ABSTRACT
Introduction
Androgen-deprivation therapy (ADT) is the main therapy for patients with advanced and metastatic prostate cancer (PCa) and, in combination with radiotherapy, for patients with localized high-risk PCa. Due to its favorable tolerability among different treatments available for ADT, leuprorelin acetate is well established as the leading luteinizing hormone-releasing hormone (LHRH) analog. The development of second-generation leuprorelin acetate (LA) depot formulation (Eligard®, Recordati S.p.A) allowed a consistent and controlled release of leuprorelin between injections and a more efficient reduction of testosterone levels with respect to conventional LHRH agonists.
Areas covered
This work provides a summary of the biological and clinical rationale for using LA to manage PCa and presents the current evidence about the therapeutic activity of the LA gel depot formulation, used as an advanced leuprorelin acetate delivery method.
Expert opinion
Results of the registration studies and post-marketing clinical trials demonstrate that the LA gel depot provides long-term efficacy in the clinical practice and a good degree of tolerability. Overall, collected data suggest that the LA gel depot can represent the ADT reference therapy in advanced PCa.
1. Introduction
Prostate cancer (PCa) represents the most frequent type of cancer in Europe. In 2020, around 470,000 new cases were estimated (20% of all new cancers) and represented the third cause of death for cancer [Citation1,Citation2]. For instance, projections of PCa incidence from 2020 to 2030 estimate an increase in the number of new cases from 473,000 to 553,000 [Citation3].
Initially, the androgen receptor (AR) signaling triggers the growth and the proliferation of prostate tumor cells. Since testosterone is a key player in the AR signaling pathway [Citation4,Citation5], the androgen-deprivation therapy (ADT), aimed at reducing serum testosterone levels to those reached after surgical castration (<20 ng/dL), is now the backbone of therapy for patients with advanced and metastatic PCa. When combined with radiotherapy (RT), ADT also represented the standard of care for localized high-risk PCa, based on the improvements observed in different randomized trials [Citation6–10].
Aside from surgical castration, three classes of hormonal treatments are available for ADT: luteinizing hormone-releasing hormone (LHRH) antagonists, LHRH agonists, and anti-androgens [Citation11–13]. In addition, new compounds that target the AR signaling pathway have also been recently developed [Citation14,Citation15].
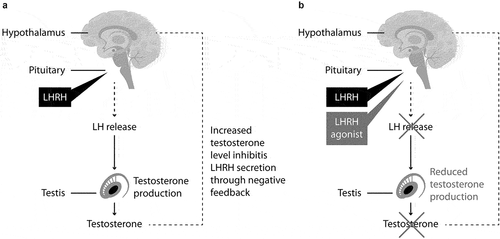
The clinical benefits of treating PCa with LHRH agonists were reported in 1982, and long-acting formulations of LHRH agonists are currently the main form of ADT used in clinical practice [Citation16]. After the injection, long-acting LHRH agonists reduce testosterone levels providing constant overstimulation of the pituitary gland, leading to inhibition of LH release and consequently to inhibition of testosterone production () [Citation16]. Their effectiveness is higher compared to antiandrogen monotherapy [Citation17–20]. With regard to LHRH antagonists, there is a lack of significant long-term data or survival evidence directly comparing the two treatment regimens. Consequently, it is impossible to define one treatment’s superiority over the other in terms of effectiveness and PCa control [Citation2]. Nevertheless, the use of LHRH agonists in clinical practice is easier compared to degarelix – the only LHRH antagonist approved in Europe for the treatment of advanced PCa. Indeed, degarelix requires the subcutaneous administration of large volumes that frequently causes adverse reactions at the injection site, limiting its use. For instance, a post-hoc meta-analysis suggested a reduction in ADT-related cardiovascular events in the subgroup of patients with preexisting cardiovascular morbidity treated with degarelix, compared to the use of LHRH agonist [Citation21]. Nevertheless, more recent studies suggested no evident difference regarding cardiovascular morbidity when comparing LHRH agonists versus antagonists [Citation22,Citation23]. This finding is also supported by the first international, randomized clinical trial that prospectively compared the cardiovascular safety of LHRH agonists and antagonists in PCa patients. There was no difference in major adverse cardiovascular events at 1 year between the two patient groups [Citation24].
Figure 1. (A) Normal control of testosterone levels and (B) mechanism of action of LHRH agonists. LH: luteinizing hormone. LHRH: luteinizing hormone-releasing hormone.
In Europe, the main available LHRH agonists are leuprorelin, goserelin, buserelin and triptorelin acetate, all based on biodegradable microspheres or implants containing the active drug, which are injected subcutaneously or intramuscularly [Citation23,Citation25]. Histrelin acetate is also available in some European countries; nevertheless, it represents a minor option since it requires a surgical procedure for the insertion. Among these different options, leuprorelin acetate (leuprolide acetate) is well established as the leading LHRH analog due to its favorable tolerability and, today, it represents the LHRH analog most prescribed worldwide [Citation12,Citation26]. Leuprorelin acetate is available as a 1-, 3-, 6- and 12-month preparation, marketed under different brand names worldwide [Citation23,Citation25]. Among them, the development of second-generation leuprorelin acetate (LA) depot formulation (Eligard®, Recordati S.p.A) allowed a consistent and controlled release of leuprorelin between injections and a more efficient reduction of testosterone levels with respect to conventional LHRH agonists [Citation27].
The LA gel depot exploits the Atrigel® delivery system (hereafter termed atrigel) to release leuprorelin in a constant way over a wide range of time periods [Citation28], thus resulting in profoundly suppressed testosterone levels with minimal ‘breakthroughs’ (intended as the experience of testosterone surges >50 ng/dL during long-term treatment) than conventional LHRH agonists, as demonstrated in clinical trials [Citation26,Citation29–31].
This review aims to present an overview of the biological and clinical rationale for the use of leuprorelin acetate to manage PCa and to provide a comprehensive and exhaustive global revision of the current literature evidence, including the discussion of pre- and post-marketing clinical trials, about the therapeutic activity of the LA gel depot formulation, used as an advanced leuprorelin acetate delivery method.
