1. Introduction
In recent years, we have witnessed a progressive improvement in early breast cancer (BC) survival rates, mostly achieved by advancements in diagnostic capabilities and treatment efficacy. Although these breakthroughs have affected triple-negative BC (TNBC), this BC subtype is still associated with the poorest survival rates, showing a marked inclination to early relapse and high tropism for visceral involvement. These peculiar traits qualify TNBC as the most challenging BC subtype, clearly outlining an unmet medical need in the contemporary landscape of early BC.
The main source of uncertainty is the suboptimal capability of accurately predicting TNBC clinical outcomes at the single patient level and modulating therapies accordingly. However, recent progress is challenging the long-lasting recognition of TNBC as an indecipherable entity. It has become increasingly evident that two main pillars contribute to a wide grayscale of clinical outcomes: disease burden and biologic heterogeneity ().
Figure 1. Prognostic stratification of triple-negative breast cancer based on tumor burden and immune features (Created with BioRender.com).
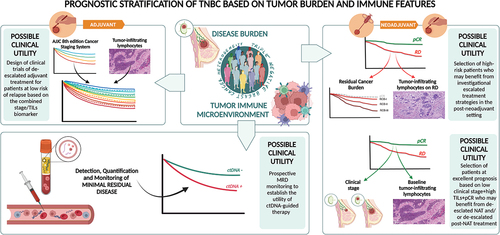
2. Prognostic stratification of TNBC based on disease burden and immune features with expert opinion
Undoubtfully, the molecular classification of TNBC in different subtypes according to gene expression profiles has improved our understanding of the complex biology of the disease [Citation1]. However, with regard to risk stratification refinement, most compelling results in terms of clinical validity and potential clinical utility have been obtained through the integration of disease burden features with tumor infiltrating lymphocytes (TILs) levels, the latter being a standardized proxy of TNBC immune microenvironment [Citation2]. Indeed, international guidelines endorse TILs as biomarkers to be routinely integrated into the pathologic report of TNBC [Citation3,Citation4], based on the demonstrated clinical validity in terms of prognostic ability [Citation4]. However, there is the as yet unanswered question of whether the use of TILs could directly guide the management of TNBC patients. So far, evidence supporting the clinical utility of this biomarker is still missing, thus making the conduction of clinical trial designed with this specific purpose highly awaited.
Within the ‘pure’ adjuvant setting, TIL levels were demonstrated to add significant prognostic information beyond that provided by traditional clinicopathologic factors [Citation5] and the adoption of a 30% TIL cutoff was proven to strongly downstage (high TILs) or upstage (low TILs) conventional pathological staging in the Pathological and Clinical Prognostic Stage Groups from the AJJC 8th edition Cancer Staging System [Citation6]. Interestingly, the integration of stage and TILs (node-negative disease + high TILs) was recently reported to be able to identify a subgroup of TNBC patients with excellent long-term survival even in the absence of systemic therapy. This approach, if transposed into the therapeutic experimental scenario of TNBC, could guide the design of clinical trials testing de-escalated adjuvant treatment for patients identified as having a low risk of relapse based on the combined stage/TIL biomarker.
The majority of TNBC candidates who receive systemic treatments for early disease are currently managed within the neoadjuvant platform. According to this, the main considerations that can be made in terms of prognostication are based on the well-established assumption that patients achieving a pathologic complete response (pCR) after neoadjuvant treatment (NAT) are expected to experience significantly more favorable outcome as compared to those showing some degree of invasive residual disease (RD). This dichotomization in pCR/no-pCR is solidly associated with outcome at a single patient level, to the extent that it has implications in terms of design of registration trials and, accordingly, from a regulatory point of view [Citation7,Citation8]. However, it is now well-accepted that not all patients achieving pCR are cured and not all patients with RD will eventually relapse.
One of the first successful attempts to optimize this stratification has been a more in-depth evaluation of RD, by both quantifying and qualifying its extent [Citation9], into the composite score of residual cancer burden (RCB). RCB was proven to be able to reliably predict long-term survival after NAT in all BC subtypes, including TNBC, and its evaluation is currently endorsed as a standardized and reproducible tool to be incorporated into post-NAT histological reports [Citation3].
In the process of refining TNBC risk stratification in the neoadjuvant setting, the weight of the immune microenvironment cannot be neglected. The baseline assessment of TILs on pre-NAT tumor samples not only is able to predict the likelihood of pCR but also shows an association with survival that could stand beyond pCR [Citation10,Citation11]. Indeed, it is becoming increasingly evident that a significant degree of heterogeneity exists within TNBC patients achieving pCR. This hitherto neglected notion may acquire crucial relevance in the contemporary scenario characterized by the availability of post-operative escalated treatments irrespective of whether pCR is achieved (namely, pembrolizumab based on KEYNOTE522 trial results [Citation11]). With this regard, the role of TILs in discriminating patients with pCR at different prognoses deserves to be clearly established by current and future research. Ideally, high baseline TILs could contribute to isolating pCR patients who are really cured from those still at risk of relapse and to modulating adjuvant treatments accordingly. In terms of design of trials testing de-escalated neoadjuvant approaches, high baseline TILs could be adopted as an inclusion criterion and the integration of information on disease burden (stage at diagnosis and/or pathologic response) could then lead to isolating patients at truly excellent prognosis who may not need additional treatments in the post-neoadjuvant setting. This approach deserves to be prioritized in the research agenda.
With regard to patients without pCR, TILs assessed on the surgical sample post-NAT are strongly prognostic. In particular, the presence of high TIL levels in RD after NAT has been proven to be associated with a reduced risk of relapse and death [Citation12,Citation13], with the highest degree of prognostic information achievable with the integration of RCB and RD TILs [Citation13]. A variation on this theme, which further solidifies the value of the immune compartment of TNBC microenvironment and highlights the complexity of the interplay between TNBC, immune system, and chemotherapy, is the observation that an increase in TIL levels from baseline biopsy to RD after NAT exposure is associated with a positive impact on prognosis [Citation14]. These data make the appraisal of RD-TILs emerging as a strategical tool for a more rational selection of high-risk patients who may deserve investigational escalated treatment strategies in the post-neoadjuvant setting.
The detection, quantification, and monitoring of molecular residual disease (MRD) by liquid biopsy following treatments with curative intent represent an opportunity to further refine the concept of disease burden. In particular, available evidence supports the detection of MRD as strongly prognostic in TNBC patients, with ctDNA positivity negatively affecting prognosis and, conversely, ctDNA negativity being associated with improved survival, even in patients with no pCR after NAT [Citation15]. Unfortunately, the attempts to translate these efforts into clinical utility in TNBC patients have been unsuccessful so far, since this biomarker failed the prospective demonstration of actionability and did not result in improved patients’ outcome [Citation16]. The success of this process will probably depend on the overcoming of the current limitations in terms of test sensitivity and specificity and the uncertainties regarding optimal timing and patient selection.
In recent years, we have witnessed a mushrooming of artificial intelligence (AI)-based tools outlining a potential future scenario where AI may actually find a placement in TNBC prognostication. In the short-term perspective, one of the most useful applications of AI to TNBC stratification may be represented by machine learning models developed to assist in the quantification of TILs, thus possibly contributing to overcoming limitations in terms of inter- and intra-observer variability. Other futuristic application of AI might be the development of risk-prediction models for specific metastatic patterns as well as artificial neural networks to identify prognostic molecular signatures [Citation17]. Of course, several unsolved technical, validation, standardization, and sustainability issues currently limit the clinical implementation of such approaches.
Declaration of interest
F.M. reports personal fees from Roche, Novartis, Gilead, Seagen, and Pfizer, outside the submitted work. M.V.D. reports personal fees from Eli Lilly, Pfizer, Novartis, Roche, Astrazeneca, Merck Sharp & Dohme, BMS, Daiichi-Sankyo, Gilead, Exact Sciences, and Seagen, outside the submitted work.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers of this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Bianchini G, Angelis CD, Licata L, et al. Treatment landscape of triple-negative breast cancer — expanded options, evolving needs. Nat Rev Clin Oncol. 2022;19(2):91–113. doi: 10.1038/s41571-021-00565-2
- Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an international TILs Working group 2014. Ann Oncol. 2015;26(2):259–271. doi: 10.1093/annonc/mdu450
- Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(8):1194–1220. doi: 10.1093/annonc/mdz173
- Burstein HJ, Curigliano G, Thürlimann B, et al. Customizing local and systemic therapies for women with early breast cancer: the St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann Oncol. 2021;32(10):1216–1235. doi: 10.1016/j.annonc.2021.06.023
- Loi S, Drubay D, Adams S, et al. Tumor-infiltrating lymphocytes and prognosis: a pooled individual patient analysis of early-stage triple-negative breast cancers. J Clin Oncol. 2019;37(7):559–569. doi: 10.1200/JCO.18.01010
- Loi S, Salgado R, Adams S, et al. Tumor infiltrating lymphocyte stratification of prognostic staging of early-stage triple negative breast cancer. NPJ Breast Cancer. 2022;8(1):3. doi: 10.1038/s41523-021-00362-1
- Prowell TM, Pazdur R. Pathological complete response and accelerated drug approval in early breast cancer. N Engl J Med. 2012;366(26):2438–2441. doi: 10.1056/NEJMp1205737
- Prowell TM, Beaver JA, Pazdur R. Residual disease after neoadjuvant therapy - developing drugs for high-risk early breast cancer. N Engl J Med. 2019;380:612–615. doi: 10.1056/NEJMp1900079
- Yau C, Osdoit M, van der NM, et al. Residual cancer burden after neoadjuvant chemotherapy and long-term survival outcomes in breast cancer: a multicentre pooled analysis of 5161 patients. Lancet Oncol. 2022;23(1):149–160. doi: 10.1016/S1470-2045(21)00589-1
- Dieci MV, Tsvetkova V, Griguolo G, et al. Integration of tumour infiltrating lymphocytes, programmed cell-death ligand-1, CD8 and FOXP3 in prognostic models for triple-negative breast cancer: analysis of 244 stage I–III patients treated with standard therapy. Eur J Cancer. 2020;136:7–15. doi: 10.1016/j.ejca.2020.05.014
- Gluz O, Nitz U, Kolberg-Liedtke C, et al. De-escalated neoadjuvant chemotherapy in early triple-negative breast cancer (TNBC): impact of molecular markers and final survival analysis of the WSG-ADAPT-TN trial. Clin Cancer Res. 2022;28(22):4995–5003. doi: 10.1158/1078-0432.CCR-22-0482
- Dieci MV, Criscitiello C, Goubar A, et al. Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Ann Oncol. 2014;25(3):611–618. doi: 10.1093/annonc/mdt556
- Luen SJ, Salgado R, Dieci MV, et al. Prognostic implications of residual disease tumor-infiltrating lymphocytes and residual cancer burden in triple-negative breast cancer patients after neoadjuvant chemotherapy. Ann Oncol. 2019;30(2):236–242. doi: 10.1093/annonc/mdy547
- Dieci MV, Carbognin L, Miglietta F, et al. Incorporating weekly carboplatin in anthracycline and paclitaxel-containing neoadjuvant chemotherapy for triple-negative breast cancer: propensity-score matching analysis and TIL evaluation. Br J Cancer. 2023;128(2):266–274. doi: 10.1038/s41416-022-02050-8
- Magbanua MJM, Swigart LB, Ahmed Z, et al. Clinical significance and biology of circulating tumor DNA in high-risk early-stage HER2-negative breast cancer receiving neoadjuvant chemotherapy. Cancer Cell. 2023;41(6):1091–1102.e4. doi: 10.1016/j.ccell.2023.04.008
- Simon R. Clinical trial designs for evaluating the medical utility of prognostic and predictive biomarkers in oncology. Per Med. 2010;7(1):33–47. doi: 10.2217/pme.09.49
- Guo J, Hu J, Zheng Y, et al. Artificial intelligence: opportunities and challenges in the clinical applications of triple-negative breast cancer. Br J Cancer. 2023;128(12):2141–2149. doi: 10.1038/s41416-023-02215-z
