ABSTRACT
Introduction
Metastatic castrate resistant prostate cancer (mCPRC) remains an aggressive form of prostate cancer that no longer responds to traditional hormonal treatment alone. Despite the advent of novel anti-androgen medications, many patients continue to progress, and as a result, there is a growing need for additional treatment options.
Areas Covered
Lutetium-177 (177Lu) – PSMA-617 has become one of the new frontline treatment options for refractory metastatic castrate resistant prostate cancer after the failure of novel anti-androgen therapy and chemotherapy. Lu-177 has been used in real-world prospective trials and is now becoming utilized in newer phase III clinical trials. Here, we present a comprehensive overview of the current literature, covering retrospective studies, prospective studies, and clinical trials that established Lutetium-177-PSMA-617 (177Lu-PSMA-617) for the treatment of mCRPC.
Expert Opinion
177Lu – PSMA-617 has been approved for treatment of mCRPC based on positive phase III studies. While this treatment is tolerable and effective, biomarkers are necessary to determine which patients will benefit. In the future, radioligand treatments will likely be utilized in earlier lines of therapy and potentially in combination with other prostate cancer treatments.
1. Introduction
Prostate cancer (PC) is the second most frequently diagnosed cancer and the fifth leading cause of cancer death among men. In 2020, there was an estimated 1.4 million new cases of prostate cancer with a total of 375,000 deaths worldwide [Citation1]. Of those diagnosed with prostate cancer, approximately 20% will have metastatic disease, with only 26–30% surviving more than 5 years [Citation2]. Androgen deprivation therapy has been the backbone of PC treatment for decades. Reducing circulating testosterone to castrate levels (<50 ng/dL) deprives cells of their primary growth stimulus and induces PC cell death [Citation3]. However, current androgen deprivation therapies are only temporarily effective and ultimately treatment resistance develops [Citation4,Citation5]. Therefore, PC cells eventually become resistant to ADT thereby becoming castration-resistant PC [Citation6–8]. Castration-resistant prostate cancer (CRPC) is defined by disease progression despite castrate levels of testosterone and may present as a continuous rise in serum prostate-specific antigen (PSA) levels, the clinical or radiographic progression of preexisting disease, and/or the appearance of new distant metastases [Citation9]. Docetaxel was the only life-prolonging treatment for metastatic castration-resistant prostate cancer (mCRPC) until the early 2010s.
In the last few years, multiple new agents have been approved by the United States Food and Drug Administration (FDA): sipuleucel-T (2010) [Citation10], cabazitaxel (2010), abiraterone (2011) [Citation11], enzalutamide (2012) [Citation12], alpha-emitter radium-223 (2013) [Citation13], rucaparib (2020) [Citation14], and olaparib (2020) [Citation15] for mCRPC [Citation16]. All these therapeutic agents have shown increased overall survival for mCRPC in phase III clinical trials [Citation2,Citation16]. Despite the availability of numerous classes of therapy that delay disease progression and prolong life, mCRPC remains incurable and often lethal [Citation17,Citation18]. Recently the Food and Drug Administration granted breakthrough therapy designation to lutetium-177 (177Lu) – PSMA-617 for treatment of mCRPC. We present a comprehensive overview of the current literature on Lutetium-177-PSMA-617 (177Lu-PSMA-617) for the treatment of mCRPC.
Radionuclides are selected according to their physical properties such as half-life, types of emission, the energy of radiation, and biochemical properties such as tissue targeting, retention of radioactivity in the tumor, in vivo stability, and toxicity [Citation19]. The ideal half-life of therapeutic radionuclides is approximately between six and seven days. A radionuclide with a very short physical half-life will be an overall ineffective treatment option. Conversely, a radionuclide with a longer half-life could increase the exposure and toxicity to the patient since higher doses will be absorbed [Citation19–22]. Lutetium-177 has consistently been shown to be efficacious in patients with neuroendocrine tumors (NET) with longer progression-free survival and high response rate [Citation23–25]. Lu has a half-life within the recommended range of 6–7 days, shorter particle emission range therefore better irradiation of small tumors and is a medium β-particle energy appropriate for therapy. These factors make 177Lu-PSMA an ideal theranostic agent for prostate cancer cells [Citation26–28]. Currently, the two theranostic PSMA-ligands that are being evaluated are PSMA I&T and PSMA-617. The difference between the two is that PSMA I&T contains a DOTAGA chelator whereas PSMA-617 contains a DOTA chelator. However, they both have an identical urea-binding [Citation4]. Radionuclide therapy has emerged as a promising treatment for patients with disease refractory to standard of care [Citation29]. The observed improvement in overall survival with 177Lu-DOTATATE in NET (neuroendocrine tumors) led to the expansion of radionuclide use in prostate cancer [Citation30]. The physical properties of some therapeutic beta-emitting radionuclides are illustrated in [Citation19,Citation26,Citation27,Citation31–33].
Table 1. Radionuclide properties considered for use in targeted therapy. (Emax: Maximum emitted energy, mm: millimeter, MeV: million electron volts).
2. Prostate specific membrane antigen (PSMA)
PSMA, also known as folate hydrolase I or glutamate carboxypeptidase II, is a type II, 750-amino acid transmembrane protein [Citation34,Citation35]. Normal, benign, and malignant prostate tissue including intraepithelial neoplasia and metastatic specimens express PSMA [Citation36]. Its function is to hydrolyze prostatic fluid peptides, generate glutamate as a cell-surface peptidase, and improve cell survival and proliferation [Citation37,Citation38]. PSMA levels have been expressed in healthy prostate, small intestine, proximal renal tubule, and salivary and lacrimal glands [Citation39–41]. PSMA is overexpressed 100 to 1000 times in prostate cancer and is further increased in metastatic and castration resistant prostate cancer [Citation37,Citation42]. It also has been shown to be expressed in other neoplasms, such as renal cell carcinoma, hepatocellular carcinoma, and colon carcinoma [Citation34,Citation43,Citation44]. There is also a direct correlation between the density of expression of PSMA on prostate cancer cells and Gleason score of prostate cancer [Citation26]. The high PSMA expression in prostate cancer makes it an appealing target for radionucleotide therapy. However, since PSMA is not prostate-specific there is a risk of delivery of radiation to other organs. Therefore, it is important to consider the side effect profile of PSMA‐targeted therapy and understand the safest dose of radiotherapy that can be delivered to the patient without causing significant radiation damage to non‐target organs [Citation26,Citation45].
3. Methods
A systematic literature review was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A search was conducted using Google Scholar and PubMed databases by two coauthors (K.P and M.K.) to identify relevant studies in the mCRPC population. Studies were included through February 1, 2023. Keywords used for search include ‘Lutetium-177 PSMA’ OR ‘Lutetium Metastatic Prostate Cancer.’ An additional search was conducted on the US National Library of Medicine database (clinicaltrials.gov) currently enrolling phase I, II, and II studies to incorporate ongoing clinical trials as well. The search was conducted by two authors independently in three stages. The first stage including removing duplicate references by PMID and title. The second stage involved screening of articles published prior to 2010, basic science articles, non-human subjects, guidelines, case reports, abstracts, editorials, replies, commentary, reviews, non-English language articles, non-prostate cancer articles, and metastatic castrate sensitive prostate cancer articles to be excluded. The third stage involved full text analysis of all remaining references performed and additional search of articles using keywords in Medline database to ensure no further articles meeting criteria for inclusion. Reviews, retrospective analyses, case series, real-world data, and phase I, II, and III prospective clinical trials were allowed. Articles were collected from January 1, 2010, to February 1, 2023.
For all eligible studies with published results, we filtered results by only metastatic castrate resistant prostate cancer. The following variables were extracted: treatment intervention, number of enrolled patients, disease setting (front line versus relapsed setting), primary endpoint, objective response rate, progression-free survival, imaging-based progression-free survival, overall survival, RECIST criteria defined response rate, PERCIST criteria SUVmax or PSMA PET/CT response rates, and ECOG were included if available. For the actively enrolling clinical trials, the trial phase, planned intervention, and primary endpoint were included variables. The risk of bias in the included studies was assessed using the Risk of Bias in Systematic Reviews (ROBIS) tool [Citation46]. The ROBIS tool is a validated instrument designed to assess the risk of bias in systematic reviews. It includes four domains: study eligibility criteria, identification and selection of studies, data collection and study appraisal, and synthesis and findings [Citation46].
4. Results
This systematic review yielded a total of 10,418 articles. Of these, 8772 were duplicates and were removed, resulting in 1646 articles. After screening for exclusions, 100 records remained and were reviewed for our study, of which 40 were unique retrospective or prospective studies (). Our review includes 16 retrospective studies, 3 real-world studies, 9 phase I/II trials, and 1 phase III clinical trial as well as 11 clinical trials that are currently enrolling. Per the ROBIS tool, a low risk of bias for all domains was evident. This means that our systematic review followed a clear and well-defined protocol, searched for relevant studies in a comprehensive and unbiased manner, assessed the quality of included studies appropriately, and synthesized the findings in a transparent and objective way.
Figure 1. Inclusion and exclusion criteria for systematic review for treatment of metastatic castrate resistant prostate cancer using Lutetium-177 PSMA.
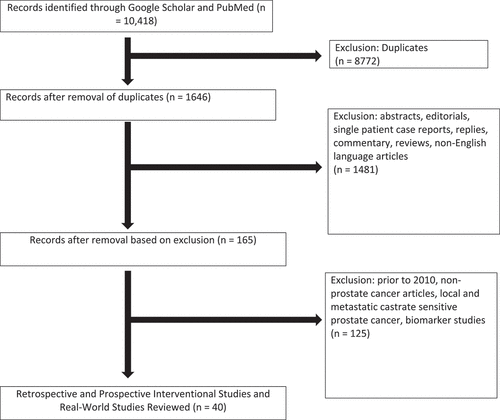
4.1. Retrospective studies ()
One study retrospectively analyzed 82 mCRPC patients who received a single dose of Lu-PSMA [Citation50]. Tolerability and response to treatment were assessed using hematologic parameters, renal scintigraphy, clinical data, and prostate-specific antigen (PSA) levels. A PSA decline from baseline was noted in 64% patients with 31% of patients having a greater than 50% decline, while 47% patients had stable disease with a 25–50% decrease in PSA levels. Only 25% of patients showed an increase in PSA levels indicating disease progression, and 7% of patients died due to extensive disease.
Table 2. Summary of retrospective studies using 177Lu-PSMA therapy in mCRPC.
A 2014–2015 retrospective multi-institutional study looked to evaluate the efficacy and safety of 177Lu-PSMA-617 in a larger population of patients. 145 patients with a median age of 73 years (range 43–88) with mCRPC were treated with a range of 1–4 cycles of treatment. Median follow-up time was sixteen weeks (range 2–30). The primary endpoint of the study was biochemical response, which was defined as a decline in PSA greater than 50% from baseline and secondary endpoints included toxicity. A total of 248 cycles of treatment were carried out in 145 patients with the overall biochemical response rate of 45% after conclusion of all treatment and 40% of patients becoming responders after only one cycle of treatment [Citation52].
A single center retrospective study reviewed 43 mCRPC patients with a median age of 71 (range 51–88) and 177Lu-PSMA-617 treatments every six to twelve weeks. A total of 112 cycles of RLT were conducted with a median of three cycles (range 1–6). Endpoints included PSA response, toxicity, median OS, and median PSA PFS. A PSA decline of greater than 90% was seen in 23% of patients, while a decline of greater than 50% was seen in 65% of patients. A median OS was 52 weeks, while a PSA PFS was found to be 20 weeks [Citation53].
Another study retrospectively studied fifty-nine mCRPC patients after receiving at least one antihormonal drug as well as chemotherapy. These patients were treated with a median of three cycles of 177Lu-PSMA-617 [Citation51]. The primary end point was overall survival, and secondary end point was decrease in PSA level. Follow-up data was available for forty-five patients with 91% showing a PSA decline. A decline in PSA levels of greater than or equal to 50% occurred in 53% of the patients. The median overall survival was 32 weeks. An initial alkaline phosphatase (ALP) level less than 220 U/L and a PSA decline after the first cycle were associated with a longer overall survival (56 vs. 28 weeks, p < 0.01, and 56 vs. 29 weeks, p = 0.04, respectively). Toxicity overview showed grade 3 leukopenia and thrombocytopenia in 3% patients and grade 3 anemia in 19%. No grade 3 or 4 nephrotoxicity or grade 4 blood toxicity was observed. This study showed a statistically significant decline in ALP levels after the first cycle of 177Lu-PSMA-617.
A retrospective analysis studied 28 patients with mCRPC who were treated with 177Lu-PSMA-617 [Citation47]. The estimated median survival was 29.4 weeks, significantly longer than survival in the supportive care group (HR, 0.44; 95% confidence interval, 0.20–0.95]; P = 0.031). Results of the study noted any PSA decline in 59% of patients after one cycle and 75% of patients after two cycles of treatment, while there was a noted PSA decline of 50% or greater in 32% of patients after one cycle of treatment and in 50% of patients after two cycles of treatment. Although this study found a 50% or greater PSA decline in majority of treated patients, 83% of patients reported a stable or improved quality of life after treatment.
177Lu-PSMA-617 was offered to thirty patients with PSMA-positive prostate tumor [Citation48]. Treatment efficacy was retrospectively assessed by PSA levels with 70% of patients demonstrating a decrease in PSA levels. 18 patients were noted to have PSA decline greater than 25%, while 13 patients had a decline greater than 50%. Six of these patients were restaged using PSMA PET/CT (positron emission tomography/computed tomography), and all six patients had a response rate of more than 50% in SUVmax (maximum standardized uptake value) of the tumor.
A single center retrospective analysis studied 62 men with mCRPC who were treated with 177Lu-PSMA-617. A median of 3 treatment cycles (2–5) were administered over 4 weeks. Progression-free survival and overall survival were 4.9 months (2.4–9.6) and 17.2 months (6–26.4) respectively. Greater than 50% PSA response was found in 58.7% of patients (p < 0.004) [Citation54].
A total of 104 patients with mCRPC who were previously treated with one line of chemotherapy (docetaxel and/or cabazitaxel) and at least one of antihormonal therapies (enzalutamide and/or abiraterone) were retrospectively studied after being treated with 177Lu-PSMA-617 RLT. A median of three cycles were administered (1–8 cycles). Results of the study noted a median overall survival of 56.0 weeks (95% CI: 50.5–61.5) and PSA decline >50% in 33% patients after receiving first cycle of treatment [Citation55].
A retrospective single center study looked at therapeutic outcomes of 177Lu-PSMA-617 in mCRPC based on post-treatment imaging findings. 36 patients (aged 67 ± 8.8 years) were included out of which 23 received 2 cycles of treatment. Eleven patients (47.8%) were considered responsive in the post-therapeutic scans by 177Lu-PSMA-617, two of which experienced complete response. Nine (39.1%) patients had stable disease while three (13%) experienced disease progression [Citation56].
Another retrospective study evaluated 56 mCPRC patients with a median age of 69.5 (range 55–84) who received one to four cycles of 177Lu-PSMA-617. Biochemical response was defined using Prostate Cancer Working Group Criteria 3 (PCWG3). A total of 139 cycles of treatment were performed with a decline of greater than 50% in 54% of patients and any PSA decline in 65% of patients. Estimated median overall survival was 16 months versus 14 months in the chemotherapy alone group. Longer OS was observed in patients with a PSA decline of >50%, a baseline ALP level <220 U/l, more than two cycles of treatment, and cumulative activity of greater than 15 GBq [Citation57].
A study sought to analyze the prognostic significance of monitoring PSA levels during 177Lu-PSMA-617 treatment. The study included thirty mCPRC patients who had baseline Ga-68 PSMA PET/CT prior to undergoing 177Lu-PSMA-617 treatment. Patients were treated with a fixed dose of 180 mCi of 177Lu-PSMA-617 every six to eight weeks. A total of 171 cycles of treatment were administered with a median of four cycles per patients (range 3–7). A PSA decline greater than 50% was seen in 33% of patients after one cycle of treatment and increased to 43% of patients at the conclusion of the last cycle of treatment. Of the 20 patients who did not have a 50% reduction in PSA levels after the first cycle, four of these patients eventually had a PSA decline of greater than 50% after the conclusion of the last cycle of treatment. The median OS was statistically significant in those who had a greater than 50% decline in PSA level 21 ± 10 (95% CI: 1.2–40.7) compared to those who did not 8.0 ± 2.6 (95% CI: 2.7–13.2) months (p = 0.012). Any PSA decline was seen in 50% of patients after just one cycle of treatment and remained stable at 46% of patients at the conclusion of treatment, but did not have a statistically significant impact on median OS; however it was significant for any PSA decline after the last cycle of treatment (13 ± 1, 95% CI: 10.9–15 months for responders versus 6.0 ± 1.9, 95% CI: 2.2–9.7 months for non-responders) [Citation58].
A retrospective study reviewed 24 mCPRC patients with a median age of 81.7 (range 75.1–91.9 years old) and median of four prior lines of treatments who were treated with 177Lu-PSMA-617. 54% of patients had bone and lymph node metastasis. Primary endpoints were response, which was defined as a decrease in PSA level over 50% from baseline and toxicity. Patients were treated with one to four cycles. A PSA decline over 50% was seen in 48% (n = 11) of patients [Citation59].
Another study retrospectively examined outcomes in 68 patients with a mean of 71 years of age (range 46–89) who were treated with a mean of three cycles of 177Lu-PSMA-617 (median of 3, range 1–7 cycles) every six weeks. The 18-month overall survival was 63.8%. Those with a baseline PSA <20 ug/L had a higher 18-month survival estimate (79.9%) versus those with PSA levels greater than 20 ug/L (53.8%, p = < 0.05). Those with an SUVmax greater than 15 had a higher 18-month survival rate of 56% compared to those with SUVmax less than 15 (p = < 0.05). Their study found any decrease in PSA levels after two cycles of treatment was indicative of greater chance of overall survival (p = < 0.01) [Citation60].
An additional retrospective study of 52 mCRPC patients (mean age of 70, range 48–87 years) evaluated the PSA response of those treated with 177Lu-PSMA-617 after one to three cycles of radioligand therapy (RLT) every eight weeks. A total of 190 cycles of RLT (3–6 cycles per patient) were given and 80% of patients showed a decline after the first cycle with 44% of patients showing a PSA response of more than 50%. After the third cycle, 73% of patients showed any PSA decline. The median OS was 60 weeks for all patients and median OS was statistically longer for those who responded with decline in PSA after first cycle compared to those without (68 vs 33 weeks) [Citation61].
Another retrospective study studied 25 patients less than 55 years of age at prostate cancer diagnosis who were treated with a median of four cycles (range 2–6) of 177Lu-PSMA-617. The median PFS was 3.8 months (95% CI 2.5–5.3) and OS was 8.5 months (95% 6.2–10.8). An initial PSA reduction of greater than 50% was seen in 36% of patients but not associated with OS benefit (p = 0.601). A PSA response greater than 50% at three months was seen in 48% of patients and associated with improvement in OS (16 months, 95% CI 7.4–24.6 versus 4.0 months, 95% CI: 1.1–6.9, p = 0.002). They also used 68Ga-PSMA-PET/CT and correlated imaging response after up to three cycles in 44% of patients. Responders had a longer median PFS (8.7 months, 95% CI 1.3–16.1 vs. 1.9 months, 95% CI 1.7–2.2, p < 0.001) and OS (16.0 months, 95% CI 7.6–24.4 vs. 4.0 months, 95% CI 0.9–7.1; p = 0.002) [Citation62].
A small two center study was conducted in 2015, which followed ten mCRPC patients treated with 177Lu-PSMA-617 [Citation49]. Response was evaluated by change in PSA. Eight weeks after therapy, seven out of the 10 study subjects, experienced a decline in PSA. Six out of ten patients had a decline more than 30%, while five patients had a decline of more than 50% in their PSA levels. Three patients showed an increase in PSA indicative of progression of disease. Post-treatment PSA levels declined significantly in this study, indicating positive treatment response.
4.2. Real-world studies ()
A real-world study evaluated 191 patients with mCPRC treated with 177Lu-PSMA-617. Patients had confirmation of PSMA expressing lesions by 68Ga-PSMA-ligand PET/CT. The majority of individuals (90%) had first- and second-line systemic treatment prior to being treated with 177Lu-PSMA-617. Patients received one to five cycles of treatment and specific endpoints included biochemical response, radiologic response, PSA PFS, and OS. A PSA RR (defined as a decline of ≥50% in PSA) was found in 56% of patients, and any PSA decline occurred in 75% of patients. The median radiographic progression-free survival was found to be six months (range 3–10), PSA PFS (n = 132) was 4 months (range 3–8), and the overall survival (n = 191) was 12 months (range 5–18) [Citation64].
Table 3. Summary of real-world analysis of 177Lu-PSMA therapy in mCRPC.
The REALITY (REgistry to Assess Outcome and Toxicity of Targeted RadionucLIde TherapY) study [Citation63] was a prospective ‘real world’ cohort of 254 patients with mCRPC who received 177Lu-PSMA-617 as experimental salvage therapy after conventional treatments had failed. The primary end points were efficacy, reflected by PSA-PFS, (prostate specific antigen-progression-free survival) OS, (overall survival) and safety which was reflected by the incidence of treatment-related (AEs). Over the entire course of 177Lu-PSMA-617 RLT (radioligand therapy), 52.0% patients had a greater than 50% decline in their PSA response. At a median (minimum – maximum) follow-up of 14.9 (5.0–64.4) months, the median PSA-PFS was 5.5 (4.4–6.6) months, while the median OS was 14.5 (11.5–17.5) months.
Another real-world prospective cohort study included twenty-one treatments refractory mCRPC patients at Bushehr Department of Nuclear Medicine between December 2016 to December 2018 treated with 177Lu-PSMA-617 every 8 weeks. Patients had to have PSMA secreting lesions detected by PSMA imaging with 68Ga-PSMA PET or 177Lu/99Tc-PSMA before to undergoing treatment. The mean age of patients was 70.3 ± 9.6 (range 54–88) with a median of two cycles of treatment (range 1–4). The primary endpoint of the study was to assess biochemical response (BCR), which was defined as a drop in the PSA of more than 50. Additional secondary endpoints included response by ECOG and overall survival. A BCR was seen in 62% of patients. The estimated overall survival was seen to be 62.69 weeks (95% confidence interval: 42.06–83.33) with a median follow-up period of 9 months (range 1–25 months) [Citation65].
4.3. Clinical trials
Currently, a limited number of trials have been published on 177Lu PSMA-617 as a treatment for mCRPC ranging from retrospective studies to Phase II and Phase III clinical trials ().
Table 4. Summary of published clinical trials studying177Lu-PSMA therapy in mCRPC.
4.3.1. Phase I/II trials
A prospective single center phase II study included 31 patients with mCRPC who had progressed despite second-line hormonal therapy and/or docetaxel chemotherapy [Citation66]. The patients underwent an average of two cycles (range 1–4) of 177Lu-DKFZ-PSMA-617 therapy. The primary endpoints of the study were to determine radiographic progression-free survival (RPFS) using PET/CT scans and overall survival (OS). The treatment response was evaluated based on biochemical response using serum PSA, molecular response using PET/CT scans, and clinical response using VAS (visual analogue scale), AS (analgesic score) and ECOG (Eastern Cooperative Oncology Group) performance status. Biochemical response showed that the mean serum PSA level after three months of initial therapy was 141.75 ± 187.43 ng/mL (range 2.5–807). The mean serum PSA level three months after the second cycle was 153.07 ± 204 ng/mL (range 1.34–762). The molecular response estimated by the mean SUVmax (maximum standardized uptake value) of tumor lesions before and after therapy was 56.7 and 18.2 respectively. In two patients it was reduced from 32.67 to 0.38 after 177Lu-DKFZ-PSMA-617 therapy, thus indicating a complete remission. The clinical response was estimated using the mean VASmax and AS. The pre-therapy VASmax was 7.5 ± 1, and post-therapy was 3 ± 0.9 (p < 0.0001), while the mean AS pre-therapy and post therapy was 2.5 ± 1.09 and 1.8 ± 0.98 (p = 0.009) respectively, and mean ECOG performance status improved from 2.54 ± 0.85 to 1.78 ± 0.92 after completion of therapy (p = 0.001). The median overall survival was 16 months, and progression-free survival was 12 months. 16.1% patients died due to disease during follow-up. This trial particularly highlighted an improvement in the ECOG performance status in patients receiving 177Lu-DKFZ-PSMA-617 for the treatment of mCRPC.
A prospective phase II pilot study enrolled fourteen patients with progressive and symptomatic mCRPC despite taxane and novel anti-androgen therapy to receive up to four cycles of 177Lu PSMA-617 every six weeks. Ten out of the 14 patients had a mean PSA reduction of 59%, with five patients having over a 50% reduction, and nine patients having over a 30% reduction in PSA levels. At baseline testing, the PSMA PET SUV predicted a reduction in PSA of more than 30%, with an SUV max value of 17 ± 9 compared to 44 ± 15 (p < 0.007), and a PSMA SUV mean of 6 ± 4 compared to 10 ± 4 (p < 0.04) [Citation67].
Fifty patients were studied prospectively with PSMA-avid prostate cancer metastases who underwent a median of three cycles (range 1–4) of 177Lu PSMA-617 therapy [Citation70]. Nine were deemed 177Lu PSMA-617 refractory prior to death with a total of twelve deaths in the study. A PSA decline was noted in 23 patients, with 11 patients having a PSA decline of ≥50% after the first cycle of treatment. 12 patients had a PSA decline of <50% ranging from 11.3 to 43.5%. The remaining 23 patients experienced a PSA increase after the first 177Lu PSMA-617 cycle (range 19.7–100%).
Another single-arm, single-center, phase 2 trial [Citation42] recruited thirty men with mCRPC and progressive disease after receiving standard treatments including taxane-based chemotherapy and second-generation anti-androgen treatment (abiraterone, enzalutamide, or both). They were administered four cycles of 177Lu-PSMA-617. The primary end points included PSA level, imaging response using bone scan, and PET/CT and quality of life. The PSA decline of greater than or equal to 50% was achieved in 57% of patients. Imaging response using PSMA PET showed a complete response in 10% of patients, a partial response in 30% of patients, and progressive disease in 27% of patients. Cognitive functioning, insomnia, and pain, which were measured using the EORTC-QLQ30 (European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire) and BPI (brief pain inventory) scoring tools showed improvement during treatment compared to baseline, thus indicating improved quality of life.
A randomized, parallel group, open label, and non-inferiority phase II trial conducted between 2019 and 2021 studied a group of thirty five chemo-naive patients with mCPRC and high expressing PSMA lesions on 68Ga-PSMA-11 on PET/CT. Patients were randomized in 1:1 ratio to either 177Lu PSMA-617 (6.0–7.4 GBq/cycle, every 8 weeks, up to 4 cycles) or docetaxel (75 mg/m2/cycle, every 3 weeks, for up to 10 cycles), with fifteen and twenty patients in each group, respectively. The primary endpoint was best PSA response rate (PSA-RR), which is defined as a ≥ 50% decline in PSA from baseline. The 177Lu PSMA-617 arm PSA-RR was 60% versus 40% in docetaxel group with a difference in PSA-RR of 20% (95% CI: 1–47, p = 0.025) and met the pre-specified criterion for non-inferiority which was defined as margin of 15% decline in PSA-RR. The PFS rate for 177Lu PSMA-617 was 30% versus 20% for docetaxel (95% CI: 18–38, p = 0.5). The number of treatment-related adverse events grade 3 or higher occurred less in the 177Lu PSMA-617 arm than the docetaxel arm (30% versus 50% respectively, p = 0.2) [Citation68].
The Thera-P trial [Citation69] was a phase II prospective multicenter trial looking at those with mCPRC who progressed after docetaxel chemotherapy and were randomly assigned to 177Lu PSMA-617 or cabazitaxel. Initial reported results found that those who were treated with 177Lu PSMA-617 treatment led to improvement in PSA response rate (66% vs 37%), RECIST response rate (49% vs 24%), PFS (HR 0.63), less G3-G4 toxicities (33% vs 53%), and overall better patient reported outcomes.
A phase II study followed forty patients with PET/CT-68Ga-PSMA positive mCRPC treated with 177Lu PSMA-617. 177Lu PSMA-617 was given for up to four cycles (median number of three cycles, range 2–5) every eight to twelve weeks. With a median follow-up of 15.5 months (range 6–22 ), 37.5% of patients had a greater than 50% PSA decline and 50% had a PSA decline greater than 30%. The median PFS was 7.5 months (95% CI: 4.8–10.5) and median OS was 12.4 months (95% CI 7.4–20.3 months) [Citation71].
A prospective trial investigated the efficacy and toxicity of 177Lu-PSMA-617 in fourteen patients with mCRPC [Citation73]. Biochemical response using PSA level and clinical symptoms were evaluated after two or more cycles of treatment. PSA levels declined in 11 out of 14 (78.6%) patients, with greater than 50% decline in 45.4% patients over a period of 8 weeks. Progressive disease, defined by a PSA increase of 25% or greater occurred in 21.4% patients. The mean serum alkaline phosphatase level declined from 569.5 U/L to 498.4 U/L, but this was not statistically significant (P = 0.17). At baseline, nine patients with bone metastases reported skeletal bone pain. Eight of these patients showed improvement in pain after treatment.
Another prospective trial studied 56 patients with progressive mCRPC and rising PSA levels who then received 177Lu-PSMA [Citation72]. Clinical efficacy was assessed using the visual analogue scale for pain, PSA levels, median overall survival, and Gallium-68–labeled prostate-specific membrane antigen (68Ga-PSMA PET/CT) for objective response. There was a decline in PSA of greater than 80% in 23.2% of patients, a decline of greater than 50% in 58.9%, and a decline of greater than 30% in 66.1% of patients. Progressive disease was noted in 10.7% of patients with a rise in PSA level greater than 25%. 68Ga-PSMA PET/CT prior to PSMA radioligand therapy showed the median SUVmax of the target lesion to be 37.5, which decreased to 15.7 after PSMA RLT. Ga-PSMA PET/CT was used to measure response rates which included a partial remission in 56%, stable disease in 8%, and progressive disease in 36% of patients. The median progression-free survival was 13.7 months, and median overall survival was not reached. PET/CT results showing a decrease in SUV max of the target lesion are indicative of a favorable objective response to Lu-PSMA therapy.
4.3.2. Phase III trials
The VISION trial was an international open-label phase 3 trial that assigned 581 mCRPC patients who previously received a novel hormonal agent and chemotherapy to either the treatment group (receiving 177Lu-PSMA-617 and standard of care) or control group (standard of care alone) [Citation17]. Primary end points were progression-free survival, median overall survival, and median follow-up. Median progression-free survival was 8.7 months in the 177Lu-PSMA-617 group, as compared with 3.4 months in the control group (HR for progression or death, 0.40; 99.2% confidence interval, 0.29 to 0.57; P < 0.001). Median overall survival was 15.3 months in the 177Lu-PSMA-617 group, as compared with 11.3 months in the control group (hazard ratio for death, 0.62; 95% CI, 0.52 to 0.74; P < 0.001). The median follow-up was 20.3 months (95% CI, 19.8 to 21.0) in the 177Lu-PSMA-617 group and 19.8 months (95% CI, 18.3 to 20.8) in the control group. Secondary end points included median time to the first symptomatic skeletal event or death and PSA level. The median time to the first symptomatic skeletal event or death was 11.5 months in the 177Lu-PSMA-617 group, as compared with 6.8 months in the control group (P < 0.001). The proportions of patients with confirmed decreases in the PSA level of at least 50% and 80% from baseline were higher in the 177Lu-PSMA-617 group than in the control group.
The VISION trial was a positive study that demonstrated an improvement in the primary endpoints of median overall survival and radiographic progression-free survival in the Lu-PSMA group compared to the control group. This led to the FDA approval of Lutetium 177 Vipivotide Tetraxetan (Pluvicto) for the treatment of adult patients with prostate-specific membrane antigen-positive mCRPC [Citation74]. It is noteworthy that a subset of patients in the VISION study had previously undergone treatment with novel antiandrogens and cabazitaxel, and the control arm of standard care precluded the administration of active cancer treatment, potentially confounding the observed outcomes.
A subgroup analysis of the VISION trial demonstrated that patients who received 177Lu-PSMA-617 plus ARPI had a lower hazard ratio for death (0.55, 95% CI 0.43–0.70) compared to those who received 177Lu-PSMA-617 without ARPI (0.70, 95% CI 0.53–0.93). The results suggest that 177Lu-PSMA-617 therapy may provide a survival benefit regardless of concurrent ARPI therapy. However, further investigation is needed to determine if the addition of ARPI could improve the efficacy of this treatment [Citation75].
4.3.3. Ongoing prospective trials
A total of 11 ongoing clinical trials studying 177Lu-PSMA-617 in patients with mCRPC have been identified, analyzed, and included in our review (). These prospective trials included single arm or multiple arm studies. Most clinical trials evaluate 177Lu-PSMA-617 in combination with other therapies used for treatment in prostate cancer, including enzalutamide, cabazitaxel, olaparib, and radium-223 [Citation78–81]. Other studies evaluate combination with treatments under investigation for use in prostate cancer, such as cabozantinib, pembrolizumab, and ipilimumab/nivolumab [Citation83–86]. Primary end points of these studies differ and include radiographic response, dose limiting toxicities, PSA response, and progression-free survival.
Table 5. Ongoing prospective trials of Lu-PSMA in prostate cancer [Citation87].
5. Quality of life
Some of the studies assessed quality of life (QoL) in patients being treated with 177Lu PSMA-617. Health-related quality of life was assessed using a scoring system and was noted that treatment with 177Lu PSMA-617 was associated with prolonged time to worsening of QoL [Citation17]. Another study specifically assessed health-related quality of life using a questionnaire at baseline and after two cycles of treatment. It was noted that compared to baseline, QoL was significantly improved at 2-month follow-up with an increase in global health status (p = 0.025), role functioning (p = 0.017), emotional functioning (0.010), and decrease in pain (p = 0.033) [Citation88]. An additional phase II trial we reported earlier conducted a pre and post therapy health QoL survey with reported score using the NCCN-FACT-FPSI17 questionnaire. They assessed disease-related symptoms from an emotional and physical perspective, functional well-being, prostate cancer–specific symptoms, and treatment-related side effects. Patients who underwent 177Lu PSMA-617 treatment were more likely to have an improvement in disease-related symptoms from a physical and emotional perspective, in treatment side effects, and prostate cancer–specific symptoms as well compared to docetaxel (p = <0.01) [Citation68]. A German study discussed performance status improvement with decline in ECOG ≥1 and pain palliation (>2 level VAS decline) being achieved in 47% and 64% of patients respectively [Citation62].
6. Toxicity
Fatigue, nausea, and dry mouth were the most commonly reported adverse events in patients receiving 177Lu-PSMA-617 [Citation17]. Hematological toxicity was noted to be one of the most commonly reported adverse side effect related to 177Lu PSMA therapy and was especially seen in patients with a heavy burden of skeletal metastases and borderline marrow function [Citation89–91]. Up to 10–25% of men had a Grade 1–2 reduction in hemoglobin or platelets [Citation89]. Anemia, leukopenia, and thrombocytopenia were infrequently observed in some patients receiving LuPSMA therapy [Citation48]. As a result of the physiologic uptake of this therapy in salivary glands, some patients have experienced mild and transient xerostomia as a side effect [Citation49]. In order to reduce these adversities, many studies used ice packs thirty minutes before and up to four hours after the injection [Citation50,Citation89]. No changes were noticed in body temperature or blood pressure [Citation73]. As per the REALITY study, grade 3 or 4 anemia, fatigue, thrombocytopenia, and lymphopenia were the four most common AEs related to 177Lu-PSMA-617 RLT and should be monitored closely during treatment [Citation63].
7. Imaging/Biomarkers
Quantitative biomarkers derived from imaging and blood tests can identify patients with poor prognosis undergoing PSMA- directed therapy and are being studied to assess response to 177Lu-PSMA-617. PSMA-PET imaging has shown heterogeneity in PSMA expression among metastases suggesting its use as a biomarker of PSA response to 177Lu-PSMA-617 [Citation92]. Another imaging modality that was analyzed was FDG-PET [Citation93,Citation94]. It provided the measure of tumor glycolysis, and in conjunction with PSMA-PET identified sites of disease that were FDG-positive but PSMA-negative [Citation95].
Amongst serum biomarkers, high ALP (alkaline phosphatase) and LDH (lactate dehydrogenase) have been associated with worse progression-free and overall survival [Citation95–98]. Furthermore, decreased pretherapeutic hemoglobin levels (HR 0.698 per g/dl; 95%-CI 0.560–0.872; p = 0.001), presence of hepatic metastasis (HR 6.981; 95%-CI 2.583–18.863; p < 0.001), and increased pretherapeutic c-reactive protein (CRP), alkaline phosphatase (ALP), and gamma-glutamyltransferase (GGT) levels were also associated with a shorter survival [Citation99].
Androgen receptor (AR) mutations have been shown to be associated with worse prognosis in mCRPC and may be a way to predict resistance to 177Lu PSMA-617 treatment. One phase II study measured AR amplification in circulating plasma DNA by ddPCR during 177Lu PSMA-617 treatment and found that those with AR gain mutations were 2.4 times less likely to have a PSA response. Twelve of 15 patients with raised AR measured by PCR and 5 of 25 patients with normal AR had early progression of disease (p = 0.0002). Raised AR gain was associated with a median PFS of 4.7 months (95% CI: 2.9–7.0 months) while those with a normal AR had a median PFS of 9.4 months (95% CI: 6.9–11.5, p = 0.020). Those with an AR gain had a median OS of 7.4 months (95% CI: 4.5–10.3 months) versus normal AR had a median OS of 19.1 months (95% CI: 10.6–20.3, p = 0.020) [Citation71].
PSMA expression in circulating tumor cells from CRPC patients has been studied as a novel prognostic biomarker. It has been shown that PSMA expression is predictive of poorer treatment response, shorter PSA progression-free survival (CI, 1.33 to 12.8; P = 0.014) and overall survival (CI, 1.08 to 153; P = 0.040) [Citation100]. A study evaluated the prognostic role of membranous PSMA (mPSMA) protein expression by immunohistochemistry in mCRPC biopsies. It was found that expression of mPSMA at diagnosis was associated with higher Gleason grade (p = 0.04) and worse overall survival (p = 0.006). The same study also looked at the association of DNA damage repair (DDR) with mPSMA protein expression and found that tumors with DDR had higher mPSMA expression compared to those without DDR (p = 0.016; 87.5 {25.0–247.5} vs 20 {0.3–98.8}) [Citation101].
Mean standardized uptake value (SUVmean) of prostate-specific membrane antigen (PSMA) has shown to be a predictive biomarker for response to 177Lu-PSMA-617 in patients with mCRPC [Citation69]. A study evaluated the association between SUVmean and clinical outcomes in mCRPC patients receiving Lu-PSMA. They found that patients in the higher quartile (SUVmean: rPFS, ≥ 10.2; OS, ≥ 9.9) had a median radiographic progression-free survival (rPFS) and overall survival (OS) of 14.1 and 21.4 months, vs 5.8 and 14.5 months for those in the lowest quartile (<6.0; < 5.7) respectively [Citation102]. Another study investigated the PSA response and found that patients with an SUVmean ≥10 had a response rate of 91% (95% CI, 76%-98%), compared to 52% (95% CI, 39%-64%) for patients with an SUVmean <10 [Citation69].
Nomograms to predict response of Lu-PSMA have been developed which include variables like time since diagnosis, history of chemotherapy, hemoglobin levels, tumor PSMA expression, and number of PSMA-positive metastatic lesions. It was noted that tumors with high PSMA expression were associated with more favorable outcomes while bone disease was less likely to be adequately controlled with Lu-PSMA [Citation103].
8. Conclusion
177Lu-PSMA-617 has shown promising results in the treatment of men with mCRPC and is likely to play a significant role in the future of prostate cancer management. Repeatedly, this novel therapy has demonstrated a low toxicity profile and appears to be well tolerated in men with refractory metastatic disease. Both retrospective studies and prospective clinical trials have shown to be an effective option for patients with mCRPC following novel hormonal agents and chemotherapy. A limitation to this review is its retrospective nature, which makes it difficult to compare patient populations, dosing cycles, and dose intensities between studies [Citation104]. Amongst the studies, there is also inherent variation in measurement/biomarkers of response, PSMA imaging modalities used, retrospective vs. prospective designs, and small recruitment size. Nonetheless, this systematic review covers the landmark early studies that led to approval of 177Lu-PSMA-617 for mCRPC and highlights the promise of this novel prostate cancer treatment.
9. Expert opinion
This review discusses the retrospective and small prospective studies that culminated in the randomized, multicenter phase III VISION trial. Based on positive results of this trial, 177Lu-PSMA-617 received approval and is now standard of care for patients who progress following chemotherapy and a novel hormonal agent for mCRPC. Another phase III study, PSMAfore has also met the primary endpoint of radiographic progression-free survival. The studies support use of this treatment, and phase II studies, including TheraP, suggest that this treatment is superior to other options for mCRPC, including chemotherapy.
While this treatment is effective, there remains some uncertainty in its adoption. Barriers have been encountered to its usage in clinical practice. Amongst studies, there has been variability in dosage as well as number of cycles of treatment used. For example dosage was 7.4 GBq for four to six cycles in the VISION study [Citation17], compared to 8.5 GBq with reduction by 0.5 GBq for up to six cycles in the TheraP study [Citation69]. There were also differences in eligibility for these studies, as the VISION study merely specified that PSMA-positive lesions were needed, whereas TheraP required SUVmax of at least 20 at one site of disease and at least 10 at all sites of disease [Citation17]. Production and distribution issues, availability at non-academic medical centers as well as globally, and cost of treatment have also been impediments to its wider adoption.
Although the use of 177Lu-PSMA-617 is currently restricted for patients with mCRPC, this will likely be adopted in earlier lines of therapy in the future. A multicenter phase III study, PSMAddition (NCT 04720157) is enrolling patients with castrate sensitive prostate cancer. This study randomizes patients to either 177Lu-PSMA in addition to standard of care or standard of care alone. Other smaller studies, including the Bullseye study (NCT04443062) are looking into effectiveness of 177Lu-PSMA-617 in the metastatic hormone sensitive setting as well. In addition, future studies will look into the efficacy of this treatment in localized and oligorecurrent prostate cancer, likely expanding its utility for treating prostate cancer in a variety of settings. To optimize efficacy of 177Lu-PSMA-617, novel combinations with hormonal therapy, poly-ribose ADP (PARP) inhibitors, chemotherapy, immunotherapy, and radiation are being explored further for metastatic and localized disease.
Unfortunately, not all patients respond to this treatment. In the VISION trial, 51% of patients had complete response, partial response, or stable disease [Citation17]. Biomarkers are necessary to determine which patients are likely to benefit from treatment. These include imaging biomarkers, such as intensity on PSMA PET, laboratory-based biomarkers, such as LDH or alkaline phosphatase, or molecular biomarkers, such as DNA damage repair mutations. Circulating tumor cells have also been investigated to determine molecular response to radioligand therapy. In addition to primary resistance, many patients encounter secondary resistance, as most patients in the VISION trial ultimately developed recurrence of their prostate cancer. Preclinical data has identified mechanisms of resistance, including TP53 loss [Citation105] and mutations in DNA damage repair genes [Citation106]. To date, biomarker studies have proven to be unreliable and are not yet ready to be adopted for determination of what patients should receive 177Lu-PSMA but provide rationale for future combinations to optimize durability of treatment. Therefore, future studies are necessary to establish predictive biomarkers for this treatment. Another positive predictive factor is subsequent anti-cancer therapy, as one study demonstrated that patients who received further treatment beyond 177Lu-PSMA has improved survival [Citation107]. Options for subsequent treatment include chemotherapy, novel hormonal therapies, poly-ribose ADP (PARP) inhibition for patients with DNA-damage repair mutations, radium-223 and clinical trials; however, more studies are necessary to determine the optimal subsequent treatment.
This review predominantly focused on 177Lu-PSMA-617 as radioligand therapy for prostate cancer; however, other radionuclides are likely to gain a foothold as viable prostate cancer treatment. In particular, the alpha-emitter actinium-225 has demonstrated efficacy when partnered with PSMA-617. Other PSMA targeted therapies have also shown promise. Bispecific T-cell engagement has been recently explored in solid tumors, and PSMA is an attractive target for prostate cancer. The United States FDA has recently cleared an investigational new drug application for JANX007, PSMA-TRACTr (Prostate-specific membrane antigen-Tumor Activated T Cell Engager) for the treatment of mCRPC [Citation108]. Given the relatively high PSA responders and low toxicity profile of this novel therapy it can prove to play a promising role in treatment of prostate cancer.
The initial approval for 177Lu-PSMA-617 for mCRPC is only the beginning for radioligand treatment of prostate cancer. Many exciting new trials and biomarker studies are underway that will determine its optimal use in the future.
Article highlights
Lutetium-177 (177Lu) – PSMA-617 is a novel radioligand therapy that has been explored for treatment of metastatic castration-resistant prostate cancer (mCRPC), an advanced cancer with limited therapeutic options.
The retrospective studies, real-world studies, and clinical trials that established the efficacy and safety of 177Lu-PSMA-617 compared to other agents for mCRPC treatment are reviewed.
Based on positive results from the phase III VISION trial, 177Lu-PSMA-617 received breakthrough therapy designation by the Food and Drug Administration and is now standard of care for patients who progress following chemotherapy and a novel hormonal agent for mCRPC.
Biomarkers derived from imaging, laboratory studies, genomics, and PSMA expression in circulating tumor cells are under development to optimize the patients that benefit from this therapy.
Ongoing clinical trials continue to investigate the potential benefits and optimal use of 177Lu-PSMA-617 in mCRPC, including combinations with other prostate cancer therapies.
Despite initial positive studies of 177Lu-PSMA-617, uncertainty remains in regard to dosage, length of treatment, mechanisms of resistance, and access to treatment.
Declaration of interest
P Mendiratta has received consultant fees from Seattle Genetics and has served on the speakers bureau for Seattle Genetics, Astellas, and AstraZeneca (unrelated to this work). PC Barata has received consultant fees from Astellas, Eisai, AVEO Oncology, Janssen, EMD Serono; Dendreon; Pfizer, Seattle Genetics, BMS, Bayer, Guardant Health, Caris Life Sciences and Myovant (unrelated to this work). DE Spratt has received personal fees from Astellas, AstraZeneca, Blue Earth, Bayer, Boston Scientific, Elekta, Gamma Tile, Varian, Pfizer, Novartis, Myovant (unrelated to this work). A Jia has served on the advisory board for Myovant (unrelated to this work). JR Brown has served on the speakers bureau for EMD Serono, and has received consulting fees from AstraZeneca (unrelated to this work).
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: gLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021 Feb 4;71(3):209–249.
- Maluf FC, Pereira FMT, Silva AG, et al. Consensus on the treatment and follow-up for metastatic castration-resistant prostate cancer: a report from the first global prostate cancer consensus conference for developing countries (PCCCDC). JCO Glob Oncol. 2021 Apr;7(7):559–571.
- Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972 Jul;22(4):232–240.
- Chatalic KL, Heskamp S, Konijnenberg M, et al. Towards personalized treatment of prostate cancer: pSMA I&T, a promising prostate-specific membrane antigen-targeted theranostic agent. Theranostics. 2016;6(6):849–861. DOI:10.7150/thno.14744
- Harris WP, Mostaghel EA, Nelson PS, et al. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol. 2009 Feb;6(2):76–85.
- Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014 Feb;65(2):467–479.
- Gravis G, Boher JM, Joly F, et al. Androgen deprivation therapy (ADT) plus docetaxel versus ADT alone in metastatic non castrate prostate cancer: impact of metastatic burden and long-term survival analysis of the randomized phase 3 GETUG-AFU15 trial. Eur Urol. 2016 Aug;70(2):256–262.
- Karantanos T, Evans CP, Tombal B, et al. Understanding the mechanisms of androgen deprivation resistance in prostate cancer at the molecular level. Eur Urol. 2015 Mar;67(3):470–479.
- Saad F, Chi KN, Finelli A, et al. The 2015 CUA-CUOG Guidelines for the management of castration-resistant prostate cancer (CRPC). Can Urol Assoc J. 2015 Mar;9(3–4):90–96.
- Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010 Jul 29;363(5):411–422.
- de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011 May 26;364(21):1995–2005.
- Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012 Sep 27;367(13):1187–1197.
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013 Jul 18;369(3):213–223.
- Abida W, Patnaik A, Campbell D, et al. Rucaparib in men with metastatic castration-resistant prostate cancer harboring a BRCA1 or BRCA2 gene alteration. J Clin Oncol. 2020 Nov 10;38(32):3763–3772.
- Keisner SV. Rucaparib and olaparib for the treatment of prostate cancer: a clinician’s guide to choice of therapy. J Oncol Pharm Pract. 2022 Apr 19;28(7):10781552221094308.
- Nuhn P, De Bono JS, Fizazi K, et al. Update on systemic prostate cancer therapies: management of metastatic castration-resistant prostate cancer in the era of precision oncology. Eur Urol. 2019 Jan;75(1):88–99.
- Sartor O, de Bono J, Chi KN, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021 Sep 16;385(12):1091–1103.
- Dong L, Zieren RC, Xue W, et al. Metastatic prostate cancer remains incurable, why? Asian J Urol. 2019 Jan;6(1):26–41.
- Altiparmak Gulec B, Yurt F. Treatment with radiopharmaceuticals and radionuclides in breast cancer: current options. Eur J Breast Health. 2021 Jul;17(3):214–219.
- Jadvar H. Targeted alpha-therapy in cancer management: synopsis of preclinical and clinical studies. Cancer Biother Radiopharm. 2020 Sep;35(7):475–484.
- Yeong CH, Cheng MH, Ng KH. Therapeutic radionuclides in nuclear medicine: current and future prospects. J Zhejiang Univ Sci B. 2014 Oct;15(10):845–863.
- Tafreshi NK, Doligalski ML, Tichacek CJ, et al. Development of targeted alpha particle therapy for solid tumors. Molecules. 2019 Nov 26;24(23):4314.
- Delpassand ES, Samarghandi A, Zamanian S, et al. Peptide receptor radionuclide therapy with 177Lu-DOTATATE for patients with somatostatin receptor-expressing neuroendocrine tumors: the first US phase 2 experience. Pancreas. 2014 May;43(4):518–525.
- Ezziddin S, Khalaf F, Vanezi M, et al. Outcome of peptide receptor radionuclide therapy with 177Lu-octreotate in advanced grade 1/2 pancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2014 May;41(5):925–933.
- Das S, Al-Toubah T, El-Haddad G, et al. (177)lu-DOTATATE for the treatment of gastroenteropancreatic neuroendocrine tumors. Expert Rev Gastroenterol Hepatol. 2019 Nov;13(11):1023–1031.
- Emmett L, Willowson K, Violet J, et al. Lutetium (177) PSMA radionuclide therapy for men with prostate cancer: a review of the current literature and discussion of practical aspects of therapy. J Med Radiat Sci. 2017 Mar;64(1):52–60.
- Sgouros G, Bodei L, McDevitt MR, et al. Radiopharmaceutical therapy in cancer: clinical advances and challenges. Nat Rev Drug Discov. 2020 Sep;19(9):589–608.
- Hennrich U, Eder M. [(177)lu]lu-PSMA-617(Pluvicto(TM)): the first FDA-Approved radiotherapeutical for treatment of prostate cancer. Pharmaceuticals (Basel). 2022 Oct 20;15(10):1292.
- Jia AK, Zaorsky NG, Spratt DE, et al. Lutetium-177 DOTATATE: a practical review. Pract Radiat Oncol. 2022;12(4):305–311.
- Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 Trial of (177)Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017 Jan 12;376(2):125–135.
- Stokke C, Kvassheim M, Blakkisrud J. Radionuclides for targeted therapy: physical properties. Molecules. 2022 Aug 25;27(17):5429.
- Boll RA, Malkemus D, Mirzadeh S. Production of actinium-225 for alpha particle mediated radioimmunotherapy. Appl Radiat Isot. 2005 May;62(5):667–679.
- Ma J, Li L, Liao T, et al. Efficacy and safety of (225)Ac-PSMA-617-targeted alpha therapy in metastatic castration-resistant prostate cancer: a systematic review and meta-analysis. Front Oncol. 2022;12:796657.
- Ahmadzadehfar H, Rahbar K, Baum RP, et al. Prior therapies as prognostic factors of overall survival in metastatic castration-resistant prostate cancer patients treated with [(177)Lu]Lu-PSMA-617. A WARMTH multicenter study (the 617 trial). Eur J Nucl Med Mol Imaging. 2021 Jan;48(1):113–122.
- Santoni M, Scarpelli M, Mazzucchelli R, et al. Targeting prostate-specific membrane antigen for personalized therapies in prostate cancer: morphologic and molecular backgrounds and future promises. J Biol Regul Homeost Agents. 2014 Oct;28(4):555–563.
- Hupe MC, Philippi C, Roth D, et al. Expression of prostate-specific membrane antigen (PSMA) on biopsies is an independent risk stratifier of prostate cancer patients at time of initial diagnosis. Front Oncol. 2018;8:623.
- Sweat SD, Pacelli A, Murphy GP, et al. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology. 1998 Oct;52(4):637–640.
- Rajasekaran AK, Anilkumar G, Christiansen JJ. Is prostate-specific membrane antigen a multifunctional protein? Am J Physiol Cell Physiol. 2005 May;288(5):C975–81.
- Troyer JK, Beckett ML, Wright GL Jr. Detection and characterization of the prostate-specific membrane antigen (PSMA) in tissue extracts and body fluids. Int J Cancer. 1995 Sep 4;62(5):552–558.
- Wright GL Jr., Haley C, Beckett ML, et al. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol Oncol. 1995 Jan;1(1):18–28.
- Parsi M, Desai MH, Desai D, et al. PSMA: a game changer in the diagnosis and treatment of advanced prostate cancer. Med Oncol. 2021 Jun 28;38(8):89.
- Hofman MS, Violet J, Hicks RJ, et al. [(177)lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018 Jun;19(6):825–833.
- Silver DA, Pellicer I, Fair WR, et al. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997 Jan;3(1):81–85.
- Tolkach Y, Gevensleben H, Bundschuh R, et al. Prostate-specific membrane antigen in breast cancer: a comprehensive evaluation of expression and a case report of radionuclide therapy. Breast Cancer Res Treat. 2018 Jun;169(3):447–455.
- Ahmadzadehfar H, Rahbar K, Essler M, et al. PSMA-Based theranostics: a step-by-step practical approach to diagnosis and therapy for mCRPC patients. Semin Nucl Med. 2020 Jan;50(1):98–109.
- Whiting P, Savovic J, Higgins JP, et al. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016 Jan;69:225–234.
- Rahbar K, Bode A, Weckesser M, et al. Radioligand therapy with 177Lu-PSMA-617 as a novel therapeutic option in patients with metastatic castration resistant prostate cancer. Clin Nucl Med. 2016 Jul;41(7):522–528.
- Kratochwil C, Giesel FL, Stefanova M, et al. PSMA-Targeted radionuclide therapy of metastatic castration-resistant prostate cancer with 177Lu-labeled PSMA-617. J Nucl Med. 2016 Aug;57(8):1170–1176.
- Ahmadzadehfar H, Rahbar K, Kurpig S, et al. Early side effects and first results of radioligand therapy with (177)Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: a two-centre study. EJNMMI Res. 2015 Dec;5(1):114.
- Rahbar K, Schmidt M, Heinzel A, et al. Response and tolerability of a single dose of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer: a multicenter retrospective analysis. J Nucl Med. 2016 Sep;57(9):1334–1338.
- Brauer A, Grubert LS, Roll W, et al. (177)lu-PSMA-617 radioligand therapy and outcome in patients with metastasized castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2017 Sep;44(10):1663–1670.
- Rahbar K, Ahmadzadehfar H, Kratochwil C, et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med. 2017 Jan;58(1):85–90.
- Zarehparvar Moghadam S, Askari E, Divband G, et al. Efficacy, safety and prognostic factors affecting overall survival among metastatic prostate cancer patients undergoing treatment with (177)Lu-PSMA-617: a single center study. Rev Esp Med Nucl Imagen Mol (Engl Ed). 2022 Jul;41(4):239–246.
- Kemppainen J, Kangasmaki A, Malaspina S, et al. Single center experience with a 4-week (177)Lu-PSMA-617 treatment interval in patients with metastatic castration-resistant prostate cancer. Cancers (Basel). 2022 Dec 14;14(24):6155.
- Rahbar K, Boegemann M, Yordanova A, et al. PSMA targeted radioligand therapy in metastatic castration resistant prostate cancer after chemotherapy, abiraterone and/or enzalutamide. A retrospective analysis of overall survival. Eur J Nucl Med Mol Imaging. 2018 Jan;45(1):12–19.
- Mirshahvalad SA, Farzanefar S, Abbasi M. Therapeutic outcomes of (177)Lu-PSMA targeted therapy in patients with metastatic castration-resistant prostate cancer: a single-center study. Asia Ocean J Nucl Med Biol. 2023;11(1):23–29.
- Schneider CA, Tager P, Hammes J, et al. Treatment outcome and identification of factors influencing overall survival after Lu-177-PSMA-617 radioligand therapy in metastatic prostate cancer. Nuklearmedizin. 2022 Feb;61(1):25–32.
- Soydal C, Araz M, Urun Y, et al. Prognostic importance of prostatic specific antigen response in patients who received Lutetium-177 prostate-specific membrane antigen treatment for castration resistant prostate cancer. Q J Nucl Med Mol Imaging. 2021 Sep;65(3):282–286.
- Leibowitz R, Davidson T, Gadot M, et al. A retrospective analysis of the safety and activity of lutetium-177-prostate-specific membrane antigen radionuclide treatment in older patients with metastatic castration-resistant prostate cancer. Oncology. 2020 Sep;25(9):787–792.
- Tatkovic A, McBean R, Wong D. Lu177-PSMA therapy for men with advanced prostate cancer: 18 months survival analysis in a single Australian tertiary institution. J Med Imaging Radiat Oncol. 2021 Oct;65(6):740–747.
- Ahmadzadehfar H, Wegen S, Yordanova A, et al. Overall survival and response pattern of castration-resistant metastatic prostate cancer to multiple cycles of radioligand therapy using [(177)Lu]Lu-PSMA-617. Eur J Nucl Med Mol Imaging. 2017 Aug;44(9):1448–1454.
- Mader N, Groener D, Tselis N, et al. Outcome of (177)Lu-PSMA-617 radioligand therapy in chemo-refractory patients with metastatic castration-resistant early-onset prostate cancer. Cancers (Basel). 2021 Aug 20;13(16):4193.
- Khreish F, Ghazal Z, Marlowe RJ, et al. 177 Lu-PSMA-617 radioligand therapy of metastatic castration-resistant prostate cancer: initial 254-patient results from a prospective registry (REALITY Study). Eur J Nucl Med Mol Imaging. 2022 Feb;49(3):1075–1085.
- Meyrick D, Gallyamov M, Sabarimurugan S, et al. Real-world data analysis of efficacy and survival after lutetium-177 labelled PSMA ligand therapy in metastatic castration-resistant prostate cancer. Target Oncol. 2021 May;16(3):369–380.
- Assadi M, Rezaei S, Jafari E, et al. Potential application of lutetium-177-labeled prostate-specific membrane antigen-617 radioligand therapy for metastatic castration-resistant prostate cancer in a limited resource environment: initial clinical experience after 2 years. World J Nucl Med. 2020 Jan;19(1):15–20.
- Yadav MP, Ballal S, Tripathi M, et al. (177)lu-DKFZ-PSMA-617 therapy in metastatic castration resistant prostate cancer: safety, efficacy, and quality of life assessment. Eur J Nucl Med Mol Imaging. 2017 Jan;44(1):81–91.
- Emmett L, Crumbaker M, Ho B, et al. Results of a prospective phase 2 pilot trial of (177)Lu-PSMA-617 therapy for metastatic castration-resistant prostate cancer including imaging predictors of treatment response and patterns of progression. Clin Genitourin Cancer. 2019 Feb;17(1):15–22.
- Satapathy S, Mittal BR, Sood A, et al. (177)lu-PSMA-617 versus docetaxel in chemotherapy-naive metastatic castration-resistant prostate cancer: a randomized, controlled, phase 2 non-inferiority trial. Eur J Nucl Med Mol Imaging. 2022 Apr;49(5):1754–1764.
- Hofman MS, Emmett L, Sandhu S, et al. [(177)lu]lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021 Feb 27;397(10276):797–804.
- McBean R, O’Kane B, Parsons R, et al. Lu177-PSMA therapy for men with advanced prostate cancer: initial 18 months experience at a single Australian tertiary institution. J Med Imaging Radiat Oncol. 2019 Aug;63(4):538–545.
- De Giorgi U, Sansovini M, Severi S, et al. Circulating androgen receptor gene amplification and resistance to (177)Lu-PSMA-617 in metastatic castration-resistant prostate cancer: results of a Phase 2 trial. Br J Cancer. 2021 Oct;125(9):1226–1232.
- Baum RP, Kulkarni HR, Schuchardt C, et al. 177lu-labeled prostate-specific membrane antigen radioligand therapy of metastatic castration-resistant prostate cancer: safety and efficacy. J Nucl Med. 2016 Jul;57(7):1006–1013.
- Aghdam RA, Amoui M, Ghodsirad M, et al. Efficacy and safety of (177)Lutetium-prostate-specific membrane antigen therapy in metastatic castration-resistant prostate cancer patients: first experience in West Asia - a prospective study. World J Nucl Med. 2019 Jul;18(3):258–265.
- FDA D.I.S.C.O Burst Edition: fDA approval of Pluvicto (lutetium Lu 177 vipivotide tetraxetan) for the treatment of adult patients with prostate-specific membrane antigen-positive metastatic castration-resistant prostate cancer who have been treated with androgen receptor pathway inhibition and taxane-based chemotherapy US food & drug administration. 2022.
- Vaishampayan N, Morris MJ, Krause BJ, et al. [177lu]lu-PSMA-617 in PSMA-positive metastatic castration-resistant prostate cancer: prior and concomitant treatment subgroup analyses of the VISION trial. J Clin Oncol. 2022;40(16_suppl):5001. DOI:10.1200/JCO.2022.40.16_suppl.5001
- LLC CU. A multi-center, open-label, randomized phase 3 trial comparing the safety and efficacy of 177Lu-PSMA-I&T versus hormone therapy in patients with metastatic castration-resistant prostate cancer. [ Updated 2023 Feb 10]. Accessed Feb 11, 2023. ClinicalTrialsgov identifier: NCT05204927 https://clinicaltrialsgov/ct2/show/NCT05204927.
- Pharmaceuticals N Psmafore: a phase III, open-label, multi-center, randomized study comparing 177Lu-PSMA-617 vs. a Change of androgen receptor-directed therapy in the treatment of taxane naïve men with progressive metastatic castrate resistant prostate cancer. [ Updated 2023 Mar 3]. Accessed Mar 7, 2023. ClinicalTrialsgov Identifier: NCT04689828 https://wwwclinicaltrialsgov/ct2/show/study/NCT04689828.
- Emmett L, Subramaniam S, Joshua AM, et al. ENZA-p trial protocol: a randomized phase II trial using prostate-specific membrane antigen as a therapeutic target and prognostic indicator in men with metastatic castration-resistant prostate cancer treated with enzalutamide (ANZUP 1901). BJU Int. 2021 Nov;128(5):642–651.
- Centre PMC. Cabazitaxel in combination with 177Lu-PSMA-617 in metastatic castration-resistant prostate cancer. [ Updated 2022 Jul 22]. Accessed Feb 8, 2023. ClinicalTrialsgov Identifier: NCT05340374 https://clinicaltrialsgov/ct2/show/NCT05340374.
- Centre PMC 177lu-PSMA-617 therapy and olaparib in patients with metastatic castration resistant prostate cancer. [ Updated 2022 Nov 17]. Accessed Feb 8, 2023. ClinicalTrialsgov Identifier: NCT03874884 https://clinicaltrialsgov/ct2/show/NCT03874884.
- Kostos L, Buteau JP, Yeung T, et al. AlphaBet: combination of radium-223 and [(17) (7)Lu]Lu-PSMA-I&T in men with metastatic castration-resistant prostate cancer (clinical trial protocol). Front Med. 2022;9:1059122.
- Koshkin V Phase I/II study of CDK4/6 Inhibition with abemaciclib to upregulate PSMA expression prior to 177Lu-PSMA-617 treatment in patients with metastatic castrate resistant prostate cancer (mcrpc) previously treated with novel hormonal agents and chemotherapy. ClinicalTrialsgov identifier: NCT05113537 https://clinicaltrialsgov/ct2/show/NCT05113537.
- Utah Uo. A phase ib dose-escalation study of cabozantinib in combination with lutetium-177 (177Lu)-PSMA-617 in patients with metastatic castration-resistant prostate cancer (mCRPC). [ Updated 2022 Nov 16]. Accessed Feb 8, 2023. ClinicalTrialsgov identifier: NCT05613894 https://clinicaltrialsgov/ct2/show/NCT05613894.
- Aggarwal R Immunogenic priming with PSMA-Targeted radioligand therapy in advanced prostate cancer: a phase 1b study of 177Lu-PSMA-617 in combination with pembrolizumab. [ Updated 2022 Apr 19]. AccessedFeb 8, 2023. ClinicalTrialsgov identifier: NCT03805594 https://clinicaltrialsgov/ct2/show/NCT03805594.
- Centre PMC. Phase Ib/II study of Radionuclide 177Lutetium-PSMA-617 therapy in combination with pembrolizumab for treatment of metastatic Castration Resistant Prostate Cancer (mCRPC). [ Updated 2022 May 25]. Accessed Feb 8, 2023. ClinicalTrialsgov identifier: NCT03658447 https://clinicaltrialsgov/ct2/show/NCT03658447.
- Group AaNZUaPCT. Phase II study of Radionuclide 177Lu-PSMA therapy versus 177Lu-PSMA in combination with ipilimumab and nivolumab for men with metastatic castration resistant prostate cancer (mCRPC). [ Updated 2022 Jun 10]. Accessed Feb 8, 2023. ClinicalTrialsgov Identifier: NCT05150236 https://clinicaltrialsgov/ct2/show/NCT05150236.
- NIH U.S. National Library of Medicine- ClinicalTrials.Gov. Accessed Jun 22, 2022. https://clinicaltrials.gov/ct2/results?cond=Prostate+Cancer&term=Lu177-PSMA&cntry=&state=&city=&dist=.
- Marinova M, Alamdar R, Ahmadzadehfar H, et al. Improving quality of life in patients with metastatic prostate cancer following one cycle of 177Lu-PSMA-617 radioligand therapy: a pilot study. Nuklearmedizin. 2020 Dec;59(6):409–414.
- Zang J, Fan X, Wang H, et al. First-in-human study of (177)Lu-EB-PSMA-617 in patients with metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2019 Jan;46(1):148–158.
- Zang J, Liu Q, Sui H, et al. (177)lu-EB-PSMA radioligand therapy with escalating doses in patients with metastatic castration-resistant prostate cancer. J Nucl Med. 2020 Dec;61(12):1772–1778.
- Plichta KA, Graves SA, Buatti JM. Prostate-Specific Membrane Antigen (PSMA) theranostics for treatment of oligometastatic prostate cancer. Int J Mol Sci. 2021 Nov 9;22(22):12095.
- Buteau JP, Martin AJ, Emmett L, et al. PSMA and FDG-PET as predictive and prognostic biomarkers in patients given [(177)Lu]Lu-PSMA-617 versus cabazitaxel for metastatic castration-resistant prostate cancer (TheraP): a biomarker analysis from a randomised, open-label, phase 2 trial. Lancet Oncol. 2022 Nov;23(11):1389–1397.
- Suman S, Parghane RV, Joshi A, et al. Therapeutic efficacy, prognostic variables and clinical outcome of (177)Lu-PSMA-617 PRLT in progressive mCRPC following multiple lines of treatment: prognostic implications of high FDG uptake on dual tracer PET-CT vis-a-vis Gleason score in such cohort. Br J Radiol. 2019 Dec;92(1104):20190380.
- Pathmanandavel S, Crumbaker M, Ho B, et al. Evaluation of (177)Lu-PSMA-617 SPECT/CT quantitation as a response biomarker within a prospective (177)Lu-PSMA-617 and NOX66 combination trial (LuPIN). J Nucl Med. 2023 Feb;64(2):221–226.
- Ferdinandus J, Violet J, Sandhu S, et al. Prognostic biomarkers in men with metastatic castration-resistant prostate cancer receiving [177Lu]-PSMA-617. Eur J Nucl Med Mol Imaging. 2020 Sep;47(10):2322–2327.
- Fizazi K, Massard C, Smith M, et al. Bone-related parameters are the main prognostic factors for overall survival in men with bone metastases from castration-resistant prostate cancer. Eur Urol. 2015 Jul;68(1):42–50.
- Ferdinandus J, Eppard E, Gaertner FC, et al. Predictors of response to radioligand therapy of metastatic castrate-resistant prostate cancer with 177Lu-PSMA-617. J Nucl Med. 2017 Feb;58(2):312–319.
- Ahmadzadehfar H, Schlolaut S, Fimmers R, et al. Predictors of overall survival in metastatic castration-resistant prostate cancer patients receiving [(177)Lu]Lu-PSMA-617 radioligand therapy. Oncotarget. 2017 Nov 28;8(61):103108–103116.
- Wrenger R, Juptner M, Marx M, et al. Pre- and intratherapeutic predictors of overall survival in patients with advanced metastasized castration-resistant prostate cancer receiving Lu-177-PSMA-617 radioligand therapy. BMC Urol. 2022 Jul 4;22(1):96.
- Nagaya N, Nagata M, Lu Y, et al. Prostate-specific membrane antigen in circulating tumor cells is a new poor prognostic marker for castration-resistant prostate cancer. PLoS ONE. 2020;15(1):e0226219. DOI:10.1371/journal.pone.0226219
- Paschalis A, Sheehan B, Riisnaes R, et al. Prostate-specific membrane antigen heterogeneity and DNA repair defects in prostate cancer. Eur Urol. 2019 Oct;76(4):469–478.
- Kuo PH, Rahbar K, Hesterman J, et al. [68ga]ga-PSMA-11 PET baseline imaging as a prognostic tool for clinical outcomes to [177Lu]Lu-PSMA-617 in patients with mCRPC: a VISION substudy. ASCO Annual Meeting. 2022;40(16):5002. DOI:10.1200/JCO.2022.40.16_suppl.5002
- Gafita A, Calais J, Grogan TR, et al. Nomograms to predict outcomes after (177)Lu-PSMA therapy in men with metastatic castration-resistant prostate cancer: an international, multicentre, retrospective study. Lancet Oncol. 2021 Aug;22(8):1115–1125.
- Sun M, Niaz MO, Nelson A, et al. Review of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer. Cureus. 2020 Jun 30;12(6):e8921.
- Stuparu AD, Capri JR, Meyer CAL, et al. Mechanisms of resistance to prostate-specific membrane antigen-targeted radioligand therapy in a mouse model of prostate cancer. J Nucl Med. 2021 Jul 1;62(7):989–995.
- Kratochwil C, Giesel FL, Heussel CP, et al. Patients resistant against PSMA-targeting alpha-radiation therapy often harbor mutations in DNA damage-repair-associated genes. J Nucl Med. 2020 May;61(5):683–688.
- Yadav MP, Ballal S, Sahoo RK, et al. Long-term outcome of 177Lu-PSMA-617 radioligand therapy in heavily pre-treated metastatic castration-resistant prostate cancer patients. PLoS ONE. 2021;16(5):e0251375. DOI:10.1371/journal.pone.0251375
- Biospace. Janux therapeutics announces FDA clearance of investigational new drug application for JANX007 Ap-TfMC-RPCM, 2022. Accessed Feb, 2023. https://www.biospace.com/article/releases/janux-therapeutics-announces-fda-clearance-of-investigational-new-drug-application-for-janx007-a-psma-tractr-for-metastatic-castration-resistant-prostate-cancer/.