2. Leuprorelin acetate for the treatment of PCa: biological and clinical rationale
Leuprorelin acetate was the first LHRH agonist to be synthesized and used for the treatment of PCa for over 30 years; its convenience and flexibility of administration are constantly improving [Citation32].
Leuprorelin acetate is a synthetic analog of the gonadotropin-releasing hormone (GnRH). Different studies have shown that its potency in LHRH receptor stimulation is greater than that of the natural hormone [Citation12,Citation33].
Since the development of leuprorelin acetate, its efficacy and tolerability in advanced PCa have been extensively confirmed [Citation34,Citation35]. These studies show that leuprorelin acetate consistently lowers testosterone to ≤50 ng/dL, delays tumor progression and alleviates symptoms, such as bone pain [Citation36,Citation37].
In addition, leuprorelin acetate is well tolerated. Most of the adverse events registered in clinical studies were mild to moderate, and a low withdrawal rate was reported in studies [Citation36,Citation37].
3. Advanced delivery of leuprorelin acetate
The LA gel depot formulation was the first long-acting leuprorelin acetate available in Europe to treat hormone-dependent advanced PCa and, in combination with RT, for the treatment of high-risk localized and locally advanced hormone-dependent PCa [Citation38].
As mentioned above, the delivery of LA within the LA gel depot formulation makes use of atrigel, which was originally developed with the capacity to release different agents, ranging from small molecules to peptides and proteins.
Atrigel comprises a biodegradable polymer of D,L-lactide-co-glycolic acid (PLGA) and a biocompatible carrier to form a liquid drug delivery system. This is then mixed with leuprorelin acetate to form an injectable solution. After the subcutaneous injection of the copolymer, the water penetrates the implant, and the organic solvent dissolves into the surrounding tissue so that the atrigel/leuprorelin acetate mixture forms a solid spherical implant in situ [Citation28,Citation39,Citation40].
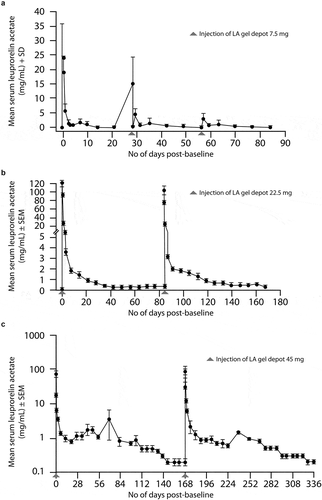
A different combination of copolymer molecular weight and solvent concentration determines a different release rate of leuprorelin acetate. Consequently, depending on the precise atrigel composition, the LA gel depot is available as a sustained-release formulation that delivers leuprorelin acetate continuously over 6 months (45 mg formulation) and is also available in 1- and 3-month formulations (7.5 mg and 22.5 mg formulations, respectively). The European Association of Urology recommendations suggest a monitoring frequency in line with these formulations [Citation2].
3.1. LA gel depot pharmacokinetic
After the LA gel depot treatment, a rise in mean serum leuprorelin acetate concentrations is observed due to burst kinetics of the delivery system, followed by a rapid decline as the atrigel depot is formed. The maximum serum concentration with the 45 mg formulation is 82 ng/mL, and ranges from 25 ng/mL with the 7.5 mg formulation to 127 ng/mL with the 22.5 mg formulation [Citation38]. The maximum serum concentration is reached within 4–8 hours after the treatment. Thereafter, serum leuprorelin acetate decreases rapidly to a constant level of 0.2–2 ng/mL (). Importantly, the leuprorelin acetate peak size does not increase with repeated dosing, and no evidence suggests an accumulation of leuprorelin acetate after a long-lasting treatment [Citation29–31].
3.2. Therapeutic activity of the LA gel depot
Up-to-date evidence indicates that the mean testosterone level after surgical castration equals 15 ng/dL [Citation41]. Therefore, a proper threshold is <20 ng/dL [Citation2]. This definition is based on the better results observed with lower testosterone levels, compared to the previously defined threshold (50 ng/dL) [Citation39,Citation42,Citation43].
The LA gel depot provides testosterone suppression far below 20 ng/dL (12 ng/dL with the 6-month formulation, 6 ng/dL with the 1-month formulation) in most patients across all doses, including the longest duration available (97% of patients with the 1-month dose, 88% of patients with the 6-month dose). Of note, reduced testosterone levels were maintained for the entire duration of the therapy (<1% of testosterone breakthroughs).
3.2.1. Pivotal trials
The therapeutic activity of the LA gel depot was studied in three pivotal trials involving men candidates for ADT with a life expectancy of at least 1 year [Citation29–31].
Exclusion criteria for the pivotal studies included standard LHRH contraindications, such as the obstruction of the urinary tract or spinal cord injuries, as well as other prostate cancer therapies.
Each of the three pivotal registration studies was completed by >90% of the enrolled patients. The main features of pivotal studies are presented in .
Table 1. Mean features of three registration studies
Overall, all patients achieved a testosterone level ≤50 ng/dL within 42 days (1-month formulation) and 35 days (3-month formulation) of the first injection of the LA gel depot. After 6 months of treatment, 97% (1-month formulation) and 94% (3-month formulation) of patients achieved a testosterone level of <20 ng/dL [Citation29,Citation30]. In 99% of patients, testosterone suppression to <50 ng/dL was reached after 12 months of treatment; 88% of patients reached a testosterone level of <20 ng/dL with the 6-month formulation [Citation31].
The reduction of testosterone levels was maintained by all patients who completed the study; in these patients, the mean testosterone levels corresponded to 6.1 ng/dL (1-month formulation) and 10.1 ng/dL (3-month formulation) after 6 months [Citation29,Citation30].
In 99% of patients (102 out of 103), testosterone levels were below the castration levels (<20 ng/dL) after the LA gel depot 6-month formulation treatment (mean testosterone levels at study end: 12.3 ng/dL) [Citation31].
As mentioned above, during treatment, minimal testosterone breakthroughs (<1% of patients) were reported [Citation29–31]. This sustained and persistent testosterone suppression has a relevant clinical interest, considering that testosterone flares are often associated with morbidity [Citation44–46]. In particular, the maintenance of low testosterone levels during ADT was associated with a reduced risk of death (HR = 0.48; 95% CI: 0.28–0.81; p = 0.006) and disease progression (HR = 0.59; 95% CI: 0.46–0.77; p < 0.0001) [Citation44,Citation47]. Moreover, the maintenance of low serum testosterone within the first year of ADT correlated with improved cause-specific survival and duration of therapy response [Citation43].
The utility of measuring serum levels of PSA for the PCa diagnosis and management was first confirmed in the late 1980s. PSA monitoring has become an important part of overall management [Citation48]. A mean decrease in PSA level of >90% was observed following 6 months of treatment with the three LA gel depot formulations [Citation29–31].
3.2.2. Post-marketing clinical trials and analyses
Several post-marketing clinical trials and post-analyses about the therapeutic activity of the LA gel depot have been carried out. Overall, these studies suggest that the treatment can provide consistent, stable and durable testosterone reduction to ≤20 ng/dL regardless of age and body weight, low nadir testosterone levels were achieved across all pivotal trials [Citation49–52].
The treatment with the LA gel depot shows a longer duration of LH suppression compared with intramuscular leuprorelin and microsphere-leuprorelin and a > 90% reduction in mean baseline PSA levels [Citation49,Citation53–55].
The LA gel depot treatment resulted in an effective reduction of testosterone levels in patients pre-treated with other LHRHs or following a switch from an alternative therapy [Citation55,Citation56]. summarizes the main features and results of these post-marketing studies, which are extensively presented in the subsequent paragraphs.
Table 2. Post-marketing clinical trials and analyses
3.2.2.1. Effectiveness and safety of the 6-month LA gel depot formulation for the treatment of advanced PCa in routine clinical practice [Citation56]
This prospective observational study confirmed the effectiveness and safety of the 6-month LA gel depot formulation in routine urological practice. A total of 1,273 PCa patients were involved and observed for 1 year.
Almost half of the subjects were naïve to the LA gel depot treatment. The other patients were previously treated with a different LA gel depot formulation (one-third of patients) or another GnRH analog (two-thirds of patients). GnRH analog was mainly administered as monotherapy; an adjunctive therapy was prescribed in 35% of cases, mostly ADT (79%).
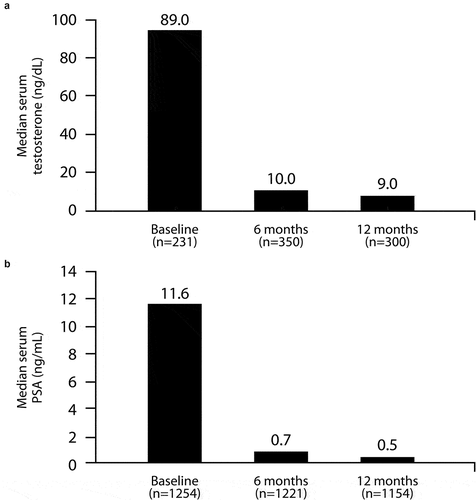
The median serum testosterone levels decreased from 89 ng/dL (baseline value) to 10 ng/dL in the first 6 months of treatment. A further decrease to 9 ng/dL was observed at the end of the observational period, corresponding to a reduction of 90% (). The median PSA values decreased from 11 ng/mL to 0.7 ng/mL in the first 6 months (94% of reduction) and to 0.5 ng/mL after 12 months (96% decrease, ).
Figure 3. Median serum concentrations of testosterone (A) and PSA (B) during treatment with the 6-month LA gel depot.
PSA and testosterone concentrations were also assessed in two additional subpopulations, treated with the 6-month formulation of the LA gel depot as monotherapy. The first was composed of patients who switched to the LA gel depot from another leuprorelin acetate product (n = 99); the second comprised patients who switched from goserelin acetate (n = 57). During the first 6 months, in the first subpopulation, the median serum testosterone levels decreased from 150 ng/dL to 17 ng/dL (88% of reduction), and the median PSA value decreased from 6 ng/mL to 0.4 ng/mL (93% of decrease). In the second subpopulation, the median serum testosterone levels decreased from 72 ng/dL to 18 ng/dL (75% of decrease). A 96% decrease in the median PSA value (from 8 ng/mL to 0.3 ng/mL) was also reported in the same population.
3.2.2.2. Effectiveness and tolerability of 1- and 3-month LA gel depot formulations for treating advanced PCa in routine clinical practice [Citation49]
This prospective observational study aimed to collect data on the effectiveness and tolerability of the 1- and 3-month LA gel depot formulations in routine clinical practice in Belgium (MANTA study – Monitoring tolerance, safety and acceptance of (Depo-)EligArd® in a Non-inTerventional triAl). A total of 243 PCa were involved and observed for a minimum period of 3 months. At the end of the observational period, a 94% decrease in the median testosterone levels was reported (from 360 ng/dL to 20 ng/dL), along with a 95% reduction in median serum PSA levels (from 12 ng/ml to 0.6 ng/ml). A period of 132 days resulted in the median patients’ follow-up time.
3.2.2.3. Effectiveness of LA gel depot to achieve low nadir testosterone in PCa patients [Citation52]
Nadir serum testosterone was related to delay in disease progression and improved survival. However, although data on nadir testosterone levels have been reported with some therapies, no data have been published on nadir testosterone levels achieved with LHRH agonist monotherapy. This study assessed nadir testosterone levels considering the pivotal trials for LA gel depot. Secondary outcomes included urinary symptoms, serum LH, bone pain and World Health Organization (WHO) performance status. Results showed that the mean baseline serum testosterone concentrations ranged from 361 to 386 ng/dL. The overall mean proportion of time testosterone suppression was maintained below each target was 100% for testosterone ≤50 ng/dL, 94% to 99% for testosterone ≤20 ng/dL, and 66% to 85% for testosterone ≤10 ng/dL ().
Figure 4. The proportion of time with testosterone levels maintained ≤10, ≤20, or ≤50 ng/dL. T: testosterone. Source: Adapted from [Citation46].
