ABSTRACT
Introduction
Despite the considerable progress made in cancer treatment through the development of target therapies, pancreatic ductal adenocarcinoma (PDAC) continues to exhibit resistance to this category of drugs. As a result, chemotherapy combination regimens remain the primary treatment approach for this aggressive cancer.
Areas covered
In this review, we provide an in-depth analysis of past and ongoing trials on both well-known and novel targets that are being explored in PDAC, including PARP, EGFR, HER2, KRAS, and its downstream and upstream pathways (such as RAF/MEK/ERK and PI3K/AKT/mTOR), JAK/STAT pathway, angiogenesis, metabolisms, epigenetic targets, claudin, and novel targets (such as P53 and plectin). We also provide a comprehensive overview of the significant trials for each target, allowing a thorough glimpse into the past and future of target therapy.
Expert opinion
The path toward implementing a target therapy capable of improving the overall survival of PDAC is still long, and it is unlikely that a monotherapy target drug will fulfill a meaningful role in addressing the complexity of this cancer. Thus, we discuss the future direction of target therapies in PDAC, trying to identify the more promising target and combination treatments, with a special focus on the more eagerly awaited ongoing trials.
1. Introduction: current therapies and genetic landscape in pancreatic cancer
The treatment of pancreatic ductal adenocarcinoma (PDAC) remains one of the greatest challenges in oncology, since, despite the numerous advances made in pharmacology with the development of new drugs, this cancer remains stubbornly impervious to oncological treatments. While target therapies have become established pillars in the treatment of other cancers like breast cancer, lung cancer, or colon cancer, PDAC still relies predominantly on chemotherapy as its treatment foundation [Citation1–3]. In the resected setting, standard adjuvant treatment is usually the chemotherapy regimen modified FOLFIRINOX (mFOLFIRINOX: fluorouracil, irinotecan, oxaliplatin), associated with overall survival (OS) of 54 months [Citation4] and survival in the locally advanced setting, even with chemotherapy (FOLFIRINOX or gemcitabine plus nab-paclitaxel) and radiation therapy is around two years [Citation5,Citation6]. In the metastatic setting, again with the same regimens of FOLFIRINOX, gemcitabine plus nab-paclitaxel, or with the recently introduced NALIRIFOX (fluorouracil, liposomal irinotecan, and oxaliplatin), median overall survival (mOS) does not reach 12 months [Citation7–9]. Regarding second or further lines of therapies, there is no established standard of care, and both chemotherapy or enrollment in clinical trials are possible options if the patient retains a sufficient performance status [Citation10].
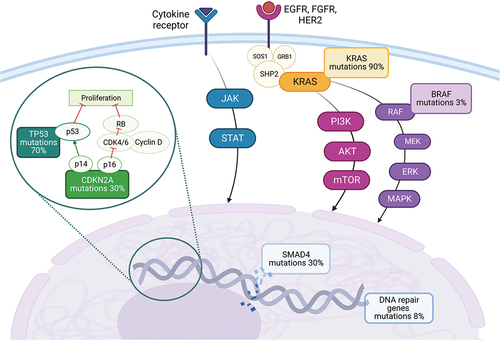
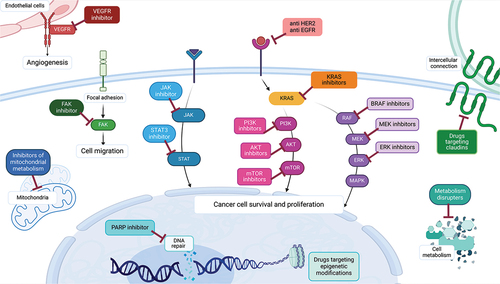
Given these meager survival data, numerous trials have been carried out with novel target drugs, hoping to improve OS and response rates. The genetic landscape of PDAC has now been widely analyzed and, despite having a low tumor mutational burden, there are several genes that are frequently mutated including KRAS (90% of cases), TP53 (70%), CDKN2A (30%), and SMAD4 (30%) (). Furthermore, there are more rarely altered genes which may potentially be associated with targeted therapeutical options, such as BRAF (3%), FGFR1 (5%), MYC (5%), MDM2 (2%), BRCA1 and 2 (1% and 4% respectively), ATM (5%), PALB2 (1%) and mismatch repair genes (2%) [Citation11–13] (). In this review, we aim to give an in-depth analysis of both well-known and novel targets that are being explored in PDAC (), including PARP, EGFR, HER2, KRAS, and its downstream and upstream pathways, JAK/STAT pathway, angiogenesis, metabolism, epigenetic targets, claudin, and novel targets [Citation14].
2. PARP
Approximately 5–20% of the patients have a genetic predisposition to PDAC development, with nearly 8–9% presenting germinal mutations of BRCA genes or exhibiting somatic mutation in DNA repair genes [Citation15,Citation16]. This information paved the way for the testing of PARP inhibitors in this setting (), drawing from the positive experience already obtained in other tumors with the same genetic signature, such as ovarian or breast cancers [Citation17,Citation18]. BRCA genes are involved in the repair of damaged DNA, particularly when double-strand breaks occur. Thus, the loss of function of these genes causes an accumulation of DNA errors, facilitating the process of oncological transformation. Simultaneously, PARP enzymes are also involved in DNA repair and the loss of BRCA makes them essential as they represent the sole way for the cell to correct DNA damage. Thus, the rationale of PARP inhibitors’ employment in neoplasia with identified BRCA mutation is to induce synthetic lethality, leading cancer cells to death and making replication processes impossible [Citation19,Citation20].
Table 1. Ongoing clinical trials in pancreatic cancer with PARP inhibitors.
In 2019, based on the results of the POLO trial, the FDA approved the first PARP inhibitor, olaparib, as maintenance therapy in BRCA-mutated metastatic PDAC patients who did not progress after ≥16 weeks on platinum-based chemotherapy [Citation21,Citation22]. Significant advantages in terms of progression-free survival (PFS) (16.6 vs 9.3 months) and preservation of quality of life were found compared to placebo, but no statistically significant differences in mOS were achieved, although survival rates at 36 months were almost doubled in the olaparib arm [Citation21]. Considering other settings, the ongoing phase II APOLLO trial (NCT04858334) [Citation23] is trying to extend olaparib administration to resected, BRCA1/2 or PALB2 mutated PDAC, following positive experience from the OlympiA trial [Citation18] in early-stage breast cancer.
Given the experience with olaparib, it is reasonable to expect that other PARP inhibitors might similarly improve oncological outcomes. For example, a single-arm phase 2 trial has recently investigated the role of rucaparib as maintenance therapy in platinum-sensitive advanced PDAC, demonstrating its effectiveness in prolonging PFS and OS in patients with BRCA1, BRCA1, or PALB2 germinal or somatic mutations [Citation24]. The phase 3 RUCAPANC trial evaluated rucaparib monotherapy in previously treated PDAC, both in metastatic and locally advanced settings, confirming a positive impact on overall response rate (ORR) (15%) and disease control rate (DCR) (16%). Notably, in this trial rucaparib was administered both in platinum-sensitive and platinum-resistant patients, with oncological benefits observed mainly in the first group [Citation16].
Other ongoing trials are also investigating the role of another PARP inhibitor, niraparib, in metastatic PDAC patients with germinal mutation in DNA repair genes, including BRCA, PALB2, ATM, NBN, ATR, BRIP1, IDH, RAD51, RAD51B/C/D, RAD54L, CDK12, BARD1, FAM175A, BAP1, CHEK1/2, GEN1, MRE11A, XRCC2, SHFM1, FANCD2, FANCA, FANCC, FANCG, RPA1, ARID1A (NCT03553004) [Citation25]. Furthermore, a single-arm phase 2 trial is recruiting chemotherapy-naïve metastatic PDAC patients with deficiency of homologous recombination (HR) genes activity to test niraparib as a first-line treatment (NCT05442749) [Citation26]. Another PARP inhibitor, veliparib, was also evaluated in PDAC. A single-arm phase 2 trial enrolled 16 patients with pretreated, BRCA1/2 or PALB2 mutated, stage III or IV PDAC who then underwent treatment with veliparib monotherapy. Unfortunately, no significant benefits on oncological outcomes were observed, even when veliparib was combined with FOLFIRI [Citation27,Citation28].
Similarly, many other ongoing trials are evaluating combination therapy, mainly associating PARP inhibitors with chemotherapy or checkpoint inhibitors. Concerning PARP-immunotherapy combinations, a phase 1b/2 trial has recently explored the addition of niraparib to nivolumab or ipilimumab as a maintenance strategy in patients with metastatic PDAC who had not progressed to platinum-based chemotherapy (regardless of DNA damage response status). At the interim analysis, positive results in terms of 6-months PFS seem to emerge, particularly for the olaparib and anti-CTLA-4 ipilimumab association (NCT03404960) [Citation29]. Another phase 2 trial is investigating whether the addition of pembrolizumab to olaparib in germline BRCA 1/2 mutated metastatic PDAC confers a longer PFS compared to olaparib monotherapy during maintenance (NCT04548752) [Citation30]. Another not-yet-recruiting trial will soon evaluate pembrolizumab combined with olaparib in metastatic PDAC with a high tumor mutation burden (>4 mutations/Mb, including Microsatellite Instability (MSI) high, MMRd) (NCT05093231) [Citation31]. Finally, one more combination trial is testing a new first-line treatment protocol with low-dose multi-drug chemotherapy followed by maintenance with olaparib and pembrolizumab [Citation32] (NCT04753879). The results of this trial are eagerly awaited.
3. EGFR
Epidermal growth factor receptor (EGFR) is a tyrosine kinase receptor that is often overexpressed in PDAC, thus numerous trials have tried to use EGFR inhibitors in this cancer (), initially in combination with chemotherapy [Citation33]. One of the most famous phase 3 trials with these target drugs included the combination of first-generation tyrosine kinase inhibitor (TKI) erlotinib plus gemcitabine versus gemcitabine monotherapy as a first-line treatment; the combination arm was formally superior to the standard treatment. However, the difference in OS was only of a few weeks (6.24 vs 5.91 months), and it was not considered clinically relevant [Citation34]. Following trials aimed at improving these results failed, such as a phase 2 trial with the combination of gemcitabine, oxaliplatin, and erlotinib [Citation35], or a phase 2 trial using second-generation TKI afatinib with gemcitabine [Citation36], both in treatment naïve advanced patients. On the other hand, the combination of erlotinib with a more updated chemotherapy regimen for first-line treatment, such as gemcitabine and nab-paclitaxel, was met with interesting clinical activity, but the reported toxicities were too high [Citation37]. The use of EGFR TKI did not have much success in the localized setting as well, as in the adjuvant therapy in combination with gemcitabine there was no improvement in survival [Citation38]. Similarly, in the neoadjuvant setting, it was combined with gemcitabine and radiotherapy, but despite interesting initial results, it was never tested in a phase 3 trial [Citation39].
Regarding the role of antibodies against EGFR, cetuximab did not meaningfully improve survival in first-line therapy alongside gemcitabine [Citation40]; however, interestingly results have been published on another antibody, nimotuzumab. First, a phase 2/3 trial saw the combination of nimotuzumab and gemcitabine performing better than gemcitabine as first-line treatment 8.6 versus 6.0 months) [Citation41], then a similar phase 3 trial including Chinese patients KRAS wild type (WT) confirmed the advantage in OS (10.9 versus 8.5 months for gemcitabine + placebo) [Citation42]. Unfortunately, to our knowledge, there are no other ongoing trials comparing more standard first-line chemotherapy regimens with or without the addition of nimotuzumab and no other promising phase 3 trials are ongoing involving EGFR inhibitors.
4. Her2
In the last decades, target therapies directed against the Human Epidermal Growth Factor Receptor-2 (HER2) have revolutionized the treatment of many tumors, radically changing patients’ prognosis in various settings [Citation43–46]. HER-2 is a tyrosine kinase receptor that mediates a signaling pathway involved in cellular growth, differentiation, and survival. When the HER-2 gene is pathologically amplified or overexpressed, the cell is prompted to proliferate, and cancer development is facilitated. The role of HER-2 in PDAC is still controversial: the frequency of overexpressed HER2 protein or amplified HER2 gene is highly variable, ranging from very low to high percentages in different data sets (6–61% and 2–24%). Its impact on prognosis is not well established, although some papers point to worse oncological outcomes in HER2-positive patients [Citation47,Citation48]. On this basis, a growing number of studies are evaluating anti-HER2 therapies in PDAC, although unfortunately only a few are specifically tailored to this tumor, and most of the evidence available mainly comes from basket trials ().
In this context, an ongoing phase 3 basket trial (DETERMINE) is testing the impact of the anti HER2 combination of antibodies trastuzumab and pertuzumab in many rare, non-breast, HER-2 positive solid neoplasia, including PDAC (NCT05786716) [Citation49]. Furthermore, the phase 2 DESTINY-PanTumor02 trial is evaluating ADC trastuzumab deruxtecan in patients with seven different types of HER2-expressing solid cancers, including PDAC (NCT04482309) [Citation50]. At the primary analysis, an ORR of 37.1% was registered but unfortunately, in the subgroup analysis, it clearly appears that the PDAC cohort obtained the lowest benefit (4%) [Citation51]. Moreover, while in other tumors an additional increase in ORR was found in the case of frankly overexpressed HER2 (3+ in immunohistochemistry), in the pancreatic cohort, this particular subgroup of patients did not show any benefit [Citation51]. The ongoing DESTINY-PanTumor01 trial similarly focuses on a wide range of solid neoplasia, including PDAC, enrolling patients with HER2 activating mutations (but no amplification): primary results were presented at ESMO 2023, confirming T-DXd’s great success in other types of cancer but not in PDAC, where the 2 included patients did not respond to treatment (NCT04639219) [Citation52]. Additionally, the KAMELEON trial tried to evaluate anti HER2-ADC trastuzumab emtansine (TDM1) in HER2-positive cancers, including bladder/urothelial cancers, cholangiocarcinoma, and PDAC. Regrettably, the study was closed prematurely due to difficulties in patient enrollment. Nevertheless, in the small cohort of patients affected by biliopancreatic tumors, a single case of partial response to TDM1 in a PDAC patient was observed, with a corresponding ORR of 14.3% [Citation53].
Other approaches that have been tried in the past decades include combination therapies of anti HER2 drugs with chemotherapy or other targets, although with disappointing results. For example, the phase 2 GATE-1 trial evaluated gemcitabine associated with trastuzumab and anti EGFR TKI erlotinib in first-line metastatic PDAC [Citation54], and the phase 1/2 THERAPY trial investigated anti EGFR antibody cetuximab plus trastuzumab in metastatic PDAC who had progressed to gemcitabine-based chemotherapy [Citation55]. None of these studies achieved substantial efficacy results or they were burdened with severe cutaneous toxicity. Another combination of anti HER2 lapatinib in combination with capecitabine was investigated, without the remarkable findings [Citation56,Citation57].
Regarding novel drugs, various combinations have been explored in the advanced setting, associating drugs that are not already approved in clinical practice with HER2-targeted monoclonal antibodies. For instance, two phase 1 basket trials tested trastuzumab associated with the farnesyl transferase inhibitor R115777 (NCT00005842) [Citation58] and with interleukin-12 (NCT00004074), respectively [Citation59]. No practice-changing results were found. Finally, there is a lack of evidence concerning the neoadjuvant and adjuvant settings. A phase 2 trial evaluated weekly trastuzumab combined with gemcitabine and concurrent radiotherapy in patients with regionally confined HER2-positive PDAC. However, the results had never been published [Citation60].
5. KRAS
The KRAS gene encodes for signal transduction molecules that can affect multiple pathways related to tumor survival, including the RAF-MEK-ERK pathway and the PI3K/AKT/mTOR pathway, moreover, several tyrosine kinase receptors have the ability to activate KRAS, through the mediation of other protein such as SHP2, GRB2, and SOS1 (, ) [Citation61]. KRAS mutations are present in about 90% of PDAC, but only 1–2% of cases have the G12C alteration susceptible to established KRAS inhibitors used in other type of cancer, such as sotorasib or adagrasib [Citation62–64]. Sotorasib has been evaluated in a phase 1/2 trial with pretreated metastatic KRAS G12C mutated PDAC patients, achieving an ORR of 21%, DCR of 84%, and OS of 6.9 months, with an acceptable safety profile [Citation65]. In the same subset of patients, adagrasib (also known as MRTX849) is being evaluated alone (NCT05634525) [Citation66] or in combination with other drugs, such as anti SHP2 inhibitor, TNO155 (NCT04330664) [Citation67], or PARP Inhibitor olaparib (NCT06130254) [Citation68]. There are also new KRAS G12C inhibitors being tested in phase 1 trials, such as RMC-6291 (NCT05462717) [Citation69], or in phase 2 trials such as JAB-21822 (NCT06008288) [Citation70], following promising results from a previous phase 1 trial including 2 PDAC patients [Citation71].
Table 2. Ongoing clinical trials in pancreatic cancer with KRAS inhibitors.
Given that most of the other cases of KRAS mutated patients exhibit KRAS mutations different from G12C, with G12D and G12V being the most frequent (40 and 30% respectively), several new specific drugs are being tested [Citation72]. These include the anti KRAS G12D drug MRTX1133 (NCT05737706) [Citation73], which had some promising preclinical data [Citation72], and the inhibitor RMC-9805 (NCT06040541) [Citation74]. An interesting 1/2a trial included the use of siG12D-LODER™, an implant with siRNA directed against KRAS G12D, in combination with chemotherapy in locally advanced PDAC (NCT01188785) [Citation75]: the combination achieved disease control in 12/15 patients and OS of 15.2 months; however, 5 patients experienced serious adverse events [Citation76]. A phase 2 trial was also performed, but the status of the trial is unknown (NCT01676259) [Citation77]. Another phase 1 trial investigating siRNA targeting KRAS G12D in metastatic PDAC patients is also ongoing, with the idea of using exosomes derived from mesenchymal stromal cells loaded with the siRNA as a method of intracellular delivery [Citation78] (NCT03608631) [Citation79]. Furthermore, specific proteolysis-targeting chimera (PROTAC) protein degraders are being tested, which have the ability to bind to their target and trigger ubiquitination; for example, ASP3082 is being tested in KRAS G12D mutated solid cancers, alone or in combination with EGFR target drugs (NCT05382559) [Citation80] and other pan-KRAS degrader are being developed [Citation81].
Drugs targeting multiple KRAS mutations are also being tested. For example, RMC-6236 has been proven to inhibit multiple KRAS mutations, suppressing the phosphorylation of the downstream effector ERK and leading to growth suppression in multiple cell lines, including PDAC [Citation82]. It is being tested in multiple advanced neoplasia including PDAC in a phase 1/1b trial (NCT05379985) [Citation83] with initial results indicating good tolerability [Citation84], and in combination with KRAS G12C specific inhibitor RMC-6291 (NCT06128551) [Citation85].
5.1. RAS-related proliferation pathways (RAF/MEK/ERK, PI3K/AKT/mTOR)
Other than inhibiting KRAS directly, several drugs have been developed to disrupt its upstream or downstream targets, including proteins involved in proliferation pathways such as RAF/MEK/ERK and PI3K/AKT/mTOR (). Binimetinib and trametinib, two MEK inhibitors that are already used in clinical practice in melanoma patients [Citation86,Citation87], have also been tested in PDAC. Binimetinib was tested in pretreated metastatic patients alongside PD-L1 inhibitor avelumab or PARP inhibitor talazoparib (NCT03637491) [Citation88]; however, the trial reported high percentages of dose-limiting toxicities [Citation89]. Similarly, in a phase 1 trial of trametinib plus CDK4/6 inhibitor ribociclib, tolerability was acceptable but efficacy was limited [Citation90]. Moreover, the combination of trametinib plus gemcitabine vs gemcitabine monotherapy did not increase survival rates or response rates, both in KRAS mutated or WT patients [Citation91]. One other ongoing trial is investigating the combination of binimetinib plus PARP inhibitor palbociclib as a neoadjuvant treatment in lung, colorectal, or PDAC (NCT04870034) [Citation92]. Furthermore, two phase 1 trials are investigating the combination of binimetinib or trametinib plus antimalarial drug hydroxychloroquine in PDAC (NCT04132505, NCT03825289) [Citation93,Citation94].
The novel MEK1/2 inhibitor selumetinib was tested in pretreated PDAC patients harboring the KRAS G12R mutation (NCT03040986) [Citation95], but with a discouraging PFS of 3 months, no objective responses and disease stability in 3 out of 8 patients [Citation96]. Nonetheless, there are other innovative MEK inhibitors being tested, including ABM-168 (NCT05831995) [Citation97] and IMM-1-104, a dual-MEK inhibitor (NCT05585320) [Citation98], in RAS mutated or RAS/RAF/NF-1 mutated advanced solid tumors, respectively.
However, most of the trials involving MEK and other KRAS downstream effectors are usually combination trials involving multiple proteins of the same pathway or several targets from different pathways. For example, a phase 1–2 trial is now exploring the combination of anti RAF/MEK inhibitor avutometinib and anti FAK (a common resistance mechanism to RAF/MEK inhibition) defactinib plus gemcitabine and nab-paclitaxel in treatment-naïve PDAC patients (NCT05669482) [Citation99], following encouraging results of preclinical studies in PDAC and in ovarian cancer [Citation100,Citation101]. However, it should be noted that a similar combination, including only target drugs, trametinib plus FAK inhibitor GSK2256098, did not show promising activity results in a phase 2 trial (NCT02428270) [Citation102,Citation103]. Similarly, another trial involving MEK inhibitor selumetinib plus EGFR inhibitor erlotinib in pretreated PDAC patients had only modest antitumoral activity [Citation104].
Another target being explored is ERK: its inhibitor ulixertinib was tested alongside gemcitabine and nab-paclitaxel in treatment naïve patients but the trial was interrupted prematurely due to the high rate of all-grade treatment toxicities [Citation105]. Ulixertinib is also being tested alongside CDK4/6 inhibitor palbociclib in solid tumors with a specific expansion cohort for metastatic PDAC independent of their mutational status (NCT03454035) [Citation106,Citation107], while another ERK inhibitor, ERAS-007, is also being tested with palbociclib in KRAS or NRAS mutated PDAC (NCT05039177) [Citation108]. Notably, the double targeting of ERK and other proteins from the same pathway was also experimented on, but in the case of ERK1/2 inhibitor GDC‐0994 and MEK inhibitor cobimetinib, the trial was terminated before completion due to high levels of adverse events [Citation109].
Several other combination drugs can be found in ongoing trials such as: MEK inhibitor trametinib and pan RAF inhibitor naporafenib (NCT05907304) [Citation110], trametinib, and ULK inhibitor DCC-3116 (ULK are kinases involved in autophagy) (NCT04892017) [Citation111,Citation112], BRAF inhibitor vemurafenib plus multi TKI sorafenib (NCT05068752) [Citation113], anti ERK inhibitor LY3214996 plus SHP2 inhibitor RMC-4630 (NCT04916236) [Citation114], and KRAS G12C inhibitor JAB-21822 in KRAS G12C mutated solid tumors (NCT05288205) [Citation115,Citation116]. As stated before, SHP2 is a mediator in the KRAS pathway, thus several basket trials are now ongoing with SHP2 inhibitors monotherapy, such as HBI-2376 in KRAS or EGFR mutated tumors (NCT05163028) [Citation117] and JAB-3312 (NCT04045496, NCT04121286) [Citation118,Citation119].
Based on preclinical data, pointing to the activation of the PI3K pathway as a possible resistance mechanism to the sole blockade of the MEK pathway [Citation120], the idea of targeting both RAF/MEK/ERK and PI3K/AKT/mTOR pathway has been tested in PDAC [Citation121], although with not very encouraging results. Data from a phase 1b trial investigating MEK inhibitor binimetinib and PI3K inhibitor buparlisib in multiple advanced cancer with RAS/RAF alterations indicate higher than expected toxicities [Citation122] and a phase 1b trial with buparlisib and trametinib reported only stable disease as the best response in PDAC [Citation123]. Similarly, the combination of MEK 1/2 inhibitor selumetinib and AKT inhibitor MK2206 was compared to mFOLFOX in pretreated metastatic PDAC patients, but the experimental arm had worse OS and tolerability data compared to chemotherapy [Citation124].
Furthermore, many other drugs involving the PI3K/AKT/mTOR that are being tested, mostly in combination with cyclin-dependent kinase (CDK) inhibitors, especially CDK4/6 inhibitors, following encouraging results in preclinical data indicating a synergistic activity [Citation125–127]. The rationale for this combination can be found in the frequent loss of the CDKN2A gene in PDAC, which normally encodes for p16, a CDK4/6 and CDK2 inhibitor. The loss of CDKN2A is thus associated with increased activation of CDK4/6; however, the use of CDK4/6 inhibitor monotherapy did not yield good results for PDAC in published data, possibly due to the increased activity of other proliferation pathways such as the PI3K/AKT/mTOR one [Citation128]. The results of a phase 1 trial of the mTOR inhibitor everolimus plus CDK4/6 inhibitor ribociclib in heavily pretreated PDAC patients indicate that the combination was well tolerated but not very effective in this setting [Citation126]. Similarly, the combination of CDK4/6 inhibitor abemaciclib with PI3K/mTOR inhibitor LY3023414 in pretreated PDAC only reached a DCR of 12%, compared to the 36% of standard of care chemotherapy [Citation129]. Following promising preclinical data [Citation130], the combination of AKT inhibitor MK2206 with CDK4/6 inhibitor dinaciclib (NCT01783171) [Citation131] was also conducted but with no reported clinical benefit [Citation132]. On the other hand, a trial with PI3K/mTOR inhibitor gedatolisib with palbociclib is still ongoing (NCT03065062) [Citation133].
6. JAK/STAT
Activated by different cytokine and growth factors’ receptors () and deeply interconnected with many other intracellular mechanisms, the JAK/STAT signaling pathway is crucial for cellular functions, such as apoptosis, inflammation, and immune response, making it a promising target in PDAC [Citation134] (). Several JAK/STAT inhibitors have been investigated in the last decade, initially in combination with chemotherapy but with poor results. Two phase 3 trials with JAK inhibitor ruxolitinib in association with capecitabine as a second-line therapy in PDAC patients failed to improve outcomes [Citation135]. Similarly, in a phase 1 trial, the combination of anti JAK/TBK1 momelotinib in combination with gemcitabine and nab-paclitaxel in metastatic treatment-naïve patients did not improve OS [Citation136]. On the other hand, the results of a phase 1b/2 trial with JAK1 inhibitor itacitinib in combination with gemcitabine and nab-paclitaxel in PDAC patients showed an interesting ORR of 24%; however, the trial was terminated early due to sponsor’s decision based on the discouraging results from the previously mentioned phase 3 trial with ruxolitinib and capecitabine [Citation137].
Table 3. Ongoing clinical trials in pancreatic cancer with JAK/STAT inhibitors.
Given these discouraging results for the combination of JAK/STAT inhibitors with chemotherapy, several ongoing trials are exploring new combinations, such as the joint blockade of both RAF/MEK/ERK pathway and JAK/STAT pathway or the addition of immunotherapy. For example, a phase 1 trial is testing the combination of JAK inhibitor ruxolitinib plus MEK inhibitor trametinib (NCT04303403) [Citation138] and another is combining ruxolitinib plus trametinib and anti PD-1 retifanlimab, following encouraging preclinical results indicating a pro-immunogenic role of these target drugs (NCT05440942) [Citation139,Citation140]. A phase 2 trial is also testing anti STAT3 danvatirsen with anti PD-L1 durvalumab in advanced PDAC, lung, and colon cancer (NCT02983578) [Citation141]. Notably, a phase 1 basket trial with itacitinib and PD-L1 inhibitor pembrolizumab was also conducted, but no results have been published (NCT02646748) [Citation142]. Interestingly, a STAT3 inhibitor TTI-101 is also being investigated in conjunction with radiation therapy in borderline resectable PDAC (NCT06141031) [Citation143].
7. Antiangiogenic therapies
Neoangiogenesis is a fundamental process in cancer, as it allows for cancer growth and metastatic spread. Based on this assumption, in the 2010s several antiangiogenic drugs have been tested in PDAC, without much success (). For example, in two phase 3 trials, the anti-vascular endothelial growth factor (VEGF) drug bevacizumab was tested alongside gemcitabine with no survival gain, and even when the combination included also the anti EGFR erlotinib, only a small gain in PFS was registered, but no OS gain [Citation144,Citation145]. Another VEGF inhibitor, aflibercept, also failed to improve survival results in combination with gemcitabine [Citation146]. Similarly, the anti VEGF receptor (VEGR) ramucirumab was tested alongside mFOLFIRINOX in a phase 2 trial for advanced PDAC patients, without any improvement in OS or ORR [Citation147].
Various multi-target TKIs affecting VEGR have been investigated, always with poor results. For example, sorafenib, a TKI targeting VEGFR, PDGFR, and BRAF, was combined both with erlotinib in pretreated patients and with gemcitabine in treatment of naïve patients, without registering significant improvements [Citation148,Citation149]. Similarly, discouraging results have also been recorded in pretreated patients with sunitinib monotherapy, an anti PFGR, VEGFR, cKIT, and RET TKI and with VEGFR, cKIT, and PDGFR inhibitor axitinib in combination with gemcitabine in treatment of naïve patients [Citation150,Citation151]. Of importance, in PDAC, despite a strong proangiogenic drive, the defective nature of the new blood vessels and the compression caused by the surrounding fibrotic stroma can create a hypoxic and hypovascular environment [Citation152], and it has been suggested that the increased hypoxia caused by antiangiogenic drug could possibly cause a more aggressive phenotype to be selected and further impede drug delivery [Citation153,Citation154]. This may be the reason no OS advantage has been recorded with antiangiogenic drugs.
Despite these initial failures, new antiangiogenic drugs are being tested, especially in China. Fruquintinib is an anti VEGFR-1/2/3 drug tested in multiple solid advanced tumors (including one PDAC case) with good tolerability and intriguing efficacy results [Citation155], thus two phase 2 trials are now ongoing in PDAC: one as monotherapy in the third-line setting (NCT05257122) [Citation156] and one in first-line therapy alongside gemcitabine and nab-paclitaxel (NCT05168527) [Citation157]. Another novel drug is anlotinib, a multi-target TKI directed against VEGFR, cKit, PDGFRα, and FGFR, that is being tested in several phase 1–2 trials in combination with immune or chemo-immune therapies. Preliminary results on a single-arm phase 2 trial on the combination of anlotinib, anti PD-1 penpulimab and gemcitabine/nab-paclitaxel in the treatment of naïve PDAC patients suggest promising efficacy, with ORR of 43% and DCR of 95% (NCT05493995) [Citation158,Citation159]. A phase 2 randomized trial is now ongoing (NCT06051851) [Citation160]. In the second-line setting, a similar combination of anlotinib, anti-PD-1 toripalimab, and nab-paclitaxel is also being tested (NCT04718701) [Citation161], as well as the combination of anlotinib, anti PD-L1 tislelizumab, and investigator choice chemotherapy (NCT05681390) [Citation162] and the combo anlotinib, anti-PD-L1/TGF-β drug TQB2858 and gemcitabine plus nab-paclitaxel (NCT05193604) [Citation163]. Trials with the combination of just TKI and immunotherapy are also ongoing, investigating combo such as anlotinib with penpulimab (NCT04803851) [Citation164] or with anti PD-1 pembrolizumab (NCT05218629) [Citation165].
8. Metabolism-based target drugs
The alterations in cancer cells’ metabolisms famously include a preference for glycolysis and anabolism, however, cancer cells also depend on mitochondrial activity such as the tricarboxylic acid cycle (TCA) to produce the precursors for fatty acid synthesis and other anabolic intermediate [Citation166,Citation167] (). Mitochondrial enzymes such as the pyruvate dehydrogenase complex, in charge of the oxidative decarboxylation of pyruvate to acetyl-CoA, and the α-ketoglutarate dehydrogenase complex, that allows cancer cells to utilize the amino acid glutamine, are essential to utilize carbon in the TCA [Citation168]. In this context, CPI-613 (also known as devimistat) is a lipoate derivative drug that can inhibit both enzymes, causing havoc in the cancer cells’ metabolic pathways that ultimately lead to apoptosis [Citation168]. The combination of CPI-613 with mFOLFIRINOX or with the combination of gemcitabine and nab-paclitaxel was investigated in two dose-finding phase 1 trials, and the results indicate a relatively well-tolerated drug with a response rate between 50 and 61%, indicating a possible synergistic effect [Citation169,Citation170]. However, a phase 3 trial investigating the combination of CPI-613 plus mFOLFIRINOX vs standard of care FOLFIRINOX failed to report a significant increase in OS, PFS, or ORR [Citation171]. A few trials are ongoing in the neoadjuvant setting for locally advanced pancreatic tumors in combination with mFOLFIRINOX (NCT03699319) [Citation172] or in combination with radiotherapy and gemcitabine (NCT05325281) [Citation173], based on synergistic effect noted in the preclinical setting [Citation174,Citation175].
Table 4. Ongoing clinical trials in pancreatic cancer with metabolism based target therapies.
Another drug targeting cancer cell energy metabolism is GP-2250, capable of inhibiting enzymes involved in glycolysis (such as hexokinase/HK2 and glyceraldehyde‐3‐phosphate dehydrogenase/GAPDH) and the TCA cycle in PDAC cell lines [Citation176]. A phase 1 trial of GP‐2250 in combination with gemcitabine in patients pretreated with 5-fluorouracil based chemotherapy is now ongoing (NCT03854110) [Citation177].
A direct consequence of the increased glycolysis observed in cancer cells is the increased levels of acidic metabolites such as lactate, protons, and carbon dioxide, leading to abnormalities in the intracellular pH levels. One mechanism to maintain pH homeostasis is to increase the levels of tumor-associated carbonic anhydrases (CAIX), thus a CAIX inhibitor called SLC-0111 has been tested in a dose finding phase 1 trial including one pancreatic patient, exhibiting a good safety profile [Citation178]. A phase 1b trial with SLC-0111 plus gemcitabine is now ongoing in metastatic pretreated cancer patients (NCT03450018) [Citation179].
Another idea to target the metabolic pathways in cancer cells is to target amino acids. Alanine-serine cysteine-preferring transporter 2 (ASCT2, also known as SLC1A5) is a neutral amino-acid transporter that is involved in the uptake and movement of amino acids that are necessary for tumor growth. Its expression is significantly increased in PDAC cell lines, and a high expression of its mRNA has been associated with poor OS in patients with PDAC [Citation180]. Inhibition of ASCT2 expression in both pancreatic cell lines and mice models has a suppressive tumor effect [Citation180]. MEDI7247 is an antibody–drug conjugate composed of a human monoclonal antibody directed against ASCT2 conjugated to a cytotoxic DNA cross-linking pyrrolobenzodiazepine. Preclinical data on MEDI7247 showed favorable activity with durable tumor regression in pancreatic patients-derived xenografts [Citation181]. A phase 1 clinical trial (NCT03811652) [Citation182] was completed in 2019; however, no published results are available. To our knowledge, no other clinical trials on this drug are ongoing.
ASCT2 can also transport the amino-acid glutamine to the mitochondrial matrix, where glutamine is then converted to glutamate by the enzyme glutaminase (GLS). Glutamate then helps maintain the redox hemostasis, an important process for cancer cell survival [Citation183]. Thus, another approach to target this metabolic pathway consists of blocking GLS. This approach was tested in KRAS-mutated pancreatic cell lines, in which the KRAS alteration has been shown to promote metabolic changes including increased use of glutamine; however, the simple blockade of GLS1 through the selective inhibitor CB-839 (also known as telaglenastat) was met with failure in PDAC mouse models due to the development of multiple compensatory pathways [Citation183,Citation184]. Thus, a phase 1b/2 trial was conducted, investigating the combination treatment of CB-839 plus CDK4/6 inhibitor palbociclib in solid tumors, including PDAC (NCT03965845) [Citation185]. The results are yet to be published.
Another drug targeting metabolism is ADI-PEG20, a pegylated arginine deiminase. In normal cells, the amino-acid arginine, involved in nitric oxide formation and protein biosynthesis, is synthesized by the enzyme argininosuccinate synthase (ASS1); however, tumor cells are often ASS1 deficient and they rely on extracellular arginine [Citation186]. ADI-PEG20 can convert arginine to citrulline, thus further depleting the storage of arginine, and it has already been studied in other cancers such as mesothelioma with promising results [Citation187]. The combination of ADI-PEG20 plus gemcitabine and nab-paclitaxel has also been explored in advanced PDAC in a phase 1/1b trial: the combination was well tolerated, with interesting survival results of 11.3 months, and ORR of 45% [Citation188]. Furthermore, the preclinical data indicate a possible synergistic effect of ADI-PEG20 with radiotherapy in ASS1-deficient PDAC cells [Citation189]. Despite these interesting data, no further trials with ADI-PEG20 in metastatic or locally advanced patients are ongoing.
Another interesting avenue of research involves lowering the high levels of polyamine that are necessary for tumor growth [Citation190]. The polyamine inhibitor SBP-101 has been tested in a phase 1 trial in conjunction with gemcitabine and nab-paclitaxel in untreated metastatic patients, demonstrating at the first interim analysis encouraging efficacy results (43% partial responses, 39% stable diseases), although concerns about retinal and hepatic toxicities have been raised (NCT03412799) [Citation191]. A phase 2/3 trial (ASPIRE, NCT05254171) [Citation192] of the combination of gemcitabine + nab-paclitaxel ± SBP-101 is now ongoing.
9. Epigenetic targets
Several post-translational modifications of DNA methylation and histone proteins, collectively known as epigenetic alterations, can be observed in cancer [Citation193] (). Starting with DNA methylation, it is favored by the activity of enzymes such as DNA methyltransferase (DNMT), and in pancreatic cell lines the inhibition of DNMT resulted in growth inhibition [Citation194]. Thus, several trials with DNMT inhibitors, such as azacitidine, decitabine, and guadecitabine, have been performed or are ongoing. Results from the use of these drugs in monotherapy in all PDAC patients have been discouraging [Citation195], although some trials are now ongoing with more selected populationsfor example, in KRAS mutated patients (NCT05360264) [Citation196]. The combination of DNMT inhibitors with chemotherapy were similarly disappointing. For example, the oral hypomethylating drug azacitidine was combined with nab-paclitaxel in pretreated PDAC, obtaining a not particularly impressive DCR of 46% [Citation197], and the results of a first-line trial with decitabine and gemcitabine were never published [Citation198]. Based on preclinical data indicating that DNMT inhibitor can increase antigen presentation and chemokine expression [Citation199], more recent trials have also explored the combination of DNMT inhibitor with immunotherapy (azacitidine – pembrolizumab and guadecitabine – durvalumab) but with again, negative results [Citation200,Citation201]. Furthermore, interim results from the multi-arm SEPION trial (NCT04257448) [Citation202] with azacitidine and histone deacetylases (HDAC) inhibitor romidepsin plus gemcitabine and nab-paclitaxel in first-line advanced PDAC indicate elevated toxicities level for the combination of both romidepsin and azacitidine. However, the arm with only azacitidine and chemotherapy was well tolerated, and the results on the dose expansion and sequential immune consolidation with durvalumab and lenalidomide are eagerly awaited [Citation203].
Epigenetic regulation also involves histone acetylation, in which the acetylated histones are associated with greater levels of DNA transcription. Thus, drugs that can inhibit the activity of enzymes such as histone deacetylases (HDAC) can promote gene expression in cancer-associated silenced genes [Citation204]. In a phase 1–2 trial, HDAC inhibitor ivaltinostat was tested as a first-line option alongside anti EGFR erlotinib and gemcitabine, obtaining encouraging results (OS of 8.6 months, ORR of 25% and DCR of 94%) [Citation205] and another phase 1–2 trial is ongoing with the combination of ivaltinostat and capecitabine as maintenance therapy in patients who had not progressed after first-line FOLFIRINOX (NCT05249101) [Citation206]. HDAC inhibitors are also being investigated as their role as radiosensitizers in locally advanced PDAC cases: a trial with vorinostat and radiotherapy (RT) was terminated due to low accrual (NCT00831493) [Citation207], while partial published results for the combination of vorinostat, capecitabine, and RT indicate good tolerability but dubious efficacy (NCT00983268) [Citation208,Citation209]. Preliminary results for a more recent trial with vorinostat, multi-TKI sorafenib, and gemcitabine with RT in locally advanced PDAC point to good tolerability and encouraging efficacy, but OS data are not yet available [Citation210]. Another HDAC inhibitor entinostat is being tested alongside anti-PD-1 nivolumab in pretreated PDAC patients (NCT03250273) [Citation211] and another interesting combination with BET inhibitor ZEN003694 is being explored in a basket trial (NCT05053971) [Citation212]. Of note, Bromodomain and Extra-Terminal Domain (BET) proteins regulate gene transcription through binding with acetylated histones [Citation193], and preclinical evidence points to the efficacy of BET inhibitors in PDAC mouse models [Citation213]. Preliminary results from a phase 1–2 trial with BET inhibitor INCB057643 showed a tolerable safety profile but the two patients with PDAC have progressive disease [Citation213].
To conclude, epigenetic changes can also occur in the post-translation phase through histone methylation, regulated by histone methyltransferases (HMTs) and demethylases (HDMs) [Citation214]. Inhibitors of Enhancer of zeste homologue 2 (EZH2), a HMTs, showed interesting in vitro activity [Citation215] and are been investigated, mostly in basket trials in combination with immunotherapy, such as tazemetostat plus durvalumab (NCT04705818) [Citation216] and CPI-1205 plus ipilimumab (NCT03525795) [Citation217].
10. Claudin
Claudins play a crucial role as transmembrane proteins in the creation and control of tight junction, governing intercellular connections. Their expression is tissue-specific, and their role is defined by post-translational modifications, such as phosphorylation and dephosphorylation, which modulate the strength of intercellular links. In the last years, claudins’ conformation has been found altered in many cancer types, including PDAC, influencing their ability to metastasize and to undergo epithelial–mesenchymal transition [Citation218]. Claudin-18.2 (CLDN18.2) is exclusively expressed in cancerous pancreatic cells, with percentages ranging from 37% up to 60–94%, depending on the staining intensity considered [Citation219]. Even if some evidence suggests an increased frequency of distant metastasis in CLDN18-positive forms, a defined impact on prognosis has not been demonstrated yet [Citation220]. Various claudin-targeted strategies are currently being investigated, all as ongoing ().
Table 5. Ongoing clinical trials in pancreatic cancer with claudin target therapies.
Zolbetuximab is a monoclonal antibody directed against the CLDN18.2. It was recently demonstrated a positive impact on CLDN18.2-positive gastric cancer when administrated associated with chemotherapy [Citation221,Citation222] and pancreatic studies are currently ongoing too. A phase 2 trial is evaluating zolbetuximab in combination with gemcitabine and nab-paclitaxel as front-line treatment for CLDN18.2-positive PDAC (NCT03816163) [Citation223]. Another CLDN18.2-targeted mAb, TST001, is being tested in a phase 1/2a basket trial that includes advanced PDAC regardless of CDNL18.2 expression in combination with gemcitabine and nab-paclitaxel (NCT04396821) [Citation224]. PT886 is a novel antibody that targets both CLDN18.2 and CD47, a transmembrane protein that is highly expressed in many solid neoplasia and that can inhibit phagocyte activity. Through its double targeting, PT866 aims to redirect phagocytes toward cancer cells, inducing apoptosis. A phase 1 trial is now evaluating its administration in pretreated advanced solid tumors, including PDAC (NCT05482893) [Citation225]. Similarly, another phase 1/2 trial is testing AZD5863, a new monoclonal antibody targeting CLDN18.2 and CD3, in CLDN18.2-positive advanced/metastatic PDAC and other gastrointestinal tumors (NCT06005493) [Citation226].
SOT102 is a novel ADC made up of a CLDN18.2-targeted monoclonal antibody linked to the anthracycline PNU-159682 payload [Citation227]. A phase 1b trial (CLAUDIO-01) is currently evaluating SOT102 as first-line treatment, alone or in combination with standard of care chemotherapy, in patients affected by advanced/metastatic gastric or PDACs, regardless of CLDN18.2 expression (NCT05525286) [Citation228] p.102] Another trial is investigating another ADC, EO-3021, in various solid CLND18.2-positive neoplasia, including PDAC (NCT05980416) [Citation229]. Concerning RNA-based strategies, BNT141 is a new drug made up of two mRNAs encoding for monoclonal antibodies targeting CDLN18.2. A phase 1/2 trial is evaluating its administration in various CLDN18.2-positive tumors, including PDAC. In the PDAC cohort, it is used as a monotherapy treatment in patients without therapeutic alternatives, or combined with gemcitabine/nab-paclitaxel (NCT04683939) [Citation230].
11. Novel targets
Research in PDAC is filled with phase 1 and 2 trials investigating novel targets, some with promising results (). For example, one novel interesting target is the TP53 gene, as it encodes for one of the most important tumor suppressor proteins, plus around 60–70% of PDAC exhibit mutations with loss of function of this gene [Citation231]. Given the relevance of this gene in tumor survival, one phase 2 trial tried to restore TP53 WT function using somatic gene therapy plus gemcitabine and nab-paclitaxel in first or second-line metastatic PDAC (NCT02340117) [Citation232]: the initial results are extremely encouraging both when used in the first-line (no patient had disease progression) and in second-line, with a mOS between 10.7 and 13.4 months [Citation233]. Another trial regarding gene transfer involves gemcitabine combined with intratumoral injection of CYL-02, a drug capable of restoring the activity of genes involved in chemotherapy resistance such as SSTR2, DCK, and UMK: in the phase 1 trial including locally advanced and metastatic patients the treatment was well tolerated and a phase 2 trial with locally advanced patients only is now ongoing (NCT02806687) [Citation234,Citation235].
Another novel target is plectin, a scaffolding protein of the cytoskeleton of normal cells, which contributes to several processes including migration, proliferation, and signal transduction [Citation236]. In cells from pancreatic adenocarcinoma, it's often erroneously placed on the cell surface, making it an interesting oncoprotein, biomarker, and therapeutic target [Citation237]. A phase 1–2 trial is exploring the role of anti-plectin antibody ZB131 in pretreated PDAC, cholangiocarcinoma, and ovarian cancers with high expression of cancer-specific plectin (NCT05074472) [Citation238] with interim results indicating good tolerability [Citation239]. Another interesting drug, also considering the good results observed in breast cancer [Citation240], is the anti-transmembrane calcium signal transducer (Trop2) ADC sacituzumab govitecan. It was investigated in a basket trial including 16 pretreated PDAC, unfortunately with no objective responses, but with 43% of stable diseases [Citation241]. However, a phase 1 trial is ongoing with the combination of sacituzumab govitecan and capecitabine in pretreated patients (NCT06065371) [Citation242].
Another potential target is the protein transporter exportin-1 (XPO1), which can be overexpressed in cancer, leading to tumor suppressing proteins being removed from the nucleus where they normally exert their functions; thus, there are various efforts underway to block the function of this transport. One such option involves the use of Selective Inhibitor of Nuclear Export (SINE) compounds such as selinexor, which has been tested in a phase 1 trial for PDAC patients alongside gemcitabine and nab-paclitaxel, with promising data on efficacy and one case of long-lasting response (OS of 22 months) [Citation243]. Other combination with XPO1 that are being explored in solid tumors include PARP inhibitors (NCT05035745) [Citation244] and immunotherapy (NCT04850755) [Citation245]. Other XPO1 inhibitor under investigation include SL-801, which was tested in a phase 1 trial for solid tumors, demonstrating good tolerability [Citation246].
Regarding PDAC exhibiting HR deficiency, other than the PARP inhibitors that we discussed previously, there are several novel target drugs that are being developed. Following promising preclinical data, the combination of ataxia telangiectasia and RAD3-related (ATR) target therapy plus PARP inhibitor has been tested, and in a basket trial including ATR inhibitor ceralasertib plus olaparib, 2 out of 6 pretreated PDAC patients achieved disease stability [Citation247,Citation248]. A trial with ceralasertib plus olaparib or durvalumab is now ongoing in various solid tumors (NCT03682289) [Citation249]. Similarly, there are other drug being tested in solid tumors with HR deficiency including PDAC, such as IDE161, a poly ADP-ribose glycohydrolase (PARG) inhibitor (NCT05787587) [Citation250] and RNA polymerase I transcription of ribosomal RNA genes inhibitor CX-5461 (NCT04890613) [Citation251].
Moreover, another interesting field includes target therapy directed against component of the pancreatic tumor microenvironment, which is characterized by a desmoplastic stroma rich in stromal cells such as cancer associated fibroblast (CAF) and a dense extracellular matrix [Citation213]. One component of this matrix is hyaluronan which can impair drug delivery, thus drug such as its degrader pegvorhyaluronidase alfa (PEGPH20) have been proven to increase response and PFS rate of standard chemotherapy in patients with hyaluronan-rich stroma, although OS was not significantly increased [Citation252]. The concept of decreasing desmoplasia however does not always lead to the expected results, for example the use of vismodegib, a sonic hedgehog inhibitor capable of decreasing CAF activity, was not associated with better OS survival in PDAC, highlighting the complexity of successfully altering PDAC microenvironment [Citation253].
Furthermore, several phase 1 trials published promising results for different drugs, for example, Bruton’s tyrosine kinase inhibitor Ibrutinib [Citation254,Citation254], anti αV integrins and neuropilin-1 peptide CEND-1 [Citation255] and stromal targeting all-trans-retinoic-acid with chemotherapy [Citation256]. However, it should be noted that for every promising phase 1 trial, many more phase 2 trials will fail their efficacy endpoint, as was the case for drugs like anti Notch2–3 antibody tarextumab [Citation257], anti-Heat Shock Protein 90 tanespimycin [Citation258], checkpoint kinase 1 inhibitor (CHK1) LY2603618 [Citation259], urokinase inhibitor upamostat [Citation260] and multitarget TKI dasatinib (anti BCR-ABL, c-Src, c-KIT, platelet-derived growth factor receptor β, and EphA2) [Citation261].
12. Conclusion
Given the aggressive nature of PDAC, often associated with a quick decline in patient’s performance status, the need for more effective and better tolerated drugs is still strong. Considering the efficacy results of target drugs in various cancer types and their frequently superior tolerability profile compared to chemotherapy, numerous trials have tried to investigate the efficacy of target therapies in PDAC. However, with the exception of PARP inhibitor olaparib as maintenance therapy in BRCA-mutated patients not progressing to first-line platinum-based chemotherapy (and even then with no advantage in OS [Citation21]), no other target drugs or combination treatments have been able to achieve better results than the standard chemotherapy regimens. Thus, the quest for effective drugs is still very much ongoing.
Given olaparib’s positive impact on patient prognosis and the ongoing trials, it is reasonable to expect that other PARP inhibitors may also be available soon, hopefully for a larger subset of patients, given that drugs such as niraparib and rucaparib are being tested in patients with different alterations of the DNA repairs mechanisms, and not just BRCA mutated patients [Citation24], [Citation25]. In these selected patients, PARP inhibitors have the potential to fill a crucial gap between chemotherapy lines, keeping the disease under control and concurrently allowing the patient to recover from the higher toxicity of chemotherapy. Furthermore, considering the aggressive nature of PDAC, combination therapies including immunotherapy may hold the key to achieving longer OS, given the promising results of the combination of olaparib with ipilimumab [Citation29], and the other ongoing trials exploring the combination of PARP inhibitors, chemotherapy, and immunotherapy [Citation30–32].
On the other hand, fewer expectations are placed on EGFR inhibitors. Despite erlotinib being one of the few target therapies associated with an increased OS when combined with gemcitabine in a phase 3 trial, the clinical benefit of only a few weeks was not clinically relevant [Citation34]. Furthermore, the majority of the trials that have been conducted in the past with classic EGFR TKI (erlotinib or afatinib) or the more recent ones with antibodies, such as nimotuzumab, are usually combined with only gemcitabine, even though the standard first-line regimens today are combination treatments (gemcitabine/nab-paclitaxel, or FOLFIRINOX); thus, the relevance of these trials is dubious. The creation of a trial with nimotuzumab combined with a more standard first-line treatment would certainly be interesting. However, a potential challenge lies in toxicity, as evidenced by the issue encountered in the sole trial involving gemcitabine/nab-paclitaxel and erlotinib [Citation37].
Similarly, anti-HER2 therapies struggle to find their place in PDAC management. While traditional anti-HER2 therapies have lost most of their appeal, even when combined with chemotherapy or other target drugs, the innovativeness of ADCs and their proven efficacy in other tumors had brought new hope for PDAC as well. However, the primary analysis of the two DESTINY-PanTumor trials [Citation51,Citation52] did not yield remarkable results in the PDAC cohort, showing that a deeper understanding of the role of HER2 expression in PDAC is warranted. The DESTINY-PanTumor02 trial explored the possibility that the oncological response to ADCs may not be closely associated with the grade of immunohistochemical HER2 overexpression in cancerous cells [Citation51]. Furthermore, it must be considered that the assessment of HER2 expression itself might be influenced by the wide heterogeneity of the tumor [Citation262]. Therefore, further investigations are needed to identify predictive markers of response to HER2 inhibitors as, at the moment, HER2 status seems to be non-exhaustive.
Regarding KRAS, the high percentages of patients exhibiting alterations to this transduction molecule have made it an interesting target; however, several problems plague this concept. Firstly, the most commonly found mutations, G12D and G12V, do not have a specific inhibitor with proven efficacy, although several molecules are in the early stages of clinical testing [Citation73,Citation74,Citation83]. As of now, only the G12C inhibitor sotorasib proved to have clinical efficacy in pretreated PDAC patients, and even in this case, the evidence is not strong, as it is based on a phase 1–2 trial [Citation65]. Secondly, cancer cells can implement many resistance mechanisms to bypass KRAS inhibition, including activation of its downstream effector (RAF/MEK/ERK and PI3K/AKT/mTOR pathways), upregulation of its tyrosine kinase receptors (such as EGFR, HER2, FGFR), intervention of mediators such as SHP2 or disruption of the cell cycle regulation through CDK4/6 alteration, caused by the often concurrent loss of function of CDKN2A [Citation263]. The knowledge of these mechanisms is the foundation for the idea of combining multiple drugs. Popular combinations include drugs targeting the KRAS system plus CDK4/6 inhibitors, chemotherapy, or PARP inhibitors, as well as multiple combinations targeting different components of the same or related pathway (Table RAF/MEK/ERK, PI3K/AKT/mTOR). For example, in KRAS G12C positive colon cancer, the efficacy of KRAS G12C inhibitor divarasib was increased when paired with anti EGFR antibody cetuximab [Citation264]. However, most of this type of combination trials are still ongoing for PDAC, and the few trials from which results are available are not encouraging, as most combinations are often either too toxic to be well tolerated or they do not exhibit strong antitumor activity [Citation90,Citation91,Citation104,Citation105,Citation109,Citation122–124,Citation126,Citation129,Citation132].
Regarding JAK/STAT inhibitors, given the poor results of these drugs with chemotherapy [Citation135,Citation136], it is unlikely that these combinations will see further clinical trials, although it should be noted that no trials have compared these target drugs with FOLFIRINOX, another first-line option nowadays. It is likely, however, that other types of combinations will continue to be explored. For example, given the role of JAK/STAT in immune response, several trials are investigating immunotherapy with checkpoint blockade drugs [Citation141,Citation142], thus it will be interesting to see if this combination can overcome the innate immune resistance of PDAC [Citation265]. Similarly, although early trials with antiangiogenic therapies have always failed to meaningfully improve OS, both alone or with chemotherapy [Citation147], new combinations with anti-angiogenic drugs associated with immunotherapies and standard of care chemotherapy have shown interestingly preliminary results and several ongoing trials may bring interestingly results [Citation157,Citation160,Citation164].
Furthermore, although many drugs have been developed with the idea of targeting the metabolic alterations that are present in pancreatic cell lines, the use of these drugs in the clinical setting is still premature. The only phase 3 trial with available results, investigating the mitochondrial disrupting drug CPI-613 in combination with mFOLFIRINOX, failed to significantly increase survival or response rates [Citation171]. The other ongoing phase 2/3 trial with the polyamine inhibitor SBP-101 plus gemcitabine and nab-paclitaxel will have to overcome the problem of retinal toxicities that have been raised in the phase 1 trial [Citation191]. Most of the other drugs are still in the early stages of testing [Citation172,Citation173,Citation177,Citation179,Citation185], while other drugs, such as MEDI7247 or ADI-PEG20, despite promising initial results have not been investigated in further clinical trials.
Regarding drugs targeting epigenetic modifications, the results of DNMT inhibitors alone or with chemotherapy have been discouraging, while the results of trials with HDAC inhibitors seem more encouraging. Although the combination of HDAC inhibitor valtinostat with EGFR inhibitor erlotinib and gemcitabine is promising [Citation205], a phase 3 trial comparing this combination with standard of care first-line chemotherapy is needed to verify the actual usefulness of this combo. On the other hand, the ongoing trial of ivaltinostat and capecitabine as maintenance therapy after first-line FOLFIRINOX [Citation206], if positive, may fill the empty niche of patients that need some recovery time from the toxicity of FOLFIROX and have not yet progressed. Furthermore, the OS data of the combination of vorinostat, multi-TKI sorafenib, and gemcitabine with RT in locally advanced PDAC, are also eagerly awaited [Citation210]. The key to implementing drugs targeting epigenetic changes may also be combining them with immunotherapies, and many phase 1–2 trials are working on this idea [Citation202,Citation211,Citation216,Citation217].
Further novel targets include claudin 18.2, due to its appeal of having a selective expression in cancer cells which makes it an ideal target for precision therapies. Even though no data are available on PDAC specifically, the promising efficacy on gastric cancer [Citation221] has led to the creation of a phase 2 trial on PDAC in combination with gemcitabine and nab-paclitaxel as front-line treatment for CLDN18.2-positive PDAC [Citation223]. Furthermore, many other drugs targeting claudin are ongoing. Regarding other innovative targets, the most promising is certainly the use of gene therapy for restoring TP53, as in a phase 2 trial, when used in combination with standard of care chemotherapy, the OS data were very encouraging even in second-line [Citation233]. Unfortunately, all other positive trials on other drugs were phase 1 or phase 1/2 trials [Citation254–256], thus more robust data are needed.
To conclude, the world of target therapies in PDAC is ever growing, both with trials investigating single target drugs and with trials evaluating the efficacy of known target drugs, such as the SMMART Adaptive Clinical Treatment (ACT) Trial (NCT05238831) [Citation266], the VISIONARY trial (NCT04584008) [Citation267] and the MATCH trial (NCT02465060) [Citation268]. However, despite this growing number of trials, no real steps forward have been taken and so far, the standard of care remains chemotherapy.
13. Expert opinion
In at least 20 years of oncological research on treating PDAC, little progress has been made and it remains one of the deadliest cancers with mOS survival below one year. So far only combination chemotherapies have had some success in treating this disease, but they are often complicated by high levels of toxicities and high rates of primary and secondary resistance [Citation269]. In recent years, several new strategies including therapies against different targets, immune checkpoint inhibitors, gene therapy, and CAR-T therapies have been developed and tested in various cancer types, but without substantial success in PDAC [Citation270]. In this manuscript, we have reviewed the major players in the field of target therapies in PDAC, from the more well-known PARP inhibitors and EGFR directed therapies, to the wide world of inhibitors against KRAS and the proteins in its pathways, and to therapies still in their infancies like the ones directed against claudin or novel targets. All in all, of this enormous number of trials, only a few drugs have managed to reach a phase 3 trial (PARP inhibitor olaparib, EGFR inhibitors erlotinib and cetuximab, JAK/STAT inhibitor ruxolitinib and JAK/TNK1 inhibitor momelotinib, anti VEGFR bevacizumab, inhibitor of mitochondrial metabolism devimistat), usually in combination with chemotherapy, and even then, they have not been able to significantly modify the survival of PDAC patients.
In our opinion, what emerges from this picture is that no single target therapy is likely to hold the key to treating this disease, at least as monotherapy treatment. It is much more conceivable to think that a combination of target therapies with chemotherapy, immunotherapy, or even both will one day be able to improve the survival rates of first-line therapy. Furthermore, the relatively recent introduction of NALIRIFOX as a possible chemotherapy option begs the question of whether the use of this regimen could change the response to target therapy in further lines or if its use in combination with target therapy may increase survival. Thus, if the correct target and the correct drugs have already been found and are just awaiting further trials remains an open question.
Increased insights from omics studies are still warranted. For instance, despite the historical lack of extensive research on KRAS WT PDAC due to its rarity, recent progress in clinically accessible next-generation sequencing platforms has significantly enhanced our comprehension of the genomics associated with this subtype. As KRAS serves as a primary pathogenic mutation that prompts the initiation, development, and progression of the majority of PDACs, it is indeed logical to propose that PDAC without KRAS mutations may constitute a unique entity characterized by distinct driver mutations and biological features. However, it is only in recent times that a handful of groundbreaking studies have indicated that KRAS WT PDAC might not only be a distinct molecular and biologic entity but might also present additional opportunities for genome-guided treatment. Specifically, recent studies highlighted that the 8–10% of PDAC patients lacking mutations in KRAS exhibit distinct molecular characteristics, underscoring the importance of appropriately stratifying these individuals for clinical trials involving target agents. Notably, these patients are more likely to be MSI-high (5% vs. 1%; p < 0.05), have a tumor mutational burden – high (4% vs. 1%; p < 0.05), and show increased infiltration of CD8+ T cells, natural killer cells, and myeloid dendritic cells [Citation271]. Furthermore, approximately 40% of KRAS WT cases display evidence of an alternative driver in the MAPK pathway, including BRAF mutations, in-frame deletions, and receptor tyrosine kinase fusions such as BRAF, FGFR2, ALK, RET, and NRG1, as well as amplification of FGF3, ERBB2, FGFR3, NTRK, and MET [Citation272]. Additionally, around 30% of MAPK-negative KRAS WT cases exhibit activating alterations in other oncogenic drivers such as GNAS, MYC, PIK3CA, and CTNNB1 [Citation272]. A retrospective chart review of clinical/molecular data using tumor molecular profiling on tissue or liquid biopsy revealed that KRAS WT cases had fewer alterations in TP53, CDKN2A, and SMAD4 and experienced improved outcomes when receiving molecularly matched treatment compared to KRAS mutated patients [Citation273]. However, it should be noted that these data come from retrospective trial and as such they such be taken with caution: for example, no distinction was made based on stage or the site of biopsy (metastatic lesion vs primary site). Furthermore, it is unclear whether the material collected during standard endoscopic biopsy of the pancreas is enough to routinely perform this type of genomic analysis in the majority of patients.
Other than data on just KRAS, another case should be made for PDAC molecular subtypes and their implication in response to treatment. Various genomic and transcriptomic studies have been conducted in PDAC, leading to the identification of different molecular subtypes, such as the classic subtype, usually associated with better prognosis, or the basal subtype, with a more dismal prognosis [Citation274]. Some evidence point to the basal subtype as being more chemoresistant to first-line FOLFIRINOX and thus several trials are now ongoing with the aim to match the molecular subtype to oxaliplatin or gemcitabine based chemotherapy [Citation275,Citation276]. Conversely, data on the correlation between each subtype and their response to target therapies are very scarce. An article on PDAC cell lines seems to indicate that the classical subtype could be more sensitive to EGFR directed therapy (such as erlotinib), however clinical data are missing [Citation277]. It would be interesting to see if a trial designed with the intent to match PDAC molecular subtypes and different target therapies could lead to better survival results in these patients.
Regarding potential strategies to select the most effective combinations, a recent study on pancreatic cell lines, organoids and patient-derived xenograft has shown that phosphoproteomic may help identifying the multiple kinases that are altered in each patient and subsequentially match it with the appropriate target drugs combination [Citation278]. Implementing this strategy in the clinical setting would allow for a truly personalized approach. Furthermore, another issue to ponder is whether PDAC should be approached like other cancers. In fact, normally a change of treatment is only decided upon after clinical or radiological proof of disease progression. However, a different approach could be to monitor the patients for early signs of disease progression, such as changes in tumor circulating DNA, allowing the clinicians to intercept the formation of cancer clones that are resistant to the ongoing treatment before they are radiologically detectable, and thus to quickly adapt with an appropriate change of treatment [Citation279]. This strategy of fast changing treatments could give new life to target therapies that are not able to completely control all tumor cells, but they could still be of help in managing this disease for short periods of time. This could also give the patients time to recover from the more classic toxicities experienced with chemotherapy, without risking losing too much time on a treatment that is destined to inevitably fail.
To conclude, the road to discovering an effective treatment involving target therapy in PDAC is still long, but it is nonetheless buzzing with a myriad of past and ongoing clinical trials and new drug development. Hopefully, one of these trials will finally unlock the door to significantly improve survival for patients affected by this neoplasia.
Article highlights
Despite the lack of OS gained with Olaparib as first-line maintenance therapy, research with PARP inhibitors is very active, and several trials are exploring the use of this drug alongside immunotherapy.
Anti HER2 drugs are still struggling to find a place in PDAC’s treatment algorithm, possibly also due to the lack of reliable predictors of response in this cancer.
The efficacy of KRAS inhibitors is hindered by the presence of many resistance mechanisms, and the use of combination to overcome this problem is often associated with high toxicity.
Although JAK/STAT inhibitor and antiangiogenic drugs have so far failed to improve OS in PDAC, several trials are exploring the use of these therapies in combination with immunotherapy and chemotherapy.
Novel targets including metabolism, epigenetic modification, TP53 and claudins are being tested in PDAC, but research in these fields is for the most part in the early stages.
Combination of target therapies with chemotherapy and/or immunotherapy are more likely to improve survival results in PDAC and there is a strong need for more personalized approaches.
Declaration of Interest
G Brandi has received a research grant from INCYTE and IPSEN and he is a member of the advisory board for INCYTE, LILLY and TAIHO. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-Mutated advanced NSCLC. N Engl J Med. 2020;382(1):41–50. doi: 10.1056/NEJMoa1913662
- Hortobagyi GN, Stemmer SM, Burris HA, et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. N Engl J Med. 2022;386(10):942–950. doi: 10.1056/NEJMoa2114663
- Kopetz S, Grothey A, Yaeger R, et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E–mutated colorectal cancer. N Engl J Med. 2019;381(17):1632–1643. doi: 10.1056/NEJMoa1908075
- Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379(25):2395–2406. doi: 10.1056/NEJMoa1809775
- Philip PA, Lacy J, Portales F, et al. Nab-paclitaxel plus gemcitabine in patients with locally advanced pancreatic cancer (LAPACT): a multicentre, open-label phase 2 study. Lancet Gastroenterol Hepatol. 2020;5(3):285–294. doi: 10.1016/S2468-1253(19)30327-9
- Kunzmann V, Siveke JT, Algül H, et al. Nab-paclitaxel plus gemcitabine versus nab-paclitaxel plus gemcitabine followed by FOLFIRINOX induction chemotherapy in locally advanced pancreatic cancer (NEOLAP-AIO-PAK-0113): a multicentre, randomised, phase 2 trial. Lancet Gastroenterol Hepatol. 2021;6(2):128–138. doi: 10.1016/S2468-1253(20)30330-7
- Wainberg ZA, Melisi D, Macarulla T, et al. NALIRIFOX versus nab-paclitaxel and gemcitabine in treatment-naive patients with metastatic pancreatic ductal adenocarcinoma (NAPOLI 3): a randomised, open-label, phase 3 trial. Lancet. 2023;402(10409):1272–1281. doi: 10.1016/S0140-6736(23)01366-1
- Goldstein D, El Maraghi RH, Hammel P, et al. Updated survival from a randomized phase III trial (MPACT) of nab-paclitaxel plus gemcitabine versus gemcitabine alone for patients (pts) with metastatic adenocarcinoma of the pancreas. J Clin Oncol. 2014;32(3_suppl):178–178. doi: 10.1200/jco.2014.32.3_suppl.178
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923
- Petrelli F, Parisi A, Tomasello G, et al. Comparison of different second line treatments for metastatic pancreatic cancer: a systematic review and network meta-analysis. BMC Gastroenterol. 2023;23(1):212. doi: 10.1186/s12876-023-02853-w
- Raphael BJ, Hruban RH, Aguirre AJ, et al. Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell. 2017;32(2):185–203.e13. doi: 10.1016/j.ccell.2017.07.007
- Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23(6):703–713. doi: 10.1038/nm.4333
- Luchini C, Paolino G, Mattiolo P, et al. KRAS wild-type pancreatic ductal adenocarcinoma: molecular pathology and therapeutic opportunities. J Exp Clin Cancer Res. 2020;39(1):227. doi: 10.1186/s13046-020-01732-6
- Caparello C, Meijer LL, Garajova I, et al. FOLFIRINOX and translational studies: towards personalized therapy in pancreatic cancer. World J Gastroenterol. 2016;22(31):6987–7005. doi: 10.3748/wjg.v22.i31.6987
- Sunkara T, Bandaru SS, Boyilla R, et al. Poly adenosine diphosphate-ribose polymerase (PARP) inhibitors in pancreatic cancer. Cureus. 2022;14(2):e22575. doi: 10.7759/cureus.22575
- Shroff RT, Hendifar A, McWilliams RR, et al. Rucaparib monotherapy in patients with pancreatic cancer and a known deleterious BRCA mutation. JCO Precis Oncol. 2018;2(2):1–15. doi: 10.1200/PO.17.00316
- Robson M, Im S-A, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a Germline BRCA Mutation. N Engl J Med. 2017;377(6):523–533. doi: 10.1056/NEJMoa1706450
- Tutt ANJ, Garber JE, Kaufman B, et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med. 2021;384(25):2394–2405. doi: 10.1056/NEJMoa2105215
- Ghosh M, Kang MS, Katuwal NB, et al. PSPC1 inhibition synergizes with Poly(ADP-ribose) polymerase inhibitors in a preclinical Model of BRCA-Mutated Breast/Ovarian cancer. Int J Mol Sci. 2023;24(23):17086. doi: 10.3390/ijms242317086
- Helleday T. The underlying mechanism for the PARP and BRCA synthetic lethality: clearing up the misunderstandings. Mol Oncol. 2011;5(4):387–393. doi: 10.1016/j.molonc.2011.07.001
- Kindler HL, Hammel P, Reni M, et al. Overall survival results from the POLO trial: a phase III study of active maintenance olaparib versus placebo for germline BRCA-Mutated metastatic pancreatic cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2022;40(34):3929–3939. doi: 10.1200/JCO.21.01604
- Research C for DE and. FDA approves olaparib for gBrcam metastatic pancreatic adenocarcinoma. FDA; 2019 [cited 2023 Nov 30]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-olaparib-gbrcam-metastatic-pancreatic-adenocarcinoma
- National Cancer Institute (NCI). APOLLO: a Randomized Phase II double-blind study of olaparib versus placebo following curative intent therapy in patients with resected pancreatic cancer and a pathogenic BRCA1, BRCA2 or PALB2 Mutation. Report No.: nCT04858334). [Internet]. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT04858334
- Reiss KA, Mick R, O’Hara MH, et al. Phase II study of maintenance rucaparib in patients with platinum-sensitive advanced pancreatic cancer and a pathogenic germline or somatic variant in BRCA1, BRCA2, or PALB2. J Clin Oncol Off J Am Soc Clin Oncol. 2021;39(22):2497–2505. doi: 10.1200/JCO.21.00003
- Kasi A, Chalise P, Williamson SK, et al. Niraparib in metastatic pancreatic cancer after previous chemotherapy (NIRA-PANC): A phase 2 trial. J Clin Oncol. 2019;37(15_suppl):TPS4168–TPS4168. doi: 10.1200/JCO.2019.37.15_suppl.TPS4168
- Centre Leon Berard. A multicentric, single arm, phase II trial assessing the efficacy of niraparib as first line therapy for patients with metastatic homologous repair-deficient pancreatic cancer [Internet]. Report No: nCT05442749. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT05442749
- National Cancer Institute (NCI). Randomized Phase II Study of 2nd Line FOLFIRI versus Modified FOLFIRI with PARP Inhibitor ABT-888 (Veliparib) (NSC-737664) in Metastatic Pancreatic Cancer [Internet]. Report No: nCT02890355. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT02890355
- Lowery MA, Kelsen DP, Capanu M, et al. Phase II Trial of Veliparib in Patients with Previously-Treated BRCA-Mutated Pancreas Ductal Adenocarcinoma. Eur J Cancer Oxf Engl 1990. 2018;89:19–26. doi: 10.1016/j.ejca.2017.11.004
- University of Pennsylvania. PARPVAX: Parpvax: a phase 1b/2, open label study of niraparib plus either ipilimumab or nivolumab in patients with advanced pancreatic cancer whose disease has not progressed on platinum-based therapy [Internet]. Report No.: nCT03404960. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT03404960
- National Cancer Institute (NCI). Randomized Phase II clinical trial of olaparib + pembrolizumab vs. Olaparib alone as maintenance therapy in metastatic pancreatic cancer patients with germline BRCA1 or BRCA2 Mutations [Internet]. Report No: nCT04548752. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT04548752
- Corrie PG. A phase II study combining pembrolizumab with olaparib in metastatic pancreatic adenocarcinoma (PDA) patients with high tumour mutation burden [Internet]. Report No: nCT05093231. clinicaltrials.gov; 2021 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT05093231
- Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins. Multi-agent low dose chemotherapy (gemcitabine, nab-paclitaxel, capecitabine, cisplatin, irinotecan) followed by maintenance olaparib and pembrolizumab in untreated metastatic pancreatic ductal adenocarcinoma. Report No: nCT04753879. [Internet]. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT04753879
- Barton CM, Hall PA, Hughes CM, et al. Transforming growth factor alpha and epidermal growth factor in human pancreatic cancer. J Pathol. 1991;163(2):111–116. doi: 10.1002/path.1711630206
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960–1966. doi: 10.1200/JCO.2006.07.9525
- Lim SH, Yun J, Lee M-Y, et al. Gemcitabine and Erlotinib with or without oxaliplatin in previously untreated advanced pancreatic cancer: a randomized phase II trial. Yonsei Med J. 2021;62(8):671–678. doi: 10.3349/ymj.2021.62.8.671
- Haas M, Waldschmidt DT, Stahl M, et al. Afatinib plus gemcitabine versus gemcitabine alone as first-line treatment of metastatic pancreatic cancer: the randomised, open-label phase II ACCEPT study of the Arbeitsgemeinschaft Internistische Onkologie with an integrated analysis of the “burden of therapy” method. Eur J Cancer Oxf Engl 1990. 2021;146:95–106.
- Leichman LP, O’Neil BH, Berlin J, et al. A phase IB study of erlotinib in combination with gemcitabine and nab-paclitaxel in patients with previously untreated advanced pancreatic cancer: an Academic GI Cancer Consortium (AGICC) study. J Clin Oncol. 2012;30(15_suppl):4052–4052. doi: 10.1200/jco.2012.30.15_suppl.4052
- Sinn M, Bahra M, Liersch T, et al. CONKO-005: adjuvant chemotherapy with gemcitabine plus erlotinib versus gemcitabine alone in patients after R0 resection of pancreatic cancer: a multicenter randomized phase III trial. J Clin Oncol Off J Am Soc Clin Oncol. 2017;35(29):3330–3337. doi: 10.1200/JCO.2017.72.6463
- Maurel J, Sánchez-Cabús S, Laquente B, et al. Outcomes after neoadjuvant treatment with gemcitabine and erlotinib followed by gemcitabine–erlotinib and radiotherapy for resectable pancreatic cancer (GEMCAD 10-03 trial). Cancer Chemother Pharmacol. 2018;82(6):935–943. doi: 10.1007/s00280-018-3682-9
- Philip PA, Benedetti J, Corless CL, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group–directed intergroup trial S0205. J Clin Oncol. 2010;28(22):3605–3610. doi: 10.1200/JCO.2009.25.7550
- Schultheis B, Reuter D, Ebert MP, et al. Gemcitabine combined with the monoclonal antibody nimotuzumab is an active first-line regimen inKRAS wildtype patients with locally advanced or metastatic pancreatic cancer: a multicenter, randomized phase IIb study. Ann Oncol. 2017;28(10):2429–2435. doi: 10.1093/annonc/mdx343
- Qin S, Bai Y, Wang Z, et al. Nimotuzumab combined with gemcitabine versus gemcitabine in K-RAS wild-type locally advanced or metastatic pancreatic cancer: a prospective, randomized-controlled, double-blinded, multicenter, and phase III clinical trial. J Clin Oncol. 2022;40(17_suppl):LBA4011–LBA4011. doi: 10.1200/JCO.2022.40.17_suppl.LBA4011
- Swain SM, Baselga J, Kim S-B, et al. Pertuzumab, Trastuzumab, and Docetaxel in HER2-Positive Metastatic Breast Cancer. N Engl J Med. 2015;372(8):724–734. doi: 10.1056/NEJMoa1413513
- Hurvitz SA, Hegg R, Chung W-P, et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet. 2023;401(10371):105–117. doi: 10.1016/S0140-6736(22)02420-5
- Shitara K, Bang Y-J, Iwasa S, et al. Trastuzumab deruxtecan in Previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382(25):2419–2430. doi: 10.1056/NEJMoa2004413
- Van Cutsem E, di Bartolomeo M, Smyth E, et al. Trastuzumab deruxtecan in patients in the USA and Europe with HER2-positive advanced gastric or gastroesophageal junction cancer with disease progression on or after a trastuzumab-containing regimen (DESTINY-Gastric02): primary and updated analyses from a single-arm, phase 2 study. Lancet Oncol. 2023;24:744–756. doi: 10.1016/S1470-2045(23)00215-2
- Ortega MA, Pekarek L, Fraile-Martinez O, et al. Implication of ERBB2 as a predictive tool for survival in patients with pancreatic cancer in histological studies. Curr Oncol. 2022;29(4):2442–2453. doi: 10.3390/curroncol29040198
- Omar N, Yan B, Salto-Tellez M. HER2: an emerging biomarker in non-breast and non-gastric cancers. Pathogenesis. 2015;2:1–9. doi: 10.1016/j.pathog.2015.05.002
- Cancer Research UK. DETERMINE (determining extended therapeutic indications for existing drugs in rare molecularly defined indications using a national evaluation platform trial): an umbrella-basket platform trial to evaluate the efficacy of targeted therapies in rare adult, paediatric and teenage/young adult (tya) cancers with actionable genomic alterations, including common cancers with rare actionable alterations [Internet]. Report No: nCT05722886. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT05722886
- AstraZeneca. A Phase 2, Multicenter, open-label study to evaluate the efficacy and safety of trastuzumab deruxtecan (t-dxd, ds-8201a) for the treatment of selected HER2 Expressing Tumors (DESTINY-PanTumor02)[Internet]. Report No.: nCT04482309. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT04482309
- Meric-Bernstam F, Makker V, Oaknin A, et al. Efficacy and safety of trastuzumab deruxtecan in patients with her2-expressing solid tumors: primary results from the DESTINY-PanTumor02 Phase II Trial. J Clin Oncol. 2024 Jan 1;42(1):47–58. doi: 10.1200/JCO.23.02005
- Li BT, Meric-Bernstam F, Bardia A, et al. 654O Efficacy and safety of trastuzumab deruxtecan (T-DXd) in patients (pts) with solid tumors harboring specific HER2-activating mutations (HER2m): Primary results from the international phase II DESTINY-PanTumor01 (DPT-01) study. Ann Oncol. 2023;34:S459–S460. doi: 10.1016/j.annonc.2023.09.1840
- de Vries EGE, Rüschoff J, Lolkema M, et al. Phase II study (KAMELEON) of single-agent T-DM1 in patients with HER2-positive advanced urothelial bladder cancer or pancreatic cancer/cholangiocarcinoma. Cancer Med. 2023;12(11):12071–12083. doi: 10.1002/cam4.5893
- Assenat E, Mineur L, Mollevi C, et al. Phase II study evaluating the association of gemcitabine, trastuzumab, and erlotinib as first-line treatment in patients with metastatic pancreatic adenocarcinoma (GATE 1). J Clin Oncol. 2015;33(3_suppl):379–379. doi: 10.1200/jco.2015.33.3_suppl.379
- Assenat E, Azria D, Mollevi C, et al. Dual targeting of HER1/EGFR and HER2 with cetuximab and trastuzumab in patients with metastatic pancreatic cancer after gemcitabine failure: results of the “Therapy”phase 1-2 trial. Oncotarget. 2015;6(14):12796–12808. doi: 10.18632/oncotarget.3473
- Wu Z, Gabrielson A, Hwang JJ, et al. Phase II study of lapatinib and capecitabine in second-line treatment for metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2015;76(6):1309–1314. doi: 10.1007/s00280-015-2855-z
- Cancer Trials Ireland. A Phase II Study of Lapatinib and Capecitabine in the Treatment of Metastatic Pancreatic Cancer [Internet]. Report No.: nCT00962312. clinicaltrials.gov; 2014 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT00962312
- National Cancer Institute (NCI). A Phase I, Pharmacokinetic, and Biologic Correlative Study of R115777 (NSC 702818) and Herceptin in Patients with Advanced Cancer [Internet]. Report No.: nCT00005842. clinicaltrials.gov; 2013 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT00005842
- National Cancer Institute (NCI). A Phase I Trial of Herceptin and Interleukin-12 [Internet]. Report No.: nCT00004074. clinicaltrials.gov; 2013 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT00004074
- National Cancer Institute (NCI). A Phase II Trial of Gemcitabine, Herceptin, and Radiation for Regionally Confined Adenocarcinoma of the Pancreas [Internet]. Report No.: nCT00005926. clinicaltrials.gov; 2008 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT00005926
- Huang L, Guo Z, Wang F, et al. KRAS mutation: from undruggable to druggable in cancer. Signal Transduct Target Ther. 2021;6(1):1–20. doi: 10.1038/s41392-021-00780-4
- Luo J. KRAS mutation in pancreatic cancer. Semin Oncol. 2021;48(1):10–18. doi: 10.1053/j.seminoncol.2021.02.003
- Skoulidis F, Li BT, Dy GK, et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med. 2021;384(25):2371–2381. doi: 10.1056/NEJMoa2103695
- Jänne PA, Riely GJ, Gadgeel SM, et al. Adagrasib in non–small-Cell lung cancer harboring a KRASG12C mutation. N Engl J Med. 2022;387(2):120–131. doi: 10.1056/NEJMoa2204619
- Strickler JH, Satake H, George TJ, et al. Sotorasib in KRAS p.G12C–mutated advanced pancreatic cancer. N Engl J Med. 2023;388(1):33–43. doi: 10.1056/NEJMoa2208470
- M.D. Anderson Cancer Center. Phase Ib Trial of the KRASG12C Inhibitor Adagrasib (MRTX849) in KRAS G12C Mutant Metastatic Pancreatic Cancer Patients [Internet]. Report No.: nCT05634525. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT05634525
- Mirati Therapeutics Inc. A Phase 1/2 Trial of MRTX849 in Combination with TNO155 in Patients with Advanced Solid Tumors with KRAS G12C Mutation KRYSTAL 2 [Internet]. Report No.: nCT04330664. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT04330664
- M.D. Anderson Cancer Center. Phase Ib Trial of the KRAS G12C Inhibitor Adagrasib (MRTX849) in Combination with the PARP Inhibitor Olaparib in Patients with KRAS G12C Mutated Advanced Solid Tumors, with a Focus on Gynecological, Breast, Pancreatic and KEAP1 Mutated Non-small Cell Lung Cancers [Internet]. Report No.: nCT06130254. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT06130254
- Revolution Medicines, Inc. Phase 1/1b, multicenter, open-label, dose escalation and dose expansion study of rmc-6291 monotherapy in subjects with advanced KRASG12C Mutant Solid Tumors [Internet]. Report No.: nCT05462717. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT05462717
- Jacobio Pharmaceuticals Co. Ltd. A Phase 2, multi-center, open-label, single-arm study to evaluate the efficacy and safety of JAB-21822 monotherapy in patients with locally advanced or metastatic kras p.g12c mutated pancreatic cancer [Internet]. Report No.: nCT06008288. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT06008288
- Li J, Zhao J, Cao B, et al. A phase I/II study of first-in-human trial of JAB-21822 (KRAS G12C inhibitor) in advanced solid tumors. J Clin Oncol. 2022;40(16_suppl):3089–3089. doi: 10.1200/JCO.2022.40.16_suppl.3089
- Hallin J, Bowcut V, Calinisan A, et al. Anti-tumor efficacy of a potent and selective non-covalent KRASG12D inhibitor. Nat Med. 2022;28(10):2171–2182. doi: 10.1038/s41591-022-02007-7
- Mirati Therapeutics Inc. A Phase 1/2 Multiple Expansion Cohort Trial of MRTX1133 in Patients with Advanced Solid Tumors Harboring a KRAS G12D Mutation [Internet]. Report No.: nCT05737706. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT05737706
- Revolution Medicines, Inc. Phase 1/1b, Multicenter, Open-Label, Study of RMC 9805 in Participants with Advanced KRASG12D-Mutant Solid Tumors [Internet]. Report No.: nCT06040541. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT06040541
- Silenseed Ltd. Phase I - Escalating Dose Study of siG12D LODER (Local Drug EluteR) in patients with locally advanced adenocarcinoma of the pancreas, and a Single Dose Study of siG12D LODER (Local Drug EluteR) in Patients with Non-operable Adenocarcinoma of the Pancreas [Internet]. Report No.: nCT01188785. clinicaltrials.gov; 2019 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT01188785
- Golan T, Khvalevsky EZ, Hubert A, et al. Rnai therapy targeting KRAS in combination with chemotherapy for locally advanced pancreatic cancer patients. Oncotarget. 2015;6(27):24560–24570. doi: 10.18632/oncotarget.4183
- Silenseed Ltd. A Prospective, Multinational, Multi-Center, Phase 2, Single-Arm, Open-Label Study Evaluating the Efficacy, Safety and Tolerability of siG12D-LODER in Combination with Standard of Care Chemotherapy in the Treatment of Patients with Locally Advanced Pancreatic Cancer [Internet]. Report No.: nCT01676259. clinicaltrials.gov; 2021 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT01676259
- Surana R, LeBleu VS, Lee JJ, et al. Phase I study of mesenchymal stem cell (MSC)-derived exosomes with KRASG12D siRNA in patients with metastatic pancreatic cancer harboring a KRASG12D mutation. J Clin Oncol. 2022;40(4_suppl):TPS633–TPS633. doi: 10.1200/JCO.2022.40.4_suppl.TPS633
- M.D. Anderson Cancer Center. Phase I Study of Mesenchymal Stromal Cells-Derived Exosomes with KrasG12D siRNA for Metastatic Pancreas Cancer Patients Harboring KrasG12D Mutation [Internet]. Report No.: nCT03608631. [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT03608631
- Astellas Pharma Inc. A Phase 1 Study of ASP3082 in Participants with Previously Treated Locally Advanced or Metastatic Solid Tumor Malignancies with KRAS G12D Mutation [Internet]. Report No.: nCT05382559. clinicaltrials.gov; 2024 [cited 2024 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT05382559
- Bery N, Miller A, Rabbitts T. A potent KRAS macromolecule degrader specifically targeting tumours with mutant KRAS. Nat Commun. 2020;11(1):3233. doi: 10.1038/s41467-020-17022-w
- Gustafson WC, Wildes D, Rice MA, et al. Direct targeting of RAS in pancreatic ductal adenocarcinoma with RMC-6236, a first-in-class, RAS-selective, orally bioavailable, tri-complex RASMULTI(ON) inhibitor. J Clin Oncol. 2022;40(4_suppl):591–591. doi: 10.1200/JCO.2022.40.4_suppl.591
- Revolution Medicines, Inc. A Multicenter Open-Label Study of RMC-6236 in Patients with Advanced Solid Tumors Harboring Specific Mutations in RAS [Internet]. Report No.: nCT05379985. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT05379985
- Bayer M. RMC-6236 shows safety, efficacy in KRAS G12X tumors. Targeted Therapies in Oncology, November II, 2023. 2023 Nov 20;12(16):21.
- Revolution Medicines, Inc. Phase 1b, multicenter, open-label, dose escalation and dose expansion study of RMC-6291 in combination with RMC-6236 in participants with advanced KRAS G12C mutant solid tumors [Internet]. Report No.: nCT06128551. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT06128551
- Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet. 2015;386(9992):444–451. doi: 10.1016/S0140-6736(15)60898-4
- Dummer R, Ascierto PA, Gogas HJ, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19(5):603–615. doi: 10.1016/S1470-2045(18)30142-6
- Pfizer. A Phase 1b/2 study to evaluate safety and clinical activity of combinations of avelumab, binimetinib and talazoparib in patients with locally advanced or metastatic Ras-mutant solid tumors [Internet]. Report No.: nCT03637491. clinicaltrials.gov; 2021 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT03637491
- Rodon Ahnert J, Tan D-W, Garrido-Laguna I, et al. Avelumab or talazoparib in combination with binimetinib in metastatic pancreatic ductal adenocarcinoma: dose-finding results from phase Ib of the JAVELIN PARP MEKi trial. ESMO Open. 2023;8(4):101584. doi: 10.1016/j.esmoop.2023.101584
- LoRusso P, Fakih M, Vos FYFLD, et al. 561P Phase Ib study of ribociclib (R) + trametinib (T) in patients (pts) with metastatic/advanced solid tumours. Annal Oncol. 2020;31:S484. doi: 10.1016/j.annonc.2020.08.675
- Infante JR, Somer BG, Park JO, et al. A randomised, double-blind, placebo-controlled trial of trametinib, an oral MEK inhibitor, in combination with gemcitabine for patients with untreated metastatic adenocarcinoma of the pancreas. Eur J Cancer Oxf Engl 1990. 2014;50(12):2072–2081. doi: 10.1016/j.ejca.2014.04.024
- Roswell Park Cancer Institute. Perioperative Analysis of Binimetinib and Palbociclib in RAS-Driven Tumors [Internet]. Report No.: nCT04870034. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT04870034
- M.D. Anderson Cancer Center. Binimetinib plus hydroxychloroquine in KRAS mutant metastatic pancreatic cancer [Internet]. Report No.: nCT04132505. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT04132505
- University of Utah. THREAD: a Phase I trial of trametinib and hydroxychloroquine in patients with advanced pancreatic cancer [Internet]. Report No.: nCT03825289. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT03825289
- National Cancer Institute (NCI). a Phase II study of selumetinib (AZD6244) for the treatment of advanced pancreas cancer harboring KRAS G12R mutations [Internet]. Report No.: nCT03040986. clinicaltrials.gov; 2021 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT03040986
- Kenney C, Kunst T, Webb S, et al. Phase II study of selumetinib, an orally active inhibitor of MEK1 and MEK2 kinases, in KRASG12R-mutant pancreatic ductal adenocarcinoma. Invest New Drugs. 2021;39(3):821–828. doi: 10.1007/s10637-020-01044-8
- ABM Therapeutics Corporation. a Phase I, first-in-human, multicenter, open label, dose escalation and dose expansion study to evaluate the safety and efficacy of ABM-168 administered orally in adult patients with advanced solid tumors [Internet]. Report No.: nCT05831995. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT05831995
- Immuneering Corporation. a Phase 1/2a, open-label, multicenter, nonrandomized, safety and anti-tumor activity study of IMM-1-104, a novel oral dual MEK1/2 inhibitor in participants with previously treated RAS-mutated advanced or metastatic solid tumors [Internet]. Report No.: nCT05585320. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT05585320
- Verastem, Inc. A Phase 1b/2a Study of Gemcitabine and Nab-paclitaxel in Combination with Avutometinib (VS-6766) and Defactinib in Patients with Previously Untreated Metastatic Adenocarcinoma of the Pancreas [Internet]. Report No.: nCT05669482. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT05669482
- Banerjee SN, Ring KL, Van Nieuwenhuysen E, et al. Initial efficacy and safety results from ENGOT-ov60/GOG-3052/RAMP 201: a phase 2 study of avutometinib (VS-6766) ± defactinib in recurrent low-grade serous ovarian cancer (LGSOC). J Clin Oncol. 2023;41(16_suppl):5515–5515. doi: 10.1200/JCO.2023.41.16_suppl.5515
- Le Large TYS, Bijlsma MF, El Hassouni B, et al. Focal adhesion kinase inhibition synergizes with nab-paclitaxel to target pancreatic ductal adenocarcinoma. J Exp Clin Cancer Res CR. 2021;40(1):91. doi: 10.1186/s13046-021-01892-z
- Aung KL, McWhirter E, Welch S, et al. A phase II trial of GSK2256098 and trametinib in patients with advanced pancreatic ductal adenocarcinoma (PDAC) (MOBILITY-002 Trial, NCT02428270). J Clin Oncol. 2018;36(4_suppl):409–409. doi: 10.1200/JCO.2018.36.4_suppl.409
- University Health Network. Toronto. Molecular Basket Trial in Multiple Malignancies with Common Target Pathway Aberrancies: A Phase II Trial of GSK2256098 and Trametinib in Patients with Advanced Pancreatic Cancer [Internet]. Report No: nCT02428270. clinicaltrials.gov; 2022 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT02428270
- Ko AH, Bekaii-Saab T, Van Ziffle J, et al. A multicenter, open-label phase II clinical trial of combined MEK plus EGFR inhibition for chemotherapy-refractory advanced pancreatic adenocarcinoma. Clin Cancer Res. 2016;22(1):61–68. doi: 10.1158/1078-0432.CCR-15-0979
- Grierson PM, Tan B, Pedersen KS, et al. Phase Ib Study of Ulixertinib Plus Gemcitabine and Nab-Paclitaxel in Patients with Metastatic Pancreatic Adenocarcinoma. Oncology. 2022;28(2):e115–e123. doi: 10.1093/oncolo/oyac237
- UNC Lineberger Comprehensive Cancer Center. A Phase I Trial of Ulixertinib (BVD-523) in Combination with Palbociclib in Patients with Advanced Solid Tumors with Expansion Cohort in Previously Treated Metastatic Pancreatic Cancer and Metastatic RAS-mutant and NF1-mutant (no Concurrent BRAFV600 Mutations) Melanoma [Internet]. Report No: nCT03454035. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT03454035
- Raybould AL, Burgess B, Urban C, et al. A phase Ib trial of ERK inhibition with ulixertinib combined with palbociclib in patients (Pts) with advanced solid tumors. J Clin Oncol. 2021;39(15_suppl):3103–3103. doi: 10.1200/JCO.2021.39.15_suppl.3103
- Erasca, Inc. A Phase 1b/2 Study of Agents Targeting the Mitogen-Activated Protein Kinase Pathway in Patients with Advanced Gastrointestinal Malignancies (HERKULES-3) [Internet]. Report No.: nCT05039177. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT05039177
- Weekes C, Lockhart A, LoRusso P, et al. A Phase Ib Study to Evaluate the MEK Inhibitor Cobimetinib in Combination with the ERK1/2 Inhibitor GDC‐0994 in Patients with Advanced Solid Tumors. Oncology. 2020;25:833–e1438. doi: 10.1634/theoncologist.2020-0292
- Erasca, Inc. An Open-label Study to Assess the Safety and Efficacy of Naporafenib (ERAS-254) Administered with Trametinib in Previously Treated Patients with Locally Advanced Unresectable or Metastatic Solid Tumor Malignancies with RAS Q61X Mutations [Internet]. Report No: nCT05907304. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT05907304
- Tolcher AW, Hong DS, Vandross AL, et al. A phase 1/2 study of DCC-3116 as a single agent and in combination with trametinib in patients with advanced or metastatic solid tumors with RAS or RAF mutations. J Clin Oncol. 2022;40(16_suppl):TPS3178–TPS3178. doi: 10.1200/JCO.2022.40.16_suppl.TPS3178
- Deciphera Pharmaceuticals LLC. A Phase 1/2, First-in-Human Study of DCC-3116 as Monotherapy and in Combination with RAS/MAPK Pathway Inhibitors in Patients with Advanced or Metastatic Solid Tumors with RAS/MAPK Pathway Mutations [Internet]. Report No: nCT04892017. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT04892017
- HonorHealth Research Institute. A Phase II Trial of Vemurafenib in Combination with Sorafenib to Treat Patients with Advanced KRAS Mutated Pancreatic Cancer: Targeting RAF Dimers to Suppress Oncogenic RAS Signaling (The Dr. Nate Nieto Study) [Internet]. Report No: nCT05068752. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT05068752
- The Netherlands Cancer Institute. Phase I/Ib Study with the Combination of RMC-4630 (SHP2 Inhibitor) and LY3214996 (ERK Inhibitor) in Metastatic KRAS Mutant CRC, PDAC and NSCLC [Internet]. Report No: nCT04916236. clinicaltrials.gov; 2022 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT04916236
- Jacobio Pharmaceuticals Co. Ltd. A Phase 1/2a Clinical Study to Evaluate the Safety, Tolerability, Pharmacokinetics and Antitumor Activity of JAB-21822 in Combination with JAB-3312 in Patients with Advanced Solid Tumors Harboring KRAS p.G12C Mutation [Internet]. Report No: nCT05288205. clinicaltrials.gov; 2022 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT05288205
- Wang J, Zhao J, Zhong J, et al. 653O Glecirasib (KRAS G12C inhibitor) in combination with JAB-3312 (SHP2 inhibitor) in patients with KRAS p.G12C mutated solid tumors. Ann Oncol. 2023;34:S459. doi: 10.1016/j.annonc.2023.09.1839
- HUYABIO International, LLC. A Phase 1, Open-Label, Dose Escalation of HBI-2376 in Patients with Advanced Malignant Solid Tumors Harboring KRAS or EGFR Mutations [Internet]. Report No: nCT05163028. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT05163028
- Jacobio Pharmaceuticals Co. Ltd. A Phase 1, Multi-Center, Open-Label Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Preliminary Evidence of Antitumor Activity of JAB-3312 in Adult Patients with Advanced Solid Tumors [Internet]. Report No: nCT04045496. clinicaltrials.gov; 2022 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT04045496
- Jacobio Pharmaceuticals Co. Ltd. A Phase 1, Multi-Center, Open-Label Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Preliminary Evidence of Antitumor Activity of JAB-3312 in Adult Patients with Advanced Solid Tumors [Internet]. Report No: nCT04121286. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT04121286
- Mirzoeva OK, Das D, Heiser LM, et al. Basal subtype and MAPK/ERK kinase (MEK)-phosphoinositide 3-kinase feedback signaling determine susceptibility of breast cancer cells to MEK inhibition. Cancer Res. 2009;69(2):565–572. doi: 10.1158/0008-5472.CAN-08-3389
- Mortazavi M, Moosavi F, Martini M, et al. Prospects of targeting PI3K/AKT/mTOR pathway in pancreatic cancer. Crit Rev Oncol Hematol. 2022;176:103749. doi: 10.1016/j.critrevonc.2022.103749
- Bardia A, Gounder M, Rodon J, et al. Phase ib study of combination therapy with MEK inhibitor binimetinib and Phosphatidylinositol 3-Kinase inhibitor Buparlisib in patients with advanced solid tumors with RAS/RAF alterations. Oncology. 2020;25(1):e160–e169. doi: 10.1634/theoncologist.2019-0297
- Bedard PL, Tabernero J, Janku F, et al. A phase ib dose-escalation study of the oral pan-PI3K inhibitor buparlisib (BKM120) in combination with the oral MEK1/2 inhibitor Trametinib (GSK1120212) in patients with selected advanced solid tumors. Clin Cancer Res. 2015;21(4):730–738. doi: 10.1158/1078-0432.CCR-14-1814
- Chung V, McDonough S, Philip PA, et al. Effect of Selumetinib and MK-2206 vs Oxaliplatin and Fluorouracil in Patients with Metastatic Pancreatic Cancer After Prior Therapy: SWOG S1115 Study Randomized Clinical Trial. JAMA Onc. 2017;3(4):516–522. doi: 10.1001/jamaoncol.2016.5383
- Franco J, Witkiewicz AK, Knudsen ES. CDK4/6 inhibitors have potent activity in combination with pathway selective therapeutic agents in models of pancreatic cancer. Oncotarget. 2014;5(15):6512–6525. doi: 10.18632/oncotarget.2270
- Weinberg BA, Wang H, Witkiewicz AK, et al. A Phase I Study of Ribociclib Plus Everolimus in Patients with Metastatic Pancreatic Adenocarcinoma Refractory to Chemotherapy. J Pancreat Cancer. 2020;6(1):45–54. doi: 10.1089/pancan.2020.0005
- Wijnen R, Pecoraro C, Carbone D, et al. Cyclin Dependent Kinase-1 (CDK-1) Inhibition as a Novel Therapeutic Strategy against Pancreatic Ductal Adenocarcinoma (PDAC). Cancers (Basel). 2021;13(17):4389. doi: 10.3390/cancers13174389
- Peguero J, Sohal DPS, O’Neil BH, et al. Tissue/Site-Agnostic Study of Ribociclib for Tumors with Cyclin D–CDK4/6 Pathway Genomic Alterations: A Phase II, Open-Label, Single-Arm Basket Study. JCO Precis Oncol. 2019;(3):1–10. doi: 10.1200/PO.18.00383
- Chiorean EG, Picozzi V, Li C-P, et al. Efficacy and safety of abemaciclib alone and with PI3K/mTOR inhibitor LY3023414 or galunisertib versus chemotherapy in previously treated metastatic pancreatic adenocarcinoma: a randomized controlled trial. Cancer Med. 2023;12(20):20353–20364. doi: 10.1002/cam4.6621
- Hu C, Dadon T, Chenna V, et al. Combined inhibition of cyclin-dependent kinases (dinaciclib) and AKT (MK-2206) Blocks Pancreatic tumor Growth and metastases in patient-derived xenograft models. Mol Cancer Ther. 2015;14(7):1532–1539. doi: 10.1158/1535-7163.MCT-15-0028
- National Cancer Institute (NCI). A Phase I Trial of Dinaciclib (SCH727965) and MK-2206 in Metastatic Pancreatic Cancer with an Expansion Cohort in Advanced Pancreatic Cancer [Internet]. Report No: nCT01783171. clinicaltrials.gov; 2017 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT01783171
- Murphy AG, Zahurak M, Shah M, et al. A phase I study of dinaciclib in combination with MK-2206 in patients with advanced pancreatic cancer. Clin Transl Sci. 2020;13(6):1178–1188. doi: 10.1111/cts.12802
- Shapiro G. Phase I Study of the CDK4/6 Inhibitor Palbociclib (PD-0332991) in Combination with the PI3K/mTOR Inhibitor Gedatolisib (PF-05212384) for Patients with Advanced Squamous Cell Lung, Pancreatic, Head & Neck and Other Solid Tumors [Internet]. Report No: nCT03065062. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT03065062
- Hu X, Li J, Fu M, et al. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Target Ther. 2021;6(1):1–33. doi: 10.1038/s41392-021-00791-1
- Hurwitz H, Van Cutsem E, Bendell JC, et al. Two randomized, placebo-controlled phase 3 studies of ruxolitinib (Rux) + capecitabine (C) in patients (pts) with advanced/metastatic pancreatic cancer (mPC) after failure/intolerance of first-line chemotherapy: JANUS 1 (J1) and JANUS 2 (J2). J Clin Oncol. 2017;35(4_suppl):343–343. doi: 10.1200/JCO.2017.35.4_suppl.343
- Ng K, Hendifar A, Starodub A, et al. Phase 1 dose-escalation study of momelotinib, a Janus kinase 1/2 inhibitor, combined with gemcitabine and nab-paclitaxel in patients with previously untreated metastatic pancreatic ductal adenocarcinoma. Invest New Drugs. 2019;37(1):159–165. doi: 10.1007/s10637-018-0650-5
- Beatty GL, Shahda S, Beck T, et al. A Phase Ib/II Study of the JAK1 Inhibitor, Itacitinib, plus nab‐Paclitaxel and Gemcitabine in Advanced Solid Tumors. Oncology. 2019;24:14–e10. doi: 10.1634/theoncologist.2017-0665
- National Cancer Centre, Singapore. Phase Ib Study Evaluating Safety and Tolerability of Combination Trametinib and Ruxolitinib in Patients with Advanced RAS Mutant Colorectal Cancer and Pancreatic Adenocarcinoma [Internet]. Report No: nCT04303403. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT04303403
- Datta J, Dai X, Bianchi A, et al. Combined MEK and STAT3 Inhibition Uncovers Stromal Plasticity by Enriching for Cancer-Associated Fibroblasts with Mesenchymal Stem Cell-Like Features to Overcome Immunotherapy Resistance in Pancreatic Cancer. Gastroenterology. 2022;163(6):1593–1612. doi: 10.1053/j.gastro.2022.07.076
- Hosein P. A Phase 1 Trial of Combined MEK, STAT3 and PD-1 Inhibition in Metastatic Pancreatic Ductal Adenocarcinoma [Internet]. Report No: nCT05440942. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT05440942
- M.D. Anderson Cancer Center. Phase II Clinical Trial Evaluating Intravenous AZD9150 (Antisense STAT3) with MEDI4736 (Anti-PD-L1) in Patients with Advanced Pancreatic, Non-Small Cell Lung Cancer, and Mismatch Repair Deficient Colorectal Cancer [Internet]. Report No: nCT02983578. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT02983578
- Incyte Corporation. A Platform Study Exploring the Safety, Tolerability, Effect on the Tumor Microenvironment, and Efficacy of Pembrolizumab + INCB Combinations in Advanced Solid Tumors [Internet]. Report No: nCT02646748. clinicaltrials.gov; 2022 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT02646748
- University of Colorado. Denver. Phase 1/2 Trial of Radiotherapy in Combination with TTI-101 in Patients with Borderline Resectable Pancreatic Cancer [Internet]. Report No: nCT06141031. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT06141031
- Van Cutsem E, Vervenne WL, Bennouna J, et al. Phase III trial of Bevacizumab in combination with gemcitabine and Erlotinib in Patients with Metastatic pancreatic cancer. J Clin Oncol. 2009;27(13):2231–2237. doi: 10.1200/JCO.2008.20.0238
- Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the cancer and leukemia group B (CALGB 80303). J Clin Oncol. 2010;28(22):3617–3622. doi: 10.1200/JCO.2010.28.1386
- Rougier P, Riess H, Manges R, et al. Randomised, placebo-controlled, double-blind, parallel-group phase III study evaluating aflibercept in patients receiving first-line treatment with gemcitabine for metastatic pancreatic cancer. Eur J Cancer. 2013;49(12):2633–2642. doi: 10.1016/j.ejca.2013.04.002
- Shaib WL, Manali R, Liu Y, et al. Phase II randomised, double-blind study of mFOLFIRINOX plus ramucirumab versus mFOLFIRINOX plus placebo in advanced pancreatic cancer patients (HCRN GI14-198). Eur J Cancer Oxf Engl 1990. 2023;189:112847. doi: 10.1016/j.ejca.2023.02.030
- Cardin DB, Goff L, Li C-I, et al. Phase II trial of sorafenib and erlotinib in advanced pancreatic cancer. Cancer Med. 2014;3(3):572–579. doi: 10.1002/cam4.208
- Gonçalves A, Gilabert M, François E, et al. BAYPAN study: a double-blind phase III randomized trial comparing gemcitabine plus sorafenib and gemcitabine plus placebo in patients with advanced pancreatic cancer. Ann Oncol. 2012;23(11):2799–2805. doi: 10.1093/annonc/mds135
- Kindler HL, Ioka T, Richel DJ, et al. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol. 2011;12(3):256–262. doi: 10.1016/S1470-2045(11)70004-3
- O’Reilly EM, Niedzwiecki D, Hall M, et al. A cancer and leukemia group B phase II study of sunitinib malate in patients with previously treated metastatic pancreatic adenocarcinoma (CALGB 80603). Oncology. 2010;15(12):1310–1319. doi: 10.1634/theoncologist.2010-0152
- Boyd LNC, Andini KD, Peters GJ, et al. Heterogeneity and plasticity of cancer-associated fibroblasts in the pancreatic tumor microenvironment. Semin Cancer Biol. 2022;82:184–196. doi: 10.1016/j.semcancer.2021.03.006
- Li S, Xu H-X, Wu C-T, et al. Angiogenesis in pancreatic cancer: current research status and clinical implications. Angiogenesis. 2019;22(1):15–36. doi: 10.1007/s10456-018-9645-2
- Annese T, Tamma R, Ruggieri S, et al. Angiogenesis in Pancreatic Cancer: Pre-Clinical and Clinical Studies. Cancers (Basel). 2019;11(3):381. doi: 10.3390/cancers11030381
- Cao J, Zhang J, Peng W, et al. A phase I study of safety and pharmacokinetics of fruquintinib, a novel selective inhibitor of vascular endothelial growth factor receptor-1, -2, and -3 tyrosine kinases in Chinese patients with advanced solid tumors. Cancer Chemother Pharmacol. 2016;78(2):259–269. doi: 10.1007/s00280-016-3069-8
- Guo W. A Phase II Clinical Trial of the Safety and Efficacy of Fruquintinib in Advanced Pancreatic Cancer Patients Who Failed Second-line Gemcitabine or 5-FU Based Chemotherapy [Internet]. Report No: nCT05257122. clinicaltrials.gov; 2022 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT05257122
- Yu X-J. An Open Single-center Phase II Clinical Study of Fruquintinib Combined with Chemotherapy in Patients with Liver Metastases from Pancreatic Cancer [Internet]. Report No: nCT05168527. clinicaltrials.gov; 2021 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT05168527
- Du J, Tong F, Sha H, et al. 155P Penpulimab combined with anlotinib and nab-paclitaxel plus gemcitabine (PAAG) as first-line treatment for advanced metastatic pancreatic cancer: A prospective, multicenter, single-arm, phase II study. Ann Oncol. 2023;34:S1535. doi: 10.1016/j.annonc.2023.10.290
- Juan D. A Multicenter Study of Penpulimab and Anlotinib in Combination with Nab-paclitaxel Plus Gemcitabine as First-line Therapy in Patients (Pts) with Metastatic Pancreatic Cancer: PAAG. [Internet]. Report No.: nCT05493995. clinicaltrials.gov; 2022 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT05493995
- The Affiliated Nanjing Drum Tower Hospital of Nanjing University Medical School. Penpulimab Combined with Anlotinib and Nab-paclitaxel Plus Gemcitabine (PAAG) as First-line Treatment for Advanced Metastatic Pancreatic Cancer: a Prospective, Multicenter, Single-arm, Phase 2 Study [Internet]. Report No: nCT06051851. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT06051851
- The First Affiliated Hospital with Nanjing Medical University. Anlotinib Plus Toripalimab and Nab-paclitaxel in Patients with Locally Advanced or Metastatic Pancreatic Cancer: an Open-label, Non-randomized, Phase II Study [Internet]. Report No: nCT04718701. clinicaltrials.gov; 2021 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT04718701
- First Affiliated Hospital Xi’an Jiaotong University. A Prospective, One-arm, Phase II Clinical Study of Tislelizumab Combined with Anlotinib and Investigator-selected Chemotherapy for Second-line Treatment of Advanced or Metastatic Pancreatic Cancer: a [Internet]. Report No: nCT05681390. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT05681390
- Chia Tai Tianqing Pharmaceutical Group Nanjing Shunxin Pharmaceutical Co. Ltd. Phase I Clinical Trial to Evaluate the Tolerability and Safety of TQB2858 Injection in Subjects with Metastatic Pancreatic Cancer [Internet]. Report No: nCT05193604. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT05193604
- Peking Union Medical College Hospital. Anlotinib Plus Anti-PD-1 Antibody AK105 as Third or More-line Therapy for Advanced Pancreatic Cancer: a Prospective, Single-arm, Open-label, Pilot Study [Internet]. Report No: nCT04803851. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT04803851
- Qingdao Central Hospital. Phase 2 Study of Anlotinib with PD-1 Inhibitor as Second Line Therapy in the Treatment of Metastatic Pancreatic Cancer [Internet]. Report No: nCT05218629. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT05218629
- Warburg O, Wind F, Negelein E. THE METABOLISM OF TUMORS IN THE BODY. J Gen Physiol. 1927;8(6):519–530. doi: 10.1085/jgp.8.6.519
- Grasso C, Jansen G, Giovannetti E. Drug resistance in pancreatic cancer: Impact of altered energy metabolism. Crit Rev Oncol Hematol. 2017;114:139–152. doi: 10.1016/j.critrevonc.2017.03.026
- Pardee TS, Lee K, Luddy J, et al. A phase I study of the First-in-Class Antimitochondrial Metabolism Agent, CPI-613, in patients with advanced hematologic malignancies. Clin Cancer Res. 2014;20(20):5255–5264. doi: 10.1158/1078-0432.CCR-14-1019
- Alistar A, Morris BB, Desnoyer R, et al. Safety and tolerability of the first-in-class agent CPI-613 in combination with modified FOLFIRINOX in patients with metastatic pancreatic cancer: a single-centre, open-label, dose-escalation, phase 1 trial. Lancet Oncol. 2017;18(6):770–778. doi: 10.1016/S1470-2045(17)30314-5
- Alistar AT, Morris B, Harrison L, et al. A single-arm, open-label, phase I study of CPI-613 (Devimistat) in combination with gemcitabine and nab-paclitaxel for patients with locally advanced or metastatic pancreatic adenocarcinoma. J Clin Oncol. 2020;38(15_suppl):4635–4635. doi: 10.1200/JCO.2020.38.15_suppl.4635
- Philip PA, Bahary N, Mahipal A, et al. Phase 3, multicenter, