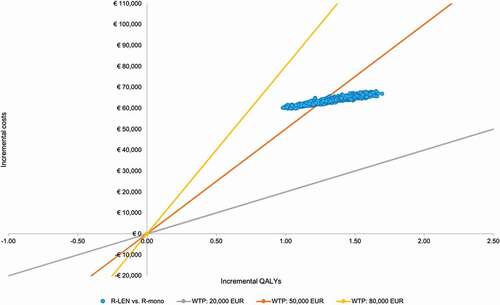
The base-case ICER was 40,493 EUR/QALY (see ). When considering health-care costs only, the ICER was 37,951 EUR/QALY gained. When future non-medical costs were considered for a societal perspective, the ICER increased to 49,296 EUR/QALY gained. In Appendix 2, the ICERs from the undiscounted analysis are presented.
Table 3. Incremental cost-effectiveness ratios
Based on the disease burden calculator, the most likely WTP-threshold was 50,000 EUR/QALY (51.6%) (see Appendix 2) [Citation34].
3.4. Deterministic sensitivity analysis
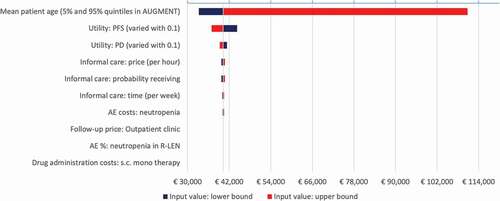
The top 10 influential parameters varied in the deterministic sensitivity analysis are summarized in . Increasing the assumed utility value by 0.1 for progression-free disease resulted in the lowest ICER (i.e. 37,116 EUR/QALY), while assuming 60% of patients receiving informal care (instead of 22% in the base-case) resulted in the highest ICER (i.e. 44,816 EUR/QALY).
3.5. Probabilistic sensitivity analysis
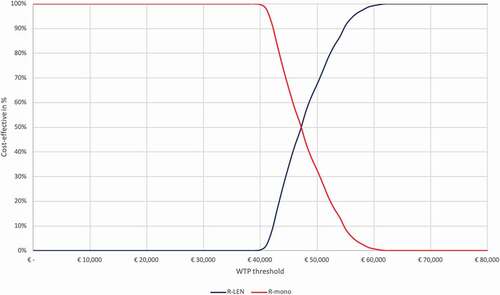
The results of the probabilistic sensitivity analysis are presented in the cost-effectiveness plane () for the base-case societal perspective (see Appendix 2 for all other perspectives). Assuming a WTP-threshold of 50,000 EUR/QALY gained, the probability of R-LEN being cost-effective was 67%. This probability was 82% when only health-care costs were considered and 3% when future non-medical costs were included in the societal perspective. The cost-effectiveness acceptability curve in depicts a range of WTP-thresholds and the probability of R-LEN being cost-effective at each of those for the base-case societal perspective (see Appendix 2 for all other perspectives).
3.6. Scenario analyses
From the base-case societal perspective, the following three scenarios exceeded the assumed WTP-threshold. First, assuming a treatment effect duration of three years yielded an ICER of 60,496 EUR/QALY gained. With an increase in the duration of the treatment effect, ICERs decreased to 33,354 EUR/QALY gained and 28,691 EUR/QALY gained after seven and 10 years of treatment effect, respectively. Assuming no treatment effect waning resulted in the lowest ICER of all scenarios (25,114 EUR/QALY gained).
Second and third, selecting either the Gompertz or Weibull distribution to model OS of R-LEN and R-mono resulted in an ICER of 64,026 EUR/QALY and 53,040 EUR/QALY gained. The results of all scenario analyses can be found in Appendix 2.
4. Discussion
4.1. Summary of results
Our analyses show that R-LEN results in incremental LYs and QALYs gained when compared to R-mono. Simultaneously, R-LEN also results in higher costs when compared to R-mono, regardless of the chosen perspective. Deterministic ICERs of R-LEN versus R-mono ranged from 37,951 EUR/QALY (health-care perspective) to 49,296 EUR/QALY gained (societal perspective including non-medical costs).
4.2. When is R-LEN cost-effective?
To determine whether R-LEN is cost-effective when compared to R-mono, the estimated ICERs need to be compared to the pertinent WTP-threshold. However, based on the disease burden calculator, two WTP-thresholds were nearly equally likely to be applicable. For this analysis, we compared the respective ICER to a 50,000 EUR/QALY gained WTP-threshold as this had the highest probability of being applicable (see Appendix). At this WTP-threshold, R-LEN is cost-effective when compared to R-mono as all deterministic ICERs (i.e. from all perspectives) were below that threshold. However, PSA simulations resulted in some variability in the modeled results (see ). The probability of R-LEN being cost-effective from a societal perspective started to increase from 0% at approximately 40,000 EUR/QALY gained to 100% at 62,000 EUR/QALY gained (see ).
4.3. Comparison with other studies
While this is the first cost-utility analysis of R-LEN for previously treated FL patients from a societal perspective, R-LEN has been evaluated in the same patient population from a UK healthcare perspective for a single technology appraisal to the NICE [Citation38]. The NICE recommended R-LEN as a treatment option, stating an ICER of 20,156 GBP (approx. 22,976 EUR) per QALY gained when R-LEN was compared to R-CVP [Citation39]. In the committee papers, an ICER of 17,233 GPB (approx. 19,644 EUR) per QALY gained can be found when R-LEN was compared to R-mono [Citation38]. Although the modeled OS estimates between the NICE assessment and our study are comparable, the estimated ICERs deviate. This may be due to several reasons. First, the UK ICERs already include a confidential price discount of lenalidomide. Second, even without such a discount, the UK list price for lenalidomide is less than the Dutch list price. Furthermore, the UK analysis comparing R-LEN to R-mono used different parametric survival models and utility values when compared to our analysis [Citation38].
Recently, another CUA was published by Zhang et al. The study concluded that R-LEN is not cost-effective with an ICER of 49,849 EUR (58,812 USD) per QALY when compared to R-mono for r/r FL from a Chinese societal perspective [Citation40]. Several methodological aspects between this and our study exist that need to be addressed. First, Zhang et al. did not have patient-level data available and therefore extracted survival data from the published Kaplan–Meier curves from the AUGMENT trial. This approach does not allow for stratification by diagnosis, and hence survival data of FL and MZL patients were used together to estimate long-term survival. Second, it was claimed that results were estimated on a lifetime horizon (i.e. until all patients had died). However, the actual time horizon used in the model was 10 years. Consequently, any costs or effects occurring after these 10 years were not captured. Third, Zhang et al. stated that costs were estimated from a societal perspective. Nevertheless, only direct medical costs were considered.
4.4. Assumptions on rituximab
Clinical experts mentioned that rituximab can be given s.c. when it is the only administered drug (as in R-mono) or when the combination drug is either administered s.c. or orally (as in R-LEN). Apart from being more time-efficient for both patients and health-care staff, s.c. administration of rituximab in general leads to lower resource use and costs when compared to i.v. administration [Citation28]. Nevertheless, the experts indicated that rituximab is usually administered i.v. since the price of its biosimilar version is lower when compared to the reference product. In contrast to this, Dutch list prices for rituximab and its biosimilars do not differ. Eventual discounts are the result of confidential price negotiations between individual hospitals and the manufacturer. To remain transparent, we adhered to the list prices for rituximab and assumed s.c. administration in both R-LEN and R-mono.
The assumption of s.c. versus i.v. administration for rituximab was tested in scenarios four and five (see Appendix) and did not lead to meaningful changes in the ICER. Assuming a lower price for rituximab (e.g. as a result of using a biosimilar) was not formally tested. However, a price discount for rituximab would affect both R-LEN and R-mono at the same extent and hence changes in the ICER are not expected.
4.5. Strength and weaknesses
In our model, incremental life years were determined by the choice of the parametric survival model to extrapolate empirical trial data and the assumption of the treatment effect waning. Ideally, long-term follow-up data would inform the distribution of patients in each health state (i.e. PF or PD). These data are, however, not available, and with limited follow-up time in AUGMENT (median = 28.3 months) [Citation10], extrapolation to a longer time horizon was necessary. Therefore, we fit several parametric survival models and consulted clinical experts to validate these long-term estimates.
Our sensitivity analyses show that the choice of a parametric survival function for OS can influence the decision whether R-LEN can be considered cost-effective at a WTP-threshold of 50,000 EUR/QALY gained. Both Gompertz and Weibull distribution resulted in an ICER above that threshold (i.e. 63,581 EUR/QALY and 52,685 EUR/QALY, respectively, from a societal perspective). Empirical survival data with a longer follow-up period than in AUGMENT might lead to different results with less uncertainty.
Furthermore, the assumption on the treatment effect duration heavily impacted both the modeled effectiveness and the ICER. Assuming an infinite treatment effect duration for R-LEN resulted in the most favorable ICER of all scenario analyses from a societal perspective (24,983 EUR/QALY), while an effect duration of three years produced the least favorable ICER of 60,181 EUR/QALY gained. Which exact time point to assume for the treatment effect waning is a matter of ongoing discussion, but the NICE committee agreed that a five year horizon was appropriate (with clinical input suggesting a treatment effect lasting between 5 and 10 years) [Citation38]. Empirical OS and PFS data of AUGMENT indicate a benefit of R-LEN over R-mono [Citation10]. After trial follow-up there are several possibilities to extrapolate this treatment effect. It can be assumed to either further increase, remain constant, or decrease. Due to the immunomodulatory effect of lenalidomide we considered a constant treatment effect after five years post treatment to be a conservative estimate.
Another important aspect of determining the modeled effectiveness is the utility value for the different model health sates (i.e. PF and PD). Based on the analyses of AUGMENT HRQoL data, we found no differences between either treatment option (i.e. R-LEN or R-mono) or health state (i.e. PFS or PD), which was in line with a previous analysis of the same data [Citation13]. No difference in HRQoL between health states seems counterintuitive. For a scenario analysis, we therefore used the study by Wild et al. (2006) with utility values of 0.805 for PF and 0.736 for PD [Citation41]. Although often used in economic evaluations, the reference is only available as a conference abstract with limited information on the methodology or utility values. However, neither the variation of utility values in the DSA nor the scenario with utility values of Wild et al. exceeded an ICER of 50,000 EUR/QALY gained. Further research is needed to determine whether there is indeed no difference in HRQoL between patients who are PF or in PD [Citation41].
The assumed average starting age of the modeled patient cohort showed to be an influential factor for the estimated ICER (see DSA results). While the mean age of FL patients in AUGMENT was approximately 61 years, clinical experts found this to be a rather young age for previously treated FL patients. Indeed, the median age at FL diagnosis for Dutch patients between 2014 and 2016 in the Netherlands was 64 years (range: 55–72, mean not reported) [Citation2]. In the DSA, the influence of assuming different ages at treatment start found that age was indeed one of the most influential parameters for the model results. It needs to be noted that changing the assumed age at treatment start in the model does not lead to different outcomes of the parametric survival estimations. The variations in age mainly influence the remaining time in the model until the lifetime horizon is reached as well as the age-adjusted utility decrements. Only empirical data from a patient cohort with an older average age than in AUGMENT would lead to different outcomes in parametric survival estimations. Such data are however not available for R-LEN versus R-mono.
4.6. Relevance of the study
Rituximab is in use since a couple of decades for previously treated FL patients but thus far, no formal cost-effectiveness analysis has been performed. Indeed, a recent systematic review in this area concluded a substantial need for further studies due to a significant evidence gap in this disease area [Citation42]. This gap seems to be most prominent in previously treated FL patients, although this population appears to be particularly burdened by high health-care resource use and costs [Citation42]. With our study we fill part of this gap, which could potentially be used as a reference point for future economic evaluations.
While we acknowledge the limited use of R-mono in (Dutch) clinical practice, there are currently no clinical data available from a phase III study comparing R-LEN to any other comparator than R-mono in previously treated FL patients. Nevertheless, the cost-effectiveness estimated for R-LEN compared to R-CHOP and R-CVP could be very relevant for clinical and reimbursement decisionmakers. For this purpose, reliable evidence needs to be available, however. The modeled effectiveness of R-mono can be seen as a conservative estimate since both R-CHOP and R-CVP are generally considered to be more effective when compared to R-mono [Citation38]. Nevertheless, results of the RELEVANCE trial comparing R-LEN to R-chemo in advanced untreated FL patients suggest similar efficacy results between these treatments [Citation43]. Whether this holds true for later treatment lines needs to be confirmed. Based on the Dutch CPG for FL, total treatment costs of an R-CHOP or R-CVP regimen are approximately 3,000 EUR or 5,800 EUR more expensive than R-mono [Citation32]. It can be hypothesized that most other cost items of R-CHOP and R-CVP will be similar or higher when compared to R-mono. Consequently, a comparison of R-LEN to R-CHOP or R-CVP will lead to lower incremental total costs when compared to R-mono. And even if effectiveness outcomes of R-CHOP or R-CVP would be similar to R-mono, the ICER of R-LEN when compared to the former two treatment options would be lower than the ICER of this analysis. Real-world evidence, for instance from clinical registries, could shed light on the difference in effectiveness between R-mono and R-CHOP/R-CVP.
As of 1 June 2020 R-LEN is reimbursed under a confidential commercial agreement between the manufacturer and the Ministry of Health in the Netherlands [Citation44]. Details of this confidential agreement (e.g. price discount on lenalidomide) are not taken into account for this evaluation. It can be assumed that a financial discount on the drug price of lenalidomide will result in a more favorable ICER compared to this analysis.
5. Conclusions
Our results show that R-LEN can be considered cost-effective at a WTP-threshold of 50,000 EUR/QALY gained from the base-case analysis. Nevertheless, this result is marked by some uncertainty. Long-term efficacy data could validate the model results and reduce this uncertainty. Although more recent and extensive data would be preferred, a further exploration of real-world evidence (e.g. from cancer registries) could be a first step.
Declaration of interest
FW Thielen reports unrestricted grants from Celgene B.V., during the conduct of the study, FW Thielen took part in a consultation with AstraZeneca on a reimbursement dossier for a treatment and disease indication unrelated to this study, and his institution was reimbursed for time spent on the consultation. MJ Kersten reports grants and personal fees from Celgene/BMS, grants and personal fees from Roche, during the conduct of the study.
CA Uyl-de Groot and HM Blommestein both received unrestricted grants from Celgene BV during the conduct of study.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (1,003.1 KB)Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Freedman A. Follicular lymphoma: 2018 update on diagnosis and management. Am J Hematol. 2018;93(2):296–305.
- Dinnessen M, Brink M, Korf-van Vliet C, et al. Het folliculair lympfoom in Nederland, 2014-2016. Landelijk rapport van het hematooncologieregister van de Nederlandse Kankerregistratie. [Internet]. 2019 [cited 2020 Apr 21]. Available from: https://www.iknl.nl/getmedia/6fbcaf47-2677-4b4e-933d-68d5be07773b/Hematologische_kankersoorten_landelijk_rapport_iknl_FL_2014_2016.pdf.
- Kharchenko EV, Sweetenham JW. Advances in relapsed/refractory follicular lymphoma therapeutics. Adv Cell Gene Ther. 2020;3(1):e74.
- Anonymous. Zevalin [Internet]. Eur. Med. Agency. 2018 [cited 2020 Mar 30]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/zevalin.
- Anonymous. Zydelig [Internet]. Eur. Med. Agency. 2018 [cited 2020 Mar 30]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/zydelig.
- Anonymous. Gazyvaro [Internet]. Eur. Med. Agency. 2018 [cited 2020 Mar 30]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/gazyvaro.
- Anonymous. MabThera [Internet]. Eur. Med. Agency. 2018 [cited 2020 Mar 29]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/mabthera.
- Anonymous. IntronA [Internet]. Eur. Med. Agency. 2018 [cited 2020 Mar 29]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/introna.
- European Medicines Agency. EMA/225905/2019. Revlimid (lenalidomide) [Internet]. [cited 2019 Dec 2]. Available from: https://www.ema.europa.eu/en/documents/overview/revlimid-epar-medicine-overview_en.pdf.
- Leonard JP, Trneny M, Izutsu K, et al. AUGMENT: a Phase III study of lenalidomide plus rituximab versus placebo plus rituximab in relapsed or refractory indolent lymphoma. J Clin Oncol Off J Am Soc Clin Oncol. 2019;37(14):1188–1199.
- Fayers P, Bottomley A. on behalf of the EORTC quality of life group and of the quality of life unit. Quality of life research within the EORTC—the EORTC QLQ-C30. Eur J Cancer. 2002;38:125–133.
- Rabin R, De CF. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337–343.
- Leonard JP, Trněny M, Izutsu K, et al. Health-Related Quality of Life (HRQoL) in Relapsed/Refractory (r/r) Indolent NHL in the Phase 3 AUGMENT Trial of Rituximab (R) plus Lenalidomide (R 2) versus R plus Placebo. Hematol Oncol. 2019;37:232–234.
- Dhanasiri S. Response to: olry de Labry Lima A et al., Cost-effectiveness of lenalidomide maintenance in patients with multiple myeloma who have undergone autologous transplant hematopoietic progenitor cells. Bone Marrow Transplant. 2019;1–2.
- Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006.
- Drummond MF, Sculpher M, Claxton K, et al. Methods for the economic evaluation of health care programmes [Internet]. Oxford: Oxford University Press; 2015 [cited 2020 Mar 26]. Available from: https://doi.org/10.1136/jech.41.4.355-a.
- Brouwer WBF, van Exel NJA, Baltussen RMPM, et al. A dollar is a dollar is a dollar—or is it? Value Health. 2006;9(5):341–347.
- Meltzer D. Accounting for future costs in medical cost-effectiveness analysis. J Health Econ. 1997;16(1):33–64.
- Feenstra TL, Van Baal PHM, Gandjour A, et al. Future costs in economic evaluation: a comment on Lee. J Health Econ. 2008;27(6):1645–1649.
- De Vries LM, Van Baal PHM, Brouwer WBF. Future costs in cost-effectiveness analyses: past, present, future. PharmacoEconomics. 2019;37(2):119–130.
- PAID [version 3.0] [Internet]. iMTA. [cited 2020 Mar 26]. Available from: https://www.imta.nl/paid/.
- Van Baal PHM, Wong A, Slobbe LCJ, et al. Standardizing the inclusion of indirect medical costs in economic evaluations. PharmacoEconomics. 2011;29(3):175–187.
- Kellerborg K, Perry-Duxbury M, De Vries L, et al. Practical guidance for including future costs in economic evaluations. Yet Publ.
- Woods B, Sideris E, Palmer S, et al. Partitioned survival analysis for decision modelling in health care: a critical review report. NICE Decis Support Unit. 2017;1–72.
- Hakkaart-van Roijen L, Van Der Linden N, Bouwamans C, et al. Kostenhandleiding: methodologie van kostenonderzoek en referentieprijzen voor economische evaluaties in de gezondheidszorg. Bijlage. 2016;1.
- Latimer NR. Survival analysis for economic evaluations alongside clinical trials - extrapolation with patient-level data: inconsistencies, limitations, and a practical guide. Med Decis Mak. 2013;33(6):743–754. 2013/01/24ed.
- Z-Index - About [Internet]. [cited 2020 Mar 26]. Available from: https://www.z-index.nl/english.
- Franken MG, Kanters TA, Coenen JL, et al. Potential cost savings owing to the route of administration of oncology drugs: a microcosting study of intravenous and subcutaneous administration of trastuzumab and rituximab in the Netherlands. Anticancer Drugs. 2018;29(8):791–801.
- Bouwmans C, Janssen J, Huijgens P, et al. Costs of haematological adverse events in chronic myeloid leukaemia patients: a retrospective cost analysis of the treatment of anaemia, neutropenia and thrombocytopenia in patients with chronic myeloid leukaemia. J Med Econ. 2009;12(2):164–169.
- Nafees B, Stafford M, Gavriel S, et al. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. 2008;6(1):84.
- Swinburn P, Lloyd A, Nathan P, et al. Elicitation of health state utilities in metastatic renal cell carcinoma. Curr Med Res Opin. 2010;26(5):1091–1096.
- Nederlandse Vereniging voor Hematologie. Richtlijn voor de diagnostiek, behandeling en follow-up van het folliculair lymfoom [Internet]. 2020 [cited 2020 Feb 19]. Available from: http://www.hovon.nl/upload/File/Richtlijnen_BehAdv/FL%20richtlijn_revisie2019.pdf.
- Statistics Netherlands. Open data [Internet]. Stat. Neth. [cited 2020 Mar 27]. Available from: https://www.cbs.nl/en-gb/our-services/open-data.
- The iMTA disease burden calculator [version 1.3 beta] [Internet]. [cited 2020 Mar 26]. Available from: www.imta.nl/idbc.
- Zorginstituut Nederland. Richtlijn voor het uitvoeren van economische evaluaties in de gezondheidszorg [Internet]. 2016 [cited 2020 Mar 11]. Available from: https://www.zorginstituutnederland.nl/publicaties/publicatie/2016/02/29/richtlijn-voor-het-uitvoeren-van-economische-evaluaties-in-de-gezondheidszorg.
- Doorduijn JK, Buijt I, Van Der Holt B, et al. Economic evaluation of prophylactic granulocyte colony stimulating factor during chemotherapy in elderly patients with aggressive non-Hodgkin’s lymphoma. Haematologica. 2004;89:1109–1117.
- Arboe B, Olsen MH, Goerloev JS, et al. Return to work for patients with diffuse large B-cell lymphoma and transformed indolent lymphoma undergoing autologous stem cell transplantation. Clin Epidemiol. 2017;9:321–329.
- Single Technology Appraisal. Lenalidomide for treated follicular lymphoma and marginal zone lymphoma [ID1374]. Committee Papers [Internet]. National Institute for Health and Care Excellence; 2020 [cited 2020 Mar 30]. Available from: https://www.nice.org.uk/guidance/gid-ta10323/documents/committee-papers.
- Final appraisal document. Lenalidomide with rituximab for previously treated follicular lymphoma. [Internet]. National Institute for Health and Care Excellence; 2020 [cited 2020 Mar 30]. Available from: https://www.nice.org.uk/guidance/gid-ta10323/documents/final-appraisal-determination-document.
- Zhang P-F, Xie D, Wen F, et al. Lenalidomide plus rituximab Vs rituximab alone in relapsed or refractory indolent lymphoma: a cost-effectiveness analysis. Cancer Med [Internet]. [cited 2020 Jun 17]; n/a. Available from: https://doi.org/10.1002/cam4.3121.
- Wild D, Walker M, Pettengell R, et al. PCN62 utility elicitation in patients with follicular lymphoma. Value Health. 2006;9(6):A294.
- Monga N, Garside J, Gurung B, et al. Cost-Effectiveness analyses, costs and resource use, and health-related quality of life in patients with follicular or marginal zone lymphoma: systematic reviews. Pharmacoecon Open. 2020;4(4):575–591.
- Morschhauser F, Fowler NH, Feugier P, et al. Rituximab plus Lenalidomide in advanced untreated follicular lymphoma. N Engl J Med. 2018;379(10):934–947.
- van Rijn M. Regeling van de Minister voor Medische Zorg van 18 mei 2020, kenmerk 1687653-205139-Z, houdende wijziging van de Regeling zorgverzekering in verband met de opname van het geneesmiddel lenalidomide in het basispakket. Staatscourant; 2020.