![Figure 4. The proportion of time with testosterone levels maintained ≤10, ≤20, or ≤50 ng/dL. T: testosterone. Source: Adapted from [Citation46].](/cms/asset/3e93366a-7bae-43d0-9901-2f6af3fa2bf7/iery_a_2082947_f0004_b.gif)
LA gel depot treatment resulted in testosterone reduction to ≤20 ng/dL within 4 weeks and ≤10 ng/dL by 5 weeks. Most testosterone levels were maintained consistently low across all formulations.
3.2.2.4. Evaluation of the LA gel depot in achieving and maintaining castrate concentrations of testosterone in patients with advanced PCa [Citation50]
Four fixed-dose, open-label studies evaluated if the LA gel depot reduced serum testosterone levels to ≤20 ng/dL. A total of 348 patients with advanced PCa, naïve to ADT, treated with the three formulations of LA gel depot were considered: 120 patients with the 1-month formulation, 117 patients with the 3-month formulation and 111 patients with the 6-month formulation. All measures were assessed at baseline, after 2, 4 and 8 hours from the treatment, 1, 2, 3, 7 days after the treatment and then every week. Patients were treated for 24 weeks with the 1- and 3-month formulations and 48 weeks with the 6- month formulation. The mean serum testosterone levels and the number of patients who achieved ≤20 ng/dL were the primary aims of the study.
The mean testosterone levels at the end of the therapy were ≤20 ng/dL in each study and corresponded to 6 ng/dL, 10 ng/dL and 13 ng/dL, respectively, for 1-month, 3-month and 6-month formulations.
Testosterone suppression to ≤20 ng/dL was achieved within 6 weeks in most patients (90–96% according to the different formulations), and 90–97% of patients maintained this result up the end of the observation period.
3.2.2.5. Evaluation of the pharmacokinetics of the LA gel depot and the impact of age and body weight on testosterone reduction [Citation51]
In this analysis, the pharmacokinetics (PK) of the LA gel depot and the possible influence of age and body weight on testosterone suppression were evaluated through two different studies: a) evaluation of the PK of the 1-month formulation LA gel depot in PCa patients subjected to bilateral surgical orchiectomy, performed at least 2 months before the study; the study period was 8 weeks; b) evaluation of the PK/pharmacodynamic (PD) in patients with advanced PCa, stratified by age and body weight, from four pivotal trials.
In the first study, an initial fast absorption of leuprorelin acetate was observed between 2–6 hours. Leuprorelin acetate levels then decreased slowly over days 2–4. A slight increase in mean leuprorelin acetate serum levels was observed from day 4 to day 7 in six out of eight patients, followed by a plateau phase. Leuprorelin acetate was then reduced slowly over 2 weeks, as usual for LHRH agonists. PK results did not vary according to age and body weight in this population.
In the second analysis, patients were naïve for ADT. Patients received a subcutaneous treatment among four formulations: 1-month preparation for 24 weeks (n = 120); 3-month preparation for 24 weeks (n = 117); 4-month preparation for 32 weeks (n = 90; formulation not marketed in the EU); 6-month preparation for 48 weeks (n = 111).
Starting from the second week of treatment and up to the end of all the considered studies, the median serum leuprorelin acetate levels were maintained between 0.05 and 1 ng/mL. Serum leuprorelin acetate levels of patients in the 1-, 3-, and 6-month pivotal studies were reported in Figure S1.
In the pivotal trials, consistent and long-lasting drug delivery over 0.05 ng/mL was reached across all doses from week 6 to week 24, along with favorable effectiveness.
Age and weight did not affect the testosterone reduction, which was attained and maintained in all subgroups, including maximum weights (>120 kg) and lowest age (<60 years).
3.2.2.6. Comparison of the pharmacokinetic and pharmacodynamics of subcutaneous and intramuscular leuprorelin acetate formulations [Citation53]
This phase I, open-label study compared the PK and PD of the 1-month LA gel depot with the 7.5 mg intramuscular-LA (IM-LA) preparation. Thirty-two patients were involved and randomly divided between the two treatments.
PK was measured through leuprorelin acetate concentrations and PD through the serum LH and testosterone concentrations.
Initially, the release of leuprorelin acetate was higher after the IM-LA treatment (the maximum concentration was equal to 27 ± 4.9 versus 19 ± 8.0 ng/ml, respectively), with a shorter Tmax (1.0 ± 0.4 versus 2.1 ± 0.8 h). The leuprorelin acetate concentration was maintained for a longer period after the 1-month LA gel depot treatment (56 versus 42 days). The LA gel depot treatment showed a long-lasting LH suppression, with median levels remaining below 1.0 IU/l up to day 56 compared with IM-LA, where LH started to rise by day 35. Consequently, serum testosterone levels increased starting from day 42 in the IM-LA patients; levels of ≤50 ng/dL were maintained only in four patients. Most of the patients treated with the LA gel depot (13 out of 16) maintained serum testosterone levels ≤50 ng/dL for over 50 days.
3.2.2.7. Evaluation of late dosing on testosterone suppression with LA gel depot and LA microsphere treatment [Citation54]
Adherence to dosing schedules to maintain testosterone suppression and the constant evaluation of testosterone and PSA levels are important to avoid the therapy failure and the consequent decrease in survival and increased healthcare costs.
The achievement and maintenance of suppressed testosterone levels determine the success of ADT for advanced PCa. Consequently, physicians should ensure that drugs are properly administered accordingly to the literature data and regulatory approval. Considering the high nonadherence rates for LHRH agonist injections in clinical practice (more than 80% according to US clinical data), this study aimed to evaluate the prevalence and the effect of late dosing on testosterone suppression for LA gel depot and LA microsphere (LA-MS) formulations in PCa patients. In this study, two definitions of ‘month’ were considered: a ‘28-day’ definition, where late dosing is intended after day 28, 84, 112 or 168 and an ‘extended’ month definition, where late dosing is intended after day 32, 97, 128 and 194. The correlation between the frequency of late dosing and testosterone levels was also assessed.
A total of 2,038 patients received the LA gel depot, and 8,360 received the LA-MS.
For the 28-day month definition, 80% and 86% of LA gel depot and LA-MS injections, respectively, were late. For extended month definition, 27% of injections were late for both. Frequencies of late injections were similar across formulations. Overall, the LA-MS treatment demonstrated higher proportions of testosterone breakthroughs if compared to LA gel depot for late injections. For the 28-day month definition, 14% of LA-MS testosterone tests vs 10% of LA gel depot testosterone tests were above 50 ng/dL (OR 1.5, 95% CI: 1.2–1.9). For the extended month definition 25% (LA-MS) vs 18% (LA gel depot) of testosterone were above 50 ng/dL (OR 1.5, 95% CI: 1.1–2.0). Of LA-MS testosterone tests, 33% were above 20 ng/dL vs 25% for LA gel depot for the 28-day month definition (OR 1.5, 95% CI: 1.3–1.8), and rates were 44% for LA-MS vs 34% for LA gel depot for the extended month (OR 1.5, 95% CI: 1.2–1.9). LA-MS was 1.5 times more expected to present testosterone above 50/20 ng/dL if compared to the LA gel depot. Least square mean ± SD testosterone was 34 ± 3 ng/dL (LA gel depot) versus 46 ± 2 ng/dL (LA-MS) for 28-day month definition (p = 0.002), and 48 ± 6 ng/dL (LA gel depot) vs 76 ± 4 ng/dL (LA-MS) for the extended month definition (p = 0.0003).
3.2.2.8. Efficacy and tolerability of 3- or 6-month LA gel depot in daily practice in Germany [Citation55]
All formulations of the LA gel depot demonstrated their effectiveness in reducing PSA and testosterone levels and presented a good tolerability profile in different clinical studies. Nevertheless, a limitation of all clinical trials is the stringent exclusion and inclusion criteria. Thus ‘real-world’ data are lacking. This was a pooled analysis comprising two observational studies aimed to investigate the effectiveness and tolerability of the LA gel depot (3- and 6-month formulations) in a real-world setting, considering patients with advanced PCa.
Overall, 1,906 subjects were involved in this study. Patients were treated with the 3-month (n = 633) or 6-month (n = 1,273) formulation for 1 year.
Median serum PSA level reduced from 12.0 ng/mL (baseline values) to 0.5 ng/mL at the end of the treatment period. This reduction was observed in both naïve and pretreated subjects.
Patients were then stratified and analyzed according to their prior therapy (no prior ADT, leuprorelin acetate, goserelin acetate/buserelin acetate). Median PSA levels declined by 96% in all patient groups at the end of the treatment (Figure S2).
3.2.2.9. Evaluation of the activity and benefits of intermittent versus continuous androgen deprivation therapy [Citation57,Citation58]
The ICELAND study was a phase III, open-label, randomized study designed to broaden the knowledge about the activity and potential benefits of intermittent androgen deprivation (IAD) compared to continuous androgen deprivation (CAD) in patients with non-metastatic relapsing or locally advanced PCa. Overall, 933 European patients (PSA levels ≤1 ng/mL following a 6-month induction with the LA gel depot 3-month formulation plus bicalutamide 50 mg/day for 1 month) were involved and randomized to CAD (n = 361) or IAD (n = 340) with leuprorelin for 36 months. The two regimens were comparable in terms of efficacy, tolerability, and quality of life outcome.
This study was followed by a post-hoc analysis aimed at correlating the serum testosterone levels during the first year of CAD to survival and time to progression (n = 345 subjects). Three subgroups of patients were considered to stratify testosterone levels into a minimum (≤20 ng/dL), median (>20 to ≤50 ng/dL), and maximum (>50 ng/dL) during the first year of CAD.
Overall, 90%, 83%, and 74% of patients receiving CAD reached a minimum, median, and maximum serum testosterone levels, respectively (Figure S3).
The three subgroups showed a comparable time to cause-specific survival and time to PSA progression, probably due to the effectiveness of leuprorelin in lowering testosterone levels.
3.3. Safety of LA gel depot
In the three pivotal clinical trials related to the LA gel depot, most adverse events were mild to moderate, and the most reported were hot flushes () [Citation29–31].
Table 3. Most reported LA gel depot treatment-related adverse events*
No reports of severe hot flushes were reported with the 6-month formulation. Only one incidence of severe hot flushes was reported in the pivotal clinical trials in a patient receiving the 7.5 mg dose [Citation25]. Notably, the three formulations presented a similar frequency or severity of adverse events. Treatment-related adverse events were noted as a cause of treatment discontinuation; vital signs remained stable during the treatment in any of the three studies [Citation29–31].
Injection site-related adverse events were typical of those observed with other preparations administered subcutaneously. The severity or duration of injection site-related events did not increase after subsequent LA gel depot injections, suggesting the tolerability of the long-term treatment.
During the three pivotal studies, symptoms or adverse events were not increased immediately after injection, and no changes in performance, pain or symptom scores in the immediate post-injection period [Citation29–31].
LHRH agonists have been associated with ‘tumor flare’ due to the transient increase in serum testosterone levels that occurs immediately after injection [Citation59,Citation60]. To prevent this, patients naïve to LHRH agonists may be prescribed a concomitant anti-androgen to block the effects of the increased testosterone level [Citation61].
As a result, monotherapy with the LA gel depot (and other LHRH agonists) is contraindicated in patients with spinal metastases or spinal cord injury. In addition, the treatment with the LA gel depot is also not indicated in pediatric patients and women, in patients with hypersensitivity to leuprorelin or to other GnRH and in patients who previously underwent orchidectomy.
4. Expert opinion
The ADT is the main therapy for patients with advanced and metastatic PCa. The hormonal treatment of PCa was transformed in the 1980s with the availability of synthetic LHRH agonists, and, among them, leuprorelin acetate is well established as the leading LHRH analog. The key challenges for a perfect ADT are a sustained release of the drug between two injections, a sufficient and rapid suppression of the serum testosterone level and the reduction of testosterone breakthroughs. The long-acting formulations of LHRH agonists have proven to meet these needs and, consequently, gained increasing acceptance from both physicians and patients. In particular, the LA gel depot represents an advanced delivery method. Indeed, the atrigel formulation of the LA gel depot was developed to ensure the controlled and sustained release of leuprorelin between injections, and clinical trials reported profoundly suppressed testosterone levels with minimal breakthroughs. Compared to conventional LHRH agonists, it has been reported that failure to reach testosterone levels of <50 ng/dL was by 2% to 17%, and <20 ng/dL by 13% to 37% of patients [Citation2,Citation12,Citation62]. However, analyses have shown that testosterone levels were lowered to <20 ng/dL in the 97% and 94% of patients treated with the LA gel depot 1- and 3-month formulation [Citation29,Citation30] and in the 88% of patients treated with the 6-month formulation [Citation27]. In addition, the LA gel depot offers a long-term experience and proven efficacy in clinical practice, with a safety profile consistent with the effects of testosterone suppression [Citation29–31]. Compared with the other formulations, the 6-month LA gel depot is associated with half the risk for patients to lose therapeutic effect in case of delayed injection, thus improving the compliance to therapy and the patients’ quality of life [Citation12].
Notably, studies have shown that the complexity of treatment regimens and the frequency of dosing can substantially impact patients’ compliance with treatment, and long-acting formulations make an important contribution to compliance and treatment outcomes in a number of therapeutic areas [Citation59]. Consequently, the availability of the 6-month formulation of the LA gel depot provides patients with a choice of the dosing interval. Perceived advantages of 6-month formulations included convenience-related aspects, improved quality of life and fewer injections [Citation63].
Even if further research is necessary to continuously improve the pharmacokinetics and pharmacodynamics of ADT substances, available evidence about the LA depot formulation suggests that this can be considered a reference ADT in advanced or metastatic PCa.
Within recent years, multiple phase III studies demonstrated that the clinical outcome for patients with metastatic hormone-sensitive PCa improved with the addition of androgen receptor-axis-targeted therapies (ARAT) or docetaxel to standard ADT [Citation64–69]. Within these studies, different types of LHRH agonists or antagonists were used in combination with ARATs or chemotherapy. The main goal is the sufficient suppression of the testosterone level, which needs to be checked regularly. However, the availability of an efficient ADT is of utmost importance as a reliable backbone of systemic therapy in metastatic hormone-sensitive and castration-resistant PCa.
5. Conclusion
ADT is the backbone therapy of advanced PCa. Among different options, long-acting LHRH agonists represent the gold standard and the most used worldwide form of ADT [Citation12,Citation21]. The LA gel depot represents a second-generation LA formulation developed to ensure a controlled and sustained release of leuprorelin between injections and to reach lower castrate testosterone levels compared to other LHRH agonists. Results from the registration and post-marketing studies suggested that the LA gel depot can provide long-term effectiveness in clinical trials and real-world practice, along with a good degree of tolerability, consistent with the effects of testosterone suppression. Taken together, collected data suggest that the LA gel depot can be considered the ADT of choice in advanced PCa.
Article highlights
Androgen-deprivation therapy (ADT) is the main therapy for patients with advanced and metastatic prostate cancer (PCa) and, in combination with radiotherapy, for patients with localized high-risk PCa.
Due to its favorable tolerability, leuprorelin acetate (LA) is well established as the leading luteinizing hormone-releasing hormone (LHRH) analog.
A second-generation LA depot formulation (Eligard®, Recordati S.p.A) has been developed to ensure a controlled and sustained release of leuprorelin between injections and to reduce testosterone levels more efficiently compared to conventional LHRH agonists.
The LA gel depot provides long-term efficacy in clinical practice and a good degree of tolerability.
The LA gel depot can be considered the reference ADT in advanced PCa.
Declaration of interest
AS Merseburger has received fees for lectures/speaker/honoraria from: AstraZeneca, Bristol-Myers Squibb, Eisai, Ferring, Ipsen, MSD, Merck Serono, Janssen, Takeda, TEVA, Astellas, Novartis, Pfizer, Recordati and Roche, has been a consultant for: AstraZeneca, Astellas, Bristol-Myers Squibb, Ferring, Ipsen, Janssen, EUSAPharm, MSD, Merck Serono, Novartis, Takeda, Teva, Pfizer, Recordati and Roche, and has served in research and clinical trials for: AstraZeneca, Astellas, Bristol-Myers Squibb, Ipsen, Janssen, EUSAPharm, MSD, Merck Serono, Novartis, Takeda, Teva, Pfizer and Roche.
MC Roesch has received fees for lectures/speaker/honoraria from Sanofi and Amgen, has been a consultant for Novartis, Ipsen, AstraZeneca, Bayer, and has received travel grants from Ipsen, Amgen and Photocure.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (242.6 KB)Acknowledgments
Editorial assistance was provided by Simonetta Papa, PhD and Aashni Shah (Polistudium SRL, Milan, Italy). Graphical assistance was provided by Massimiliano Pianta (Polistudium SRL, Milan, Italy).
Supplementary material
Supplemental data for this article can be accessed here
Additional information
Funding
References
- Bray F, Kiemeney LA. Epidemiology of prostate cancer in europe: patterns, trends and determinants. In: Bolla M, van Poppel H, editors. Management of prostate cancer. Cham: Springer International Publishing; 2017: 1–27. https://doi.org/10.1007/978-3-319-42769-0_1.
- Mottet N, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update. part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79(2):243–262.
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249.
- Gonthier K, Poluri RTK, Audet-Walsh É. Functional genomic studies reveal the androgen receptor as a master regulator of cellular energy metabolism in prostate cancer. J Steroid Biochem Mol Biol. 2019;191:105367.
- Hou X, Flaig TW. Redefining hormone sensitive disease in advanced prostate cancer. Adv Urol. 2012;2012:1–6.
- Barata P, Swami U, Agarwal N. The addition of apalutamide to ADT in the treatment of metastatic castration-sensitive prostate cancer: safety and efficacy. Expert Rev Anticancer Ther. 2020;20(3):147–150.
- Merseburger AS, Alcaraz A, von Klot CA. Androgen deprivation therapy as backbone therapy in the management of prostate cancer. Onco Targets Ther. 2016;9:7263–7274
- Bolla M, Van Tienhoven G, Warde P, et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol. 2010;11:1066–1073.
- Pilepich MV, Winter K, Lawton CA, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma--long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys. 2005;61(5):1285–1290.
- Roach M 3rd, Bae K, Speight J, et al. Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: long-term results of RTOG 8610. J Clin Oncol. 2008;26(4):585–591.
- Perlmutter MA, Lepor H. Androgen deprivation therapy in the treatment of advanced prostate cancer. Rev Urol. 2007;9 Suppl 1:S3–8.
- Crawford ED. Leuprorelin depot injection: patient considerations in the management of prostatic cancer. Ther Clin Risk Manag. 2008;4:513–526.
- Hupe MC, Merseburger AS. Advanced prostate cancer. World J Urol. 2021;39(2):295–296.
- Saranyutanon S, Srivastava SK, Pai S, et al. Therapies targeted to androgen receptor signaling axis in prostate cancer: progress, challenges, and hope. Cancers (Basel). 2019;12(1):51.
- Agarwal N, Azad A, Carles J, et al. A phase III, randomized, open-label, study (CONTACT-02) of cabozantinib plus atezolizumab versus second novel hormone therapy (NHT) in patients (pts) with metastatic, castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2021;39(6_suppl): TPS190–TPS190.
- Tolkach Y, Joniau S, Van Poppel H. Luteinizing hormone-releasing hormone (LHRH) receptor agonists vs antagonists: a matter of the receptors? BJU Int. 2013;111(7):1021–1030.
- Agarwal N, Azad A, Carles J, et al. A phase III, randomized, open-label, study (CONTACT-02) of cabozantinib plus atezolizumab versus second novel hormone therapy (NHT) in patients (pts) with metastatic, castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2021;39(6_suppl): TPS190–TPS190.
- Lepor H, Shore ND. LHRH agonists for the treatment of prostate cancer: 2012. Rev Urol. 2012;14:1–12.
- Park HS, Shin HB, Woo SH, et al. Combined androgen blockade (CAB) versus luteinizing hormone-releasing hormone (LHRH) agonist monotherapy for androgen deprivation therapy. World J Urol. 2020;38(4):971–979.
- Tolis G, Ackman D, Stellos A, et al. Tumor growth inhibition in patients with prostatic carcinoma treated with luteinizing hormone-releasing hormone agonists. Proc Natl Acad Sci USA. 1982;79(5):1658–1662.
- Albertsen PC, Klotz L, Tombal B, et al. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol. 2014;65(3):565–573.
- Hupe MC, Hammerer P, Ketz M, et al. Retrospective analysis of patients with prostate cancer initiating GnRH agonists/antagonists therapy using a German claims database: epidemiological and patient outcomes. Front Oncol. 2018;8:543.
- Hupe MC, Hammerer P, Ketz M, et al. Retrospektive GKV-Versorgungsforschungsstudie über GnRH-Antagonisten/-Agonisten zur initialen Therapie des fortgeschrittenen Prostatakarzinoms – verordnungsmuster und Krankenhauskosten in Deutschland [Retrospective SHI (Statutory Health Insurances) real-world study on initial GnRH antagonist and agonist therapy for advanced prostate cancer: prescription patterns and hospital costs in Germany]. Aktuelle Urol. 2020;51(3):275–284. German
- Lopes RD, Higano CS, Slovin SF, et al. Cardiovascular safety of degarelix versus leuprolide in patients with prostate cancer: the primary results of the PRONOUNCE randomized trial. Circulation. 2021;144(16):1295–1307.
- Solarić M, Bjartell A, Thyroff-Friesinger U, et al. Testosterone suppression with a unique form of leuprorelin acetate as a solid biodegradable implant in patients with advanced prostate cancer: results from four trials and comparison with the traditional leuprorelin acetate microspheres formulation. Ther Adv Urol. 2017;9:127–136.
- Özyiğit G, Akyol F. Cost-effectiveness analysis of leuprorelin acetate atrigel in the treatment of prostate cancer. Turk J Oncol. 2020;35(4):430–437.
- Tombal B, Berges R. How good do current LHRH agonists control testosterone? Can this be improved with Eligard®? Eur Urol Suppl. 2005;4:30–36 .
- Cox C. Treatment options gel with innovative drug delivery systems. Drug Delivery Tech. 2003;3:8 .
- Perez-Marreno R, Chu FM, Gleason D, et al. A six-month, open-label study assessing a new formulation of leuprolide 7.5 mg for suppression of testosterone in patients with prostate cancer. Clin Ther. 2002;24(11):1902–1914.
- Chu FM, Jayson M, Dineen MK, et al. A clinical study of 22.5 mg. La-2550: a new subcutaneous depot delivery system for leuprolide acetate for the treatment of prostate cancer. J Urol. 2002;168:1199–1203.
- Crawford ED, Sartor O, Chu F, et al. A 12-month clinical study of LA-2585 (45.0 mg): a new 6-month subcutaneous delivery system for leuprolide acetate for the treatment of prostate cancer. J Urol. 2006;175:533–536.
- Persad R. Leuprorelin acetate in prostate cancer: a European update. Int J Clin Pract. 2002;56:389–396.
- Furman BL. Leuprorelin. In Enna S.J, and David B.B, editors. XPharm: the Comprehensive Pharmacology Reference. Amsterdam; Boston: Elsevier; 2007. p. 1–4. https://doi.org/10.1016/B978-008055232-3.62026-4.
- Chung BH, Horie S, Chiong E. Clinical studies investigating the use of leuprorelin for prostate cancer in Asia. Prostate Int. 2020;8:1–9.
- Lama G, Papi M, Angelucci C, et al. Leuprorelin acetate long-lasting effects on GnRH receptors of prostate cancer cells: an atomic force microscopy study of agonist/receptor interaction. PLoS ONE. 2013;8:e52530.
- Shore ND, Guerrero S, Sanahuja RM, et al. A New Sustained-release, 3-month leuprolide acetate formulation achieves and maintains castrate concentrations of testosterone in patients with prostate cancer. Clin Ther. 2019;41:412–425.
- Crawford ED, Moul JW, Sartor O, et al. Extended release, 6-month formulations of leuprolide acetate for the treatment of advanced prostate cancer: achieving testosterone levels below 20 ng/dl. Expert Opin Drug Metab Toxicol. 2015;11:1465–1474.
- Summary of Product Characteristics
- Malik K, Singh I, Nagpal M, et al. Atrigel: a potential parenteral controlled drug delivery system. Der Pharmacia Sinica. 2010;1(1):74–81.
- Sartor O. Eligard® 6: a new form of treatment for prostate cancer. Eur Urol Suppl. 2006;5:905–910.
- Oefelein MG, Feng A, Scolieri MJ, et al. Reassessment of the definition of castrate levels of testosterone: implications for clinical decision making. Urology. 2000;56(6):1021–1024.
- Pickles T, Hamm J, Morris WJ, et al. Incomplete testosterone suppression with luteinizing hormone-releasing hormone agonists: does it happen and does it matter? BJU Int. 2012;110(11 Pt B):E500–7.
- Klotz L, O’Callaghan C, Ding K, et al. Nadir testosterone within first year of androgen-deprivation therapy (ADT) predicts for time to castration-resistant progression: a secondary analysis of the PR-7 trial of intermittent versus continuous ADT. J Clin Oncol. 2015;33(10):1151–1156.
- Morote J, Planas J, Salvador C, et al. Individual variations of serum testosterone in patients with prostate cancer receiving androgen deprivation therapy. BJU Int. 2009;103(3):332–5; discussion 335.
- Yassin A, AlRumaihi K, Alzubaidi R, et al. Testosterone, testosterone therapy and prostate cancer. Aging Male. 2019;22(4):219–227.
- Saad F, Fleshner N, Pickles T, et al. Testosterone breakthrough rates during androgen deprivation therapy for castration sensitive prostate cancer. J Urol. 2020;204(3):416–426.
- Perachino M, Cavalli V, Bravi F. Testosterone levels in patients with metastatic prostate cancer treated with luteinizing hormone-releasing hormone therapy: prognostic significance? BJU Int. 2010;105(5):648–651.
- Uhr A, Glick L, Gomella LG. An overview of biomarkers in the diagnosis and management of prostate cancer. Can J Urol. 2020;27(S3):24–27.
- Braeckman J, Michielsen D. Efficacy and tolerability of 1- and 3-month leuprorelin acetate depot formulations (Eligard ® /Depo-Eligard ®) for advanced prostate cancer in daily practice: a Belgian prospective non-interventional study. AOMS. 2014;3:477–483.
- Shore ND, Chu F, Moul J, et al. Polymer-delivered subcutaneous leuprolide acetate formulations achieve and maintain castrate concentrations of testosterone in four open-label studies in patients with advanced prostate cancer. BJU Int. 2017;119:239–244.
- Renzulli JF, Tagawa ST, Atkinson SN, et al. Subcutaneous in situ gel delivered leuprolide acetate’s consistent and prolonged drug delivery maintains effective testosterone suppression independent of age and weight in men with prostate cancer. BJUI Compass. 2020;1:64–73.
- Pieczonka CM, Twardowski P, Renzulli J 2nd, et al. Effectiveness of subcutaneously administered leuprolide acetate to achieve low nadir testosterone in prostate cancer patients. Rev Urol. 2018;20(2):63–68.
- Saltzstein D, Shore ND, Moul JW, et al. Pharmacokinetic and pharmacodynamic comparison of subcutaneous versus intramuscular leuprolide acetate formulations in male subjects. Ther Adv Urol. 2017;10(2):43–50.
- Crawford ED, Hafron JM, Tagawa ST, et al. Impact of late dosing on testosterone suppression with 2 different leuprolide acetate formulations: in situ gel and microsphere. An analysis of United States clinical data. J Urol. 2021;205:554–560.
- Ohlmann C-H, Gross-Langenhoff M. Efficacy and tolerability of leuprorelin acetate (Eligard®) in daily practice in Germany: pooled data from 2 prospective, non-interventional studies with 3- or 6-month depot formulations in patients with advanced prostate cancer. Urol Int. 2018;100:66–71.
- Tunn UW. A 6-month depot formulation of leuprolide acetate is safe and effective in daily clinical practice: a non-interventional prospective study in 1273 patients. BMC Urol. 2011;11:15.
- Tombal B, Cornel EB, Persad R, et al. Clinical outcomes and testosterone levels following continuous androgen deprivation in patients with relapsing or locally advanced prostate cancer: a post hoc analysis of the Iceland study. J Urol. 2017;198(5):1054–1060.
- Schulman C, Cornel E, Matveev V, et al.Intermittent versus continuous androgen deprivation therapy in patients with relapsing or locally advanced prostate cancer: a phase 3b randomised study (Iceland).Eur Urol.2016;69:720–727
- Vis AN, van der Sluis TM, Al-Itejawi HHM, et al. Risk of disease flare with LHRH agonist therapy in men with prostate cancer: myth or fact? Urol Oncol. 2015 Jan;33(1):7–15.
- Scaletscky R, Smith JA. Disease flare with gonadotrophin-releasing hormone (GnRH) analogues. How serious is it? Drug Saf. 1993;8:265–270.
- Garnick MB, Mottet N. New treatment paradigm for prostate cancer: abarelix initiation therapy for immediate testosterone suppression followed by a luteinizing hormone-releasing hormone agonist. BJU Int. 2012;110:499–504.
- Twardowski P, Atkinson S, Boldt-Houle D, et al. Late dosing of luteinizing hormone-releasing hormone agonists (LHRH) and testosterone (T) levels >20 ng/dL in prostate cancer (PCa). J Clin Oncol. 2020;38:31–32.
- Montorsi F, Tomlinson P. Which luteinising hormone-releasing hormone agonist injection schedule do men with prostate cancer prefer? Results of a European patient survey. Eur Urol. 2015;67(1):177–179.
- Fizazi K, Tran N, Fein L, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20:686–700.
- Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37:2974–2986.
- Agarwal N, McQuarrie K, Bjartell A, et al. Health-related quality of life after apalutamide treatment in patients with metastatic castration-sensitive prostate cancer (TITAN): a randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2019;20:1518–1530.
- Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381:121–131.
- STAMPEDE investigators, James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163–1177.
- Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737–746.