ABSTRACT
Introduction Metastatic triple-negative breast cancer (TNBC) is an aggressive cancer with poor survival that is difficult to treat due to a lack of targeted options. Conventional therapies targeting hormone receptors (HR) and human epidermal growth factor 2 (HER2) are ineffective and often chemotherapy is standard-of-care. Sacituzumab govitecan is an antibody drug conjugate (ADC) comprised of an active metabolite of irinotecan, SN-38, bound to a humanized monoclonal antibody targeting trophoblastic cell-surface antigen 2 (Trop-2). Trop-2 is highly expressed on the surface of TNBC cells, making it an attractive target. Areas covered We explore the mechanism, pharmacology, efficacy, safety, and tolerability of sacituzumab govitecan. A literature search was conducted via PubMed using keywords such as ‘sacituzumab govitecan,’ and ‘metastatic TNBC.’ Expert opinion Sacituzumab govitecan has promising survival benefits in patients with previously treated mTNBC based on data from the ASCENT trial. Common adverse effects were neutropenia, diarrhea, and nausea, however these effects were manageable with supportive care. Sacituzumab govitecan has shown promise in cancers outside of TNBC, such as urothelial and lung and is being evaluated in HR-positive breast cancers. It is likely we will see this therapy used in combination with other novel targeted agents as current clinical trials mature.
1. Introduction
Triple-negative breast cancer (TNBC) is an aggressive sub-type of breast cancer which lacks the expression of the estrogen receptor (ER), progesterone receptor (PR), and overexpression of human epidermal growth factor 2 (HER2). Worldwide, TNBC accounts for around 15% of breast cancers [Citation1]. TNBC is more commonly diagnosed in women of African American descent and in younger, pre-menopausal patients [Citation2]. Given the aggressive nature of the cancer, women with TNBC are more likely to have distant recurrence and death within 5 years when compared to other breast cancer sub-types [Citation3]. Overall survival (OS) in patients with metastatic TNBC (mTNBC) remains poor with median estimated survival of around eighteen months [Citation4]. Given this dismal survival, more needs to be done to develop and implement targeted therapies to improve outcomes in patients with mTNBC.
Checkpoint inhibitors, specifically programmed cell death ligand-1 (PD-L1) and programmed cell death-1 (PD-1), in combination with chemotherapy have shown benefit in PD-L1 expressing mTNBC tumors. Recently, the KEYNOTE 355 trial demonstrated a significant improvement in progression free survival (PFS) and OS in patients with a PD-L1 combined positive score (CPS) ≥10, mTNBC treated with pembrolizumab in combination with chemotherapy [Citation5,Citation6]. Pembrolizumab in combination with chemotherapy was found to reduce the risk of death by 27% (hazard ratio 0.73; 95% CI 0.55–0.95; p = 0.0093) [Citation6]. The IMpassion 130 trial demonstrated improved PFS in the intention to treat (ITT), PD-L1 positive (>1% PD-L1 expression), mTNBC population when treated with the PD-L1 inhibitor atezolizumab in combination with nab-paclitaxel [Citation7]. However, this trial did not meet the co-primary endpoint of OS in the ITT population. Furthermore, recent data from IMpassion 131, found that the combination of atezolizumab with paclitaxel did not improve PFS or OS when compared to paclitaxel alone in an advanced PD-L1 positive, TNBC population [Citation8]. In August 2021, the sponsor (Genentech) withdrew their FDA accelerated approval of atezolizumab in the treatment of PD-L1 positive TNBC. Nevertheless, immunotherapy still represents a promising therapy for the treatment of mTNBC, as shown by KEYNOTE-355.
Poly (ADP-ribose) polymerase (PARP) inhibitors are also effective in the breast cancer susceptibility gene (BRCA) mutated, mTNBC population [Citation9–11]. The OlympiAD trial, which evaluated patients with mTNBC and germline BRCA mutations treated previously with an anthracycline and a taxane, demonstrated improved PFS in patients treated with the PARP inhibitor olaparib when compared to patients who received standard-of-care chemotherapy [Citation10]. However, there was no improvement in OS [Citation12]. Similarly, the EMBRACA trial found improved PFS (but not OS) in patients with germline BRCA1/2 mutations with advanced breast cancer who were treated with the PARP inhibitor talazoparib when compared to chemotherapy [Citation9,Citation13]. While PARP inhibitors do have clinical activity, as evidence by prolongation of PFS, they do not prolong OS. Acquired resistance to therapy is universal, and there are concerns regarding efficacy beyond platinum compounds and potential overlapping mechanisms of resistance [Citation13].
Molecular subtypes of TNBC, represent another potential for targeted therapy. From gene analyses, it has been found that distinct subtypes of TNBC can predict cytotoxic susceptibility as well as prognosis [Citation14]. As an example, mesenchymal (M) subtypes of TNBC were found to have some sensitivity to phosphatidylinositol-3 kinase/mechanistic target of rapamycin (PI3K/mTOR) inhibitors and basal-like-1 (BL1) subtypes of TNBC were found to be sensitive to genotoxic agents. In addition, BL1, immunomodulatory (IM), mesenchymal stem-like (MSL) subtypes, had nearly double median OS and disease-free survival (DFS) when compared to basal-like-2 (BL2), luminal androgen receptor (LAR), and M subtypes [Citation14]. The unique biology associated with each subtype, offers another option for molecularly targeted therapy.
Despite these exciting advances, there remains limited treatment options for patients with TNBC and chemotherapy remains standard-of-care for most mTNBC [Citation15,Citation16]. While chemotherapy can provide benefit for some patients, this response is often short and riddled with side-effects [Citation17]. Further research needs to be done to continue to produce targeted therapies for the mTNBC population. This review will explore the antibody-drug conjugate (ADC) sacituzumab govitecan (IMMU-132), another encouraging advancement toward the treatment of mTNBC.
2. Antibody-drug conjugate sacituzumab govitecan
2.1. Mechanism of action
ADCs are comprised of a monoclonal antibody that is linked to a cytotoxic payload [Citation18]. The antibody portion recognizes cell-surface proteins, allowing access of the ADC compound into the cell. Once in the cell the linker is cleaved, releasing the cytotoxic agent into the tumor environment. ADCs are able to have higher potency and specificity than traditional treatment agents given their targeted approach [Citation18]. Via an antibody mechanism, the goal of ADCs is to lower exposure of cytotoxic therapy to non-cancerous cells therefore leading to less systemic toxicity [Citation15,Citation19]. Many ADCs have been approved by the FDA for clinical use including brentuximab vedotin (lymphoma), ado-trastuzumab emtansine (HER2-positive metastatic breast cancer), inotuzumab ozogamicin (acute lymphoblastic leukemia), gemtuzumab ozogamicin (acute myeloid leukemia), moxetumomab pasudotox (hairy cell leukemia), enfortumab vedotin (urothelial cancer), belantamab mafodotin-blmf (multiple myeloma), loncastuximab tesirine-Ipyl (large B-cell lymphoma), and polatuzumab vedotin-piiq (diffuse large B-cell lymphoma) [Citation20–24].
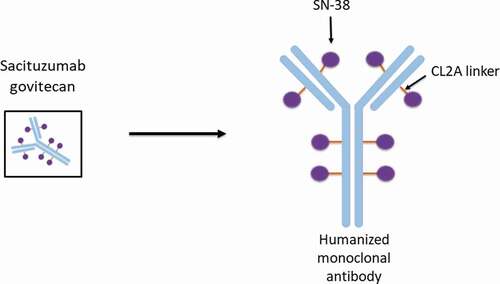
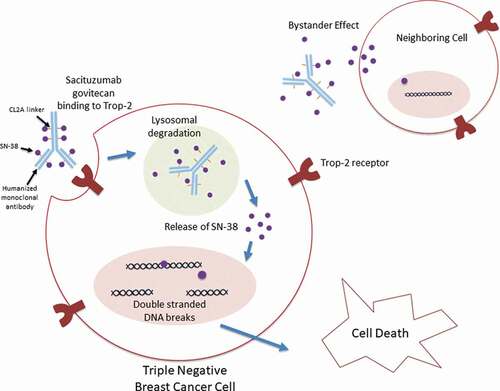
In sacituzumab govitecan, the cytotoxic payload is the active metabolite of irinotecan, called SN-38 [Citation25]. On average, there are 7–8 molecules of SN-38 per antibody structure. SN-38 works as an anti-cancer therapy by inhibiting topoisomerase I (Topo I) [Citation26]. Topo I works to cleave and reanneal the DNA double helix during cell replication [Citation27]. When this enzyme is inhibited, the DNA strands break which results in cell death [Citation27]. SN-38 is linked to a humanized monoclonal antibody (hRS7 IgG1κ) by a hydrolysable CL2A linker () [Citation25,Citation28]. This linker is of intermediate stability, allowing slow release of the cytotoxic payload [Citation29]. Given the cleavable properties of this linker, the payload SN-38, is released both intracellularly and into the tumor microenvironment [Citation30]. This allows for the delivery of the payload and therefore presumed death of the targeted cancer cell as well as cells in the adjacent tumor environment, the bystander effect [Citation30,Citation31].
The antibody target of sacituzumab govitecan is trophoblastic cell-surface antigen 2 (Trop-2). Trop-2 is a glycoprotein which assists with cell migration and anchorage-independent growth () [Citation30,Citation32]. When compared to normal tissue, Trop-2 is found to have higher expression in a vast array of cancers including breast, lung, gastric, colorectal, pancreatic, prostate, cervical, head-and-neck, and ovarian [Citation30]. Breast cancer cells with higher expression of Trop-2 have been associated with more aggressive disease and poor prognosis [Citation30]. TNBC has been shown to have greater than 85% expression of Trop-2, making this protein an attractive target [Citation30,Citation33,Citation34].
SN-38 delivered via ADC has several advantages when compared to SN-38 with irinotecan therapy. Unlike SN-38 with irinotecan therapy, SN-38 bound to the antibody conjugate is protected from de-activation via glucuronidation and is able to circulate in an inactive state, leading to less toxicity [Citation31]. In addition, the targeted mechanism of the ADC sacituzumab govitecan results in superior drug delivery when compared to irinotecan with area under the curve (AUC) analysis showing 20-fold to 136-fold more SN-38 when delivered via ADC [Citation31]. These results have been encouraging as a major goal of anti-cancer therapy is to limit toxicity and improve efficacy.
2.2. Pharmacology
To determine appropriate dosing of sacituzumab govitecan, twenty-five patients with previously treated metastatic epithelial cancers (including four with TNBC) were treated with intra-venous (IV) sacituzumab govitecan over 2–3 hours at doses of 8 mg/kg, 10 mg/kg, 12 mg/kg, and 18 mg/kg in a Phase I dose-finding trial [Citation35]. Drug was given on day 1 and day 8 of a 3 week cycle, with the goal to administer 8 cycles. Neutropenia was found to be the primary dose limiting toxicity and the maximum tolerated dose was 12 mg/kg. To prevent delays between cycles and to ensure patients would receive multiple infusions of the drug, the doses of 8 mg/kg and 10 mg/kg were selected for further analysis [Citation35]. A Phase II dose expansion trial evaluated 8 mg/kg and 10 mg/kg of sacituzumab govitecan in 178 patients with previously treated metastatic epithelial cancers (including 53 with TNBC) [Citation36]. The dose expansion of this trial was part of the same single arm Phase I/II basket study. Manageable adverse effects were observed in both doses and the dose of 10 mg/kg was selected for further clinical use.
Pharmacokinetics of sacituzumab govitecan were further assessed in the Phase II dose expansion trial [Citation36]. Half-life of sacituzumab govitecan at a dose of 10 mg/kg was noted to be around 11.7 hours compared to 102.7 hours for unconjugated IgG. Less than 5% of SN-38 in the serum was free, with most bound to IgG. Amounts of free SN-38 and glucuronidated SN-38 (SN-38 G) were similar, indicating that the SN-38 which was attached to IgG, remained protected from glucuronidation. Higher levels of SN-38 G in the serum has been associated with worsened diarrhea [Citation37]. The SN-38 payload is released gradually with around half of the payload released from the IgG conjugate every 24 hours [Citation36]. Given this gradual release, and low level of SN-38 G detected in the serum, the amount of diarrhea could be lessened. In the Phase II dose expansion trial, there was not found to be a relationship between free SN-38 and neutropenia. In addition, no antibody response to sacituzumab govitecan was noted in the 167 patients that were sampled.
For patients with renal impairment, there are no recommended dosage adjustments. However, if patients have moderate or severe hepatic impairment (bilirubin greater than 1.5 times the upper limit of normal, AST and ALT greater than 3 times the upper limit of normal, or AST and ALT greater than 5 times the upper limit of normal with associated liver metastases) treatment with sacituzumab govitecan is not recommended.
In patients treated with irinotecan, the UGT1A1*28 genotype has been associated with more severe toxicities and the UGT1A1*28 homozygotes have been strongly linked to neutropenia [Citation38]. In the Phase I/II basket trial, analysis of the three haplotypes of UGT1A1 (*1*1, *1*28, and *28,*28) found there was numerically higher all-grade neutropenia in UGT1A1*28 homozygotes but did not find a significant relationship between allele expression and diarrhea in patients treated with sacituzumab govitecan [Citation36,Citation39]. Currently, there are no recommendations to test for this genotype in patients starting sacituzumab govitecan. However, patients that have known UGT1A1 homozygosity should be monitored closely for neutropenia [Citation39]. Drugs to avoid concomitant use with sacituzumab govitecan include UGT1A1 inhibitors (amitriptyline, ketoconazole, ketoprofen, atazanavir) and UGT1A1 inducers (clofibrate, cortisol, phenobarbital, spironolactone, streptozotocin, rifampin, St. John’s wort) [Citation40].
2.3. Adverse events
Hypersensitivity within 24 hours of dosing was noted in 37% of patients treated with sacituzumab govitecan, however Grade 3–4 hypersensitivity reactions were rare occurring in only 1% of patients [Citation40]. In addition, in patients with mTNBC, nausea occurred in 69% of patients and grade 3 nausea occurred in 6% of patients. Vomiting occurred in about half (49%) of patients with mTNBC treated with sacituzumab govitecan, however grade 3 vomiting only occurred in 6% of patients. As such, premedication and/or concomitant medication with antipyretics and H1/H2 antagonists is recommended when dosing sacituzumab govitecan. Specifically, the FDA recommends premedicating with a two or three drug combination regimen. Corticosteroids may also be used if patients have experienced infusion-related reactions [Citation40].
Grade 1–4 neutropenia was present in 64% of patient mTNBC in the Phase I/II trial, though febrile neutropenia only occurred in 8% of patients [Citation40]. It is highly recommended that patients experiencing grade 3 or 4 neutropenia receive granulocyte colony stimulating factor (G-CSF). In addition, diarrhea occurred in 63% of mTNBC patients, however grade 3 or 4 diarrhea was rare occurring in only 9% of patients [Citation40]. For patients experiencing diarrhea, loperamide may be used. In patients with excessive cholinergic reactions or early diarrhea, atropine premedication can be considered. Both neutropenia and diarrhea are FDA boxed warnings and should be monitored. Alopecia is another adverse event that has been noted with sacituzumab govitecan, though this is expected. Longer-term efficacy of scalp cooling for drug-induced alopecia needs to be explored [Citation41,Citation42].
3. Clinical efficacy and adverse effects of sacituzumab govitecan in clinical trials
3.1. Phase I/II clinical trial
The Phase I dose-finding trial (NCT01631552) evaluated the treatment of sacituzumab govitecan in twenty-five patients, four of which had TNBC [Citation35]. Of the two patients in this study that achieved a partial response, one had TNBC and survived for >20 months from the start of therapy. Sixteen other patients were found to have stable disease. Patients in the study had manageable side-effects, and at lower doses there were no treatment-related grade 4 toxicities [Citation35]. These results showed promise in a TNBC population as well as other difficult to treat cancers, and lead to a Phase II expansion.
A phase I/II, basket design, single-arm, multicenter trial (IMMU-132-01; NCT01631552) evaluated heavily pretreated mTNBC patients who received 10 mg/kg of sacituzumab govitecan on days 1 and 8 of a 21-day cycle [Citation33]. Of the 108 patients identified for this trial, they received a median of 3 prior treatments [range, 2–10], including some patients who had received immune check point inhibitors [Citation43]. Of the cohort, 98% had previously received a taxane and 86% had received an anthracycline. Patients were treated for a median of 5.1 months [range, 0.03–36.1] with a mean of 18.7 doses of sacituzumab govitecan. The most-common side effects were nausea (67%), neutropenia (64%), diarrhea (62%), and fatigue (55%). During the trial only 3% of patients had side-effects which led to discontinuation and 4% of patients died during treatment. However, all deaths were felt to be secondary to disease progression rather than administration of sacituzumab govitecan. Of the cohort, the response rate was 33.3% and three patients had a complete response. Median PFS was found to be 5.5 months [95% CI 4.1–6.3] and median OS was 13.0 months [95% CI 11.2–13.7][Citation33]. Sacituzumab govitecan had a median duration of treatment that was twice as long (5.1 months) when compared to other therapeutic options (2.5 months)[Citation33].
Overall, this Phase I/II study demonstrated favorable efficacy of sacituzumab govitecan in mTNBC with manageable adverse reactions. The results of this study led to the accelerated approval by the FDA of sacituzumab govitecan in April 2020 for patients with mTNBC who had received at least two prior anti-cancer agents [Citation44]. In addition, the success of this trial led to the development of the Phase III ASCENT trial (NCT02574455).
3.2. Phase III ASCENT trial
The Phase III ASCENT trial was a randomized, open-label, multi-center trial which evaluated the efficacy of sacituzumab govitecan compared to physician’s choice single-agent chemotherapy (eribulin, vinorelbine, capecitabine, or gemcitabine)[Citation45]. The trial enrolled 529 patients with mTNBC though 468 patients without brain metastases were analyzed for the primary efficacy endpoint of PFS. Patients were randomly assigned in a ratio of 1:1 to receive sacituzumab govitecan or single-agent chemotherapy. Patients received a median of three previous anticancer therapies (range 1–16 for sacituzumab govitecan group and 1–12 for chemotherapy group) with 100% of patients having previously received taxanes, 82% anthracyclines, 66% carboplatin, 27% a PD-L1/PD-1 inhibitor, and 7% a PARP inhibitor [Citation45].
The most common side-effects in sacituzumab govitecan treatment group were neutropenia (63%), diarrhea (59%), nausea (57%), and alopecia (46%) (). The most common grade 3 and 4 adverse events included neutropenia (51%), leukopenia (10%), and diarrhea (10%). The single-agent chemotherapy treatment group had a lower percentage of adverse effects when compared to sacituzumab govitecan. In both the sacituzumab govitecan and chemotherapy group around a quarter of patients required dose reductions due to adverse events. Of patients who received sacituzumab govitecan, 49% received G-CSF compared to 23% in the chemotherapy group. Only 5% (12 patients) in the sacituzumab govitecan group had an adverse effect which led to stopping treatment. During the trial, three patients in the sacituzumab govitecan group and three patients in the chemotherapy group died. The three deaths in the sacituzumab govitecan group were not treatment related whereas one death in the chemotherapy group was related to neutropenic sepsis from treatment [Citation45].
Table 1. Common adverse events in 258 patients with metastatic triple-negative breast cancer receiving saituzumab govitecan and 224 patients receiving single-agent chemotherapy (eribulin, vinorelbine, capecitabine, gemcitabine) in the Phase III clinical trial. [45]
In terms of efficacy, sacituzumab govitecan showed superiority when compared to standard-of-care chemotherapy. In patients without baseline brain metastases, median PFS in patients treated with sacituzumab govitecan was 5.6 months [95% CI 4.3–6.3] and 1.7 months [95% CI 1.5–2.6] in the chemotherapy group. The hazard ratio (HR) for disease progression or death of 0.41 [95% CI 0.32–0.52, p < 0.001] (). Median OS for the sacituzumab govitecan group was 12.1 months [95% CI 10.7–14.0] and 6.7 months [95% CI 5.8–7.7] in the chemotherapy group. HR for death was 0.48 [95% CI 0.38–0.59, p < 0.001]. Patients treated with sacituzumab govitecan had an objective response of 35% compared to 5% of those who received standard-of-care chemotherapy [Citation45]. The results of the ASCENT trial led to regular approval by the FDA for use in patients with unresectable locally advanced or mTNBC who have received at least two prior lines of therapy, with at least one given in metastatic disease [Citation46].
Table 2. Phase III results of patients treated with sacituzumab govitecan and single-agent chemotherapy (eribulin, vinorelbine, capecitabine, gemcitabine) in metastatic triple-negative breast cancer. [45]
Efficacy was also assessed in patients with brain metastases. In a subgroup analysis evaluating patients with brain metastases from ASCENT, patients treated with sacituzumab govitecan were found to have an improved median PFS of 2.8 months [95% CI, 1.5–3.9] compared to 1.6 months [95% CI, 1.3–2.9] for standard-of-care chemotherapy. [Citation47] In addition, patients treated with sacituzumab govitecan had an objective response rate (ORR) of 3% and clinical benefit rate of 9.4% compared to an ORR of 0% and clinical benefit rate of 3.4% for standard-of-care chemotherapy. There was not found to be an improvement in OS with the treatment of sacituzumab govitecan in patients with brain metastases [Citation47].
3.3. Efficacy outside of mTNBC
Given the favorable outcomes sacituzumab govitecan demonstrated in mTNBC, multiple other clinical trials are under way examining sacituzumab govitecan as single-agent or in combination for other treatment purposes. These trials are aiming to assess the utility of sacituzumab govitecan as a neoadjuvant therapy in early TNBC, in combination with immunotherapy or a PARP inhibitor, and in hormone receptor (HR)-positive/HER2-negative breast cancer [Citation45]. Outside of breast cancer, sacituzumab govitecan has also shown promise in Phase II trials evaluating its use in small cell lung cancer (SCLC), non-small cell lung cancer (NSCLC), and urothelial cancer [Citation48–50]. Recently, the FDA granted approval of the use of sacituzumab govitecan in locally advanced or metastatic urothelial cancer [Citation51]. Furthermore, there are current clinical trials evaluating the use of sacituzumab govitecan in glioblastoma, head and neck squamous cell carcinoma, endometrial, and prostate cancer.
4. Conclusion
Sacituzumab govitecan (IMMU-132) is an ADC which targets Trop-2 for the delivery of the active metabolite of irinotecan, SN-38. Trop-2 is highly expressed in many different tumor types and notably was found to have high expression in aggressive TNBC tumor cells. Metastatic TNBC remains difficult to treat with patients often progressing past standard-of-care chemotherapies. Efficacy of sacituzumab govitecan was shown to be superior when compared to several single-agent standard-of-care chemotherapies in heavily pretreated patients without brain metastases in the Phase III ASCENT clinical trial. Adverse effects including neutropenia, diarrhea, and nausea were noted, however were manageable with supportive care and pre or post infusion treatment. This trial led to regular FDA approval of sacituzumab govitecan in the treatment of mTNBC patients who have received two or more prior systemic therapies, with at least one in the metastatic setting. Multiple trials are underway examining the use of sacituzumab govitecan in other breast cancer types, in combination with other agents, and in other tumor types.
5. Expert opinion
Metastatic TNBC remains difficult to treat due to the lack of HR and HER2 targeted therapies. Progress has been made toward more targeted therapies with the arrival of immunotherapy. Pembrolizumab in combination with chemotherapy, is FDA approved for the treatment of mTNBC in tumors that express PD-L1. In addition, the PARP inhibitors olaparib and talazoparib are now FDA approved for BRCA-mutated HER2-negative metastatic breast cancer. While these are exciting advances, only 40% of TNBC patients express PD-L1 and only 15% have a germline BRCA mutation [Citation15,Citation52]. Furthermore, resistance has become a major obstacle with patients experiencing early relapse and metastasis [Citation53]. Many patients are left with chemotherapy as their only therapeutic option. Second and third lines of palliative chemotherapy has a limited median PFS of 2–3 months [Citation54]. This demonstrates the need for newer therapies and brings excitement to the success of sacituzumab govitecan.
Sacituzumab govitecan has a more robust drug delivery and manageable side-effect profile than conventional irinotecan. Much of this is owed to the unique cleavable linker that has intermediate stability of 1–2 days and allows for gradual release of SN-38[Citation29]. This linker allows the payload, SN-38, to be released both intracellularly as well as into the adjacent tumor environment. This allows for less systemic toxicity while also promoting robust anti-cancer activity. The slow release of the payload also has been found to increase potency and tolerability by slowly releasing SN-38 G into the gastrointestinal tract [Citation29]. Irinotecan is thought to release high levels of SN-38 G into the serum, leading to more gastrointestinal upset. Furthermore, other ADCs can be tightly bound to their payload, therefore missing out on the bystander effect observed in sacituzumab govitecan.
Both the Phase I/II and Phase III clinical trials demonstrated remarkable efficacy of sacituzumab govitecan as a single-agent anti-cancer therapy in mTNBC. In the Phase III trial, patients in the sacituzumab govitecan group had received a median of three prior anticancer regimens and 29% of patients in this group had received >3 chemotherapy regimens [Citation45]. Despite this, median PFS and OS in patients without brain metastases was found to be impressive with survival at 5.6 months [95% CI 4.3–6.3] and 12.1 months [95% CI 10.7–14.0] respectively. This is beyond the median expected PFS of 2–3 months for patients who have received two or three lines of chemotherapy and is similar to expected OS [Citation4,Citation54]. A subgroup analyses of patients with brain metastases from the Phase III ASCENT trial also showed promise [Citation47]. This analysis found that patients with brain metastases treated with sacituzumab govitecan numerically had improved tumor response and PFS when compared to physician’s choice of single agent chemotherapy [Citation47]. However, data from this population was limited by prognosis and small sample size. A phase II clinical trial is underway (NCT04647916) investigating response of patients with brain metastases and HER2-negative breast cancers treated with sacituzumab govitecan. In addition, another trial is investigating the use of sacituzumab govitecan given preoperatively in patients with glioblastoma or metastatic brain tumors from breast cancer (NCT03995706).
Sacituzumab govitecan also showed impressive clinical benefit when compared to single-agent standard-of-care anti-cancer agents. Patients who had previously received a PD-1 or PD-L1 inhibitor found benefit with sacituzumab govitecan with a PFS of 4.2 [95% CI 3.2–5.6] compared to 1.6 [95% CI 1.4–2.3] in the chemotherapy group [Citation45]. In addition, when compared to single-arm chemotherapy, sacituzumab govitecan again showed benefit with an ORR of 35% compared to just 5% for the chemotherapy group [Citation45]. This highlights the possible utility and benefit of using sacituzumab govitecan in a heavily pre-treated mTNBC population.
Given these data, it is warranted to investigate sacituzumab govitecan as a possible first-line treatment in mTNBC or in localized TNBC. Indeed, there are clinical trials under-way investigating the possible benefit of this ADC as first-line therapy in a metastatic and localized setting. Two Phase II trials (NCT04468061 and NeoSTAR; NCT04230109) are looking at the use of sacituzumab govitecan as a single-agent and in combination with pembrolizumab. The first is investigating outcomes in PD-L1 negative, mTNBC patients who have not yet received treatment for their metastatic disease and the second is evaluating localized TNBC. Given the benefit seen with PARP inhibitors as well as immunotherapy agents, it would be beneficial to investigate these agents in combination with sacituzumab govitecan. Again, several clinical trials are under-way to examine the outcomes of this pairing in mTNBC patients. Morpheus-TNBC (NCT03424005) is a Phase Ib/II trial which aims to evaluate the efficacy of immunotherapy paired with several anti-cancer agents, including sacituzumab govitecan, InCITe (NCT03971409) is a Phase II trial evaluating avelumab with binimetinib, sacituzumab govitecan or liposomal doxorubicin in mTNBC, and there is a Phase I/II (NCT04039230) trial investigating the combination of sacituzumab govitecan with the PARP inhibitor talazoparib.
Sacituzumab govitecan has also shown encouraging results in heavily pretreated HR-positive/HER2-negative metastatic breast cancer patients [Citation55]. Fifty-four patients with metastatic HR-positive/HER2-negative breast cancer who had received at least two lines of chemotherapy in the metastatic setting were evaluated in the Phase I/II basket trial (IMMU-132-01; NCT01631552). ORR was found to be 31.5% (seventeen patients) [95% CI 19.5%-45.6%]. Median PFS was 5.5 months [95% CI 3.6–7.6] and median OS was 12 months [95% CI 9.0–18.2][Citation55]. Given this, it is also prudent to further investigate sacituzumab govitecan in HR-positive/HER2-negative breast cancers. Two Phase III studies (TROPiCS-02; NCT03901339, NCT04639986) are comparing the use of sacituzumab govitecan to standard-of-care regimens in patients with metastatic HR-positive/HER2-negative breast cancers. One Phase III trial (NCT04595565) is evaluating sacituzumab govitecan in HER2-negative breast cancer with residual disease after neoadjuvant chemotherapy. A Phase II study (NCT04448886) is also investigating sacituzumab govitecan as single agent or in combination with pembrolizumab in metastatic HR-positive/HER2-negative breast cancer.
In terms of adverse effects, treatment with sacituzumab govitecan is manageable and comparable to other standard-of-care therapies. Neutropenia, leukopenia, and diarrhea were the most common grade 3 and 4 adverse effects seen in the Phase III trial [Citation45]. Neutropenia was often handled with dose reduction and/or delay with growth-factor support which is also common in standard-of-care chemotherapies. Only 10% of patients had diarrhea that was a grade 3 toxicity and none had grade 4 toxicity. This is significantly lower than the 31% of patients who had a grade 3 or 4 toxicity when treated with irinotecan in monotherapy [Citation56]. Sacituzumab govitecan also scored two points for the treatment of adult patients with mTNBC who have received at least two prior therapies for metastatic disease according to the ESMO-Magnitude of Clinical Benefit Scale (MCBS). Given this, quality of life studies of patients receiving sacituzumab govitecan are still needed.
Access and cost may still represent barriers to some patients wishing to obtain sacituzumab govitecan. Sacituzumab govitecan is estimated to cost $11,195 a dose for an 80-kilogram person (this price does not include pre-medications, infusion chair time, nursing time, or other components related to care). This cost is in part due the complicated production of sacituzumab govitecan, which requires expensive resources [Citation57]. All major insurances will cover the cost of the infusion if the patient has been on two previous therapies with at least one in the metastatic setting. To help with cost, the manufacturer has reimbursement support services which offer guidance on issues ranging from coverage verification to alternate assistance options, and more [Citation58]. In addition, there are other support services such as The Gilead Patient Assistance Program that is designed for patients who are uninsured or underinsured and the Sacituzumab Govitecan Savings Program which assists with the cost of the medication [Citation58,Citation59].
Beyond sacituzumab govitecan, other novel ADCs targeting Trop-2 are being investigated. RN927C (or PF-06664178) is an ADC, also targeting Trop-2, made of a humanized antibody linked to auristatin microtubule inhibitor, PF-0638010 [Citation60]. When tested in pancreatic, ovarian, lung, and TNBC xenograft tumors, regression was noted [Citation60]. When evaluated in a Phase I dose-escalation study, RN927C was found to result in stable disease [Citation61]. However, at higher dose levels, RN927C demonstrated numerable toxicities [Citation61]. Other ADCs targeting Trop-2 include BAT8003 and DS-1062a [Citation29]. BAT8003 is conjugated via novel uncleavable linker to maytansine (a microtubule inhibitor) and DS-1062a is bound via cleavable peptide linker to DXd, a topoisomerase-I inhibitor [Citation62,Citation63]. BAT8003 was evaluated in patients with advanced epithelial cancer in a Phase I clinical trial (NCT03884517), though the outcomes of that study are not yet reported. In an ongoing Phase I trial TROPION-PanTumor01 (NCT03401385), evaluating DS-1062a in patients with advanced solid tumors, DS-1062a has shown stable disease and partial responses in several patients [Citation64]. DS-1062a is currently undergoing evaluation in patients with advanced or metastatic non-small cell lung cancer with actionable genomic alterations in the Phase II study TROPION-Lung05 (NCT04484142).
Overall, sacituzumab govitecan showed durable clinical benefit in mTNBC in Phase I/II and Phase III clinical trials. Though this therapy is not without side-effects, they are not beyond what would be expected in other standard-of-care therapies. The use of this ADC is currently being explored in HR-positive/HER2-negative breast cancers and has the potential to benefit other tumor types, including lung and urothelial malignancies. In five-years it is possible this therapy may be approved in cancers beyond TNBC. It is also likely that benefit will have been derived from sacituzumab govitecan in combination with immunotherapy agents and PARP inhibitors. It is anticipated that further targeted therapies will emerge, which also may prove to be fruitful in combination with sacituzumab govitecan.
Article highlights
Triple-negative breast cancer (TNBC) is a sub-type of breast cancer which lacks the estrogen receptor (ER), progesterone receptor (PR), and overexpression of the human epidermal growth factor 2 (HER2). Given lack of targets, TNBC is difficult to treat and overall survival (OS) remains poor.
Targeted therapies are now available for patients with metastatic TNBC (mTNBC) with expression of programmed death-ligand 1 (PD-L1) and BRCA mutations. However, the majority of patients with mTNBC are PD-L1-negative and/or BRCA wildtype. Resistance is also common with these new targeted therapies.
Sacituzumab govitecan is an antibody drug conjugate (ADC) which targets trophoblastic cell surface antigen 2 (Trop-2). Sacituzumab govitecan is comprised of SN-38, an active metabolite of irinotecan, a hydrolysable linker, and a humanized monoclonal antibody.
Trop-2 is highly expressed on the surface of TNBC cells. As such, sacituzumab govitecan was widely studied in mTNBC.
The Phase I/II clinical trial evaluating the use of sacituzumab govitecan in patients with previously treated advanced epithelial cancers, demonstrated benefit in OS and progression free survival (PFS) in patients with mTNBC when compared to historical controls. These positive outcomes led to the accelerated approval for the use of sacituzumab govitecan in pre-treated mTNBC by the Food and Drug Administration (FDA).
The Phase III ASCENT trial confirmed the benefit of sacituzumab govitecan, in patients without brain metastasis, after at least two prior treatments, one which could be in the neoadjuvant or adjuvant setting with a superior OS and PFS when compared to physician’s choice standard-of-care chemotherapies. The results of this trial led to regular approval by the FDA.
Sacituzumab govitecan is FDA approved for patients with locally advanced or mTNBC who have received two or more prior therapies with at least one in the metastatic setting.
The most common adverse events observed with sacituzumab govitecan were neutropenia, diarrhea, and nausea. Many patients had adverse events but most improved with supportive care.
Sacituzumab govitecan has become standard-of-care for patients with mTNBC who have received prior chemotherapy.
Sacituzumab govitecan has shown encouraging results in hormone receptor positive breast cancers and across other epithelial cancers. In addition, clinical trials are underway to evaluate sacituzumab govitecan in combination with immunotherapy and poly (ADP-ribose) polymerase (PARP) inhibitors.
Declaration of interest
The author(s) have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Information resources
For further reading, please visit clinicaltrial.gov to read more about current clinical trials investigating sacituzumab govitecan. In addition, visit PubMed and search TROPiCS-02 to read about an on-going clinical trial evaluating sacituzumab govitecan in HR-positive/HER2-negative metastatic breast cancer.
Additional information
Funding
References
- S S. Triple-negative breast cancer: metastatic risk and role of platinum agents. ASCO Clinical Science Symposium 2008 Chicago, IL.
- Trivers KF, Lund MJ, Porter PL, et al. The epidemiology of triple-negative breast cancer, including race. Cancer Causes Control. 2009 Sep;20(7):1071–1082.
- Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res off J Am Assoc Cancer Res. 2007 Aug 1;13(15 Pt 1):4429–4434.
- Vagia E, Mahalingam D, Cristofanilli M. The landscape of targeted therapies in TNBC. Cancers (Basel). 2020 Apr 8;12(4):916.
- Cortes J, Cescon DW, Rugo HS, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020 Dec 5;396(10265):1817–1828.
- H.S. Rugo, J., Cortés,D.W., Cescon . KEYNOTE-355: final results from a randomized, double-blind phase 3 study of first-line pembrolizumab + chemotherapy vs placebo + chemotherapy for metastatic TNBC. ESMO Congress 2021, Abstract LBA16. Annals of Oncology (2021) 32 (suppl_5): S1283-S1346. https://doi.org/10.1016/annonc/annonc741.
- Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018 Nov 29;379(22):2108–2121.
- Miles D, Gligorov J, André F, et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann Oncol. 2021 Aug;32(8):994–1004.
- Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018 Aug 23;379(8):753–763.
- Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017 Aug 10;377(6):523–533.
- Poggio F, Bruzzone M, Ceppi M, et al. Single-agent PARP inhibitors for the treatment of patients with BRCA-mutated HER2-negative metastatic breast cancer: a systematic review and meta-analysis. ESMO Open. 2018;3(4):e000361.
- Robson ME, Tung N, Conte P, et al. OlympiAD final overall survival and tolerability results: olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol. 2019 Apr 1;30(4):558–566.
- Litton JK, Hurvitz SA, Mina LA, et al. Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: final overall survival results from the EMBRACA trial. Ann Oncol. 2020 Nov;31(11):1526–1535.
- Lehmann BD, Pietenpol JA, Tan AR. Triple-negative breast cancer: molecular subtypes and new targets for therapy. Am Soc Clin Oncol Educ Book. 2015;35:e31–9. DOI:https://doi.org/10.14694/EdBook_AM.2015.35.e31
- Weiss J, Glode A, Messersmith WA, et al. Sacituzumab govitecan: breakthrough targeted therapy for triple-negative breast cancer. Expert Rev Anticancer Ther. 2019 Aug;19(8):673–679.
- Yin L, Duan JJ, Bian XW, et al. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020 Jun 9;22(1):61.
- Khosravi-Shahi P, Cabezón-Gutiérrez L, Custodio-Cabello S. Metastatic triple negative breast cancer: optimizing treatment options, new and emerging targeted therapies. Asia Pac J Clin Oncol. 2018 Feb;14(1):32–39.
- Thomas A, Teicher BA, Hassan R. Antibody-drug conjugates for cancer therapy. Lancet Oncol. 2016 Jun;17(6):e254–e262.
- Beck A, Goetsch L, Dumontet C, et al. Strategies and challenges for the next generation of antibody-drug conjugates. Nat Rev Drug Discov. 2017 May;16(5):315–337.
- Chau CH, Steeg PS, Figg WD. Antibody-drug conjugates for cancer. Lancet. 2019 Elsevier Ltd;394(10200):793–804.
- FDA approves moxetumomab pasudotox-tdfk for hairy cell leukemia. US Food & Drug Administration. Accessed 2021 Sept 1. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-moxetumomab-pasudotox-tdfk-hairy-cell-leukemia
- FDA grants regular approval to enfortumab vedotin-ejfv for locally advanced or metastatic urothelial cancer. US Food & Drug Administration. Accessed 2021 Aug 1. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-enfortumab-vedotin-ejfv-locally-advanced-or-metastatic-urothelial-cancer
- FDA granted accelerated approval to belantamab mafodotin-blmf for multiple myeloma. US Food & Drug Administration. Accessed 2021 Aug 1. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-granted-accelerated-approval-belantamab-mafodotin-blmf-multiple-myeloma
- FDA grants accelerated approval to loncastuximab tesirine-lpyl for large B-cell lymphoma. US Food & Drug Administration. Accessed 2021 Aug 1. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-loncastuximab-tesirine-lpyl-large-b-cell-lymphoma
- Syed YY. Sacituzumab govitecan: first approval. Drugs. 2020 Jul;80(10):1019–1025.
- Ramesh M, Ahlawat P, Srinivas NR. Irinotecan and its active metabolite, SN-38: review of bioanalytical methods and recent update from clinical pharmacology perspectives. Biomed Chromatogr. 2010 Jan;24(1):104–123.
- Gokduman K. Strategies targeting DNA topoisomerase I in cancer chemotherapy: camptothecins, nanocarriers for camptothecins, organic non-camptothecin compounds and metal complexes. Curr Drug Targets. 2016;17(16):1928–1939.
- Cardillo TM, Govindan SV, Sharkey RM, et al. Humanized anti-Trop-2 IgG-SN-38 conjugate for effective treatment of diverse epithelial cancers: preclinical studies in human cancer xenograft models and monkeys. Clin Cancer Res off J Am Assoc Cancer Res. 2011 May 15;17(10):3157–3169.
- Goldenberg DM, Sharkey RM. Antibody-drug conjugates targeting TROP-2 and incorporating SN-38: a case study of anti-TROP-2 sacituzumab govitecan. MAbs. 2019 Aug/Sep;11(6):987–995.
- Goldenberg DM, Cardillo TM, Govindan SV, et al. Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC). Oncotarget. 2015 Sep 8;6(26):22496–22512.
- Sharkey RM, McBride WJ, Cardillo TM, et al. Enhanced delivery of SN-38 to human tumor xenografts with an anti-Trop-2-SN-38 antibody conjugate (sacituzumab govitecan). Clin Cancer Res off J Am Assoc Cancer Res. 2015 Nov 15;21(22):5131–5138.
- Cardillo TM, Govindan SV, Sharkey RM, et al. Sacituzumab govitecan (IMMU-132), an anti-Trop-2/SN-38 antibody-drug conjugate: characterization and efficacy in pancreatic, gastric, and other cancers. Bioconjug Chem. 2015 May 20;26(5):919–931.
- Bardia A, Mayer IA, Vahdat LT, et al. Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med. 2019 Feb 21;380(8):741–751.
- Goldenberg DM, Stein R, Sharkey RM. The emergence of trophoblast cell-surface antigen 2 (TROP-2) as a novel cancer target. Oncotarget. 2018 Jun 22;9(48):28989–29006.
- Starodub AN, Ocean AJ, Shah MA, et al. First-in-human trial of a novel anti-Trop-2 antibody-SN-38 conjugate, sacituzumab govitecan, for the treatment of diverse metastatic solid tumors. Clin Cancer Res off J Am Assoc Cancer Res. 2015 Sep 1;21(17):3870–3878.
- Ocean AJ, Starodub AN, Bardia A, et al. Sacituzumab govitecan (IMMU-132), an anti-Trop-2-SN-38 antibody-drug conjugate for the treatment of diverse epithelial cancers: safety and pharmacokinetics. Cancer. 2017 Oct 1;123(19):3843–3854.
- Xie R, Mathijssen RH, Sparreboom A, et al. Clinical pharmacokinetics of irinotecan and its metabolites in relation with diarrhea. Clin Pharmacol Ther. 2002 Sep;72(3):265–275.
- Kweekel D, Guchelaar HJ, Gelderblom H. Clinical and pharmacogenetic factors associated with irinotecan toxicity. Cancer Treat Rev. 2008 Nov;34(7):656–669.
- Bardia A, Messersmith WA, Kio EA, et al. Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann Oncol. 2021 Jun;32(6):746–756.
- Immunomedics I. TRODELVY (sacituzumab govitecan-hziy) [package insert]. US Food and Drug Administration Website. Revised April, 2020. Accessed Aug, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761115s000lbl.pdf
- Nangia J, Wang T, Osborne C, et al. Effect of a scalp cooling device on Alopecia in women undergoing chemotherapy for breast cancer: the SCALP randomized clinical trial. Jama. 2017 Feb 14;317(6):596–605.
- Rugo HS, Klein P, Melin SA, et al. Association between use of a scalp cooling device and alopecia after chemotherapy for breast cancer. Jama. 2017 Feb 14;317(6):606–614.
- Bardia A, Mayer IA, Diamond JR, et al. Efficacy and safety of anti-Trop-2 antibody drug conjugate sacituzumab govitecan (IMMU-132) in heavily pretreated patients with metastatic triple-negative breast cancer. J Clin Oncol. 2017 Jul 1;35(19):2141–2148.
- Wahby S, Fashoyin-Aje L, Osgood CL, et al. FDA approval summary: accelerated approval of sacituzumab Govitecan-hziy for third-line treatment of metastatic triple-negative breast cancer. Clin Cancer Res off J Am Assoc Cancer Res. 2021 Apr 1;27(7):1850–1854.
- Bardia A, Hurvitz SA, Tolaney SM, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021 Apr 22;384(16):1529–1541.
- FDA grants regular approval to sacituzumab govitecan for triple-negative breast cancer. US Food and Drug Administration. Accessed 2021 Aug 3. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-sacituzumab-govitecan-triple-negative-breast-cancer
- Diéras V, Weaver R, Tolaney SM, et al. Abstract PD13-07: subgroup analysis of patients with brain metastases from the phase 3 ASCENT study of sacituzumab govitecan versus chemotherapy in metastatic triple-negative breast cancer. Cancer Res. 2021;81(4Supplement): PD13-07.
- Gray JE, Heist RS, Starodub AN, et al. Therapy of small cell lung cancer (SCLC) with a topoisomerase-I-inhibiting antibody-drug conjugate (ADC) targeting Trop-2, sacituzumab govitecan. Clin Cancer Res off J Am Assoc Cancer Res. 2017 Oct 1;23(19):5711–5719.
- Heist RS, Guarino MJ, Masters G, et al. Therapy of advanced non-small-cell lung cancer with an SN-38-anti-Trop-2 drug conjugate, sacituzumab govitecan. J Clin Oncol. 2017 Aug 20;35(24):2790–2797.
- Tagawa ST, Balar AV, Petrylak DP, et al. TROPHY-U-01: a phase II open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors. J Clin Oncol. 2021 Apr 30:Jco2003489. DOI:https://doi.org/10.1200/jco.20.03489.
- FDA grants accelerated approval to sacituzumab govitecan for advanced urothelial cancer. U.S. Food & Drug Administration. Accessed 2021 Jul 22. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-sacituzumab-govitecan-advanced-urothelial-cancer
- Bardia A, Tolaney SM, Punie K, et al. Biomarker analyses in the phase III ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Ann Oncol. 2021 Sep;32(9):1148–1156.
- Han Y, Yu X, Li S, et al. New perspectives for resistance to PARP inhibitors in triple-negative breast cancer. Front Oncol. 2020;10:578095.
- Kassam F, Enright K, Dent R, et al. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clin Breast Cancer. 2009 Feb;9(1):29–33.
- Kalinsky K, Diamond JR, Vahdat LT, et al. Sacituzumab govitecan in previously treated hormone receptor-positive/HER2-negative metastatic breast cancer: final results from a phase I/II, single-arm, basket trial. Ann Oncol. 2020 Dec;31(12):1709–1718.
- Pfizer. CAMPTOSAR- irinotecan hydrochloride injection, solution [prescribing information, package insert]. Food and Drug Administration Website. Revised December, 2014. Accessed Jul, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/020571s048lbl.pdf
- Bednova O, Leyton JV. Targeted molecular therapeutics for bladder cancer—a new option beyond the mixed fortunes of immune checkpoint inhibitors? Int J Mol Sci. 2020 Oct 1;21(19):7268.
- TRODELVY ACCESS SUPPORT. Gilead Sciences, Inc. Accessed 2021 Oct 7. https://trodelvy.com/patient/mTNBC/access-support
- Financial support for the uninsured—multiple options available for enrollment. Gilead Sciences, Inc. Accessed 2021 Oct 7. https://www.gileadadvancingaccess.com/financial-support/uninsured
- Strop P, Tran TT, Dorywalska M, et al. RN927C, a site-specific trop-2 antibody-drug conjugate (ADC) with enhanced stability, is highly efficacious in preclinical solid tumor models. Mol Cancer Ther. 2016 Nov;15(11):2698–2708.
- King GT, Eaton KD, Beagle BR, et al. A phase 1, dose-escalation study of PF-06664178, an anti-Trop-2/Aur0101 antibody-drug conjugate in patients with advanced or metastatic solid tumors. Invest New Drugs. 2018 Jan 15;36(5):836–847.
- Tang W, Huang X, Ou Z, et al. Abstract P6-20-16: BAT8003, a potent anti-Trop-2 antibody-drug conjugate, for the treatment of triple negative breast cancer. Cancer Res. 2019;79(4Supplement): P6-20-16.
- Okajima D, Yasuda S, Yokouchi Y, et al. Preclinical efficacy studies of DS-1062a, a novel TROP2-targeting antibody-drug conjugate with a novel DNA topoisomerase I inhibitor DXd. J clin oncol. 2018;36(15_suppl):e24206–e24206.
- Sands JM, Shimizu T, Garon EB, et al. First-in-human phase 1 study of DS-1062a in patients with advanced solid tumors. J clin oncol. 2019;37(15_suppl):9051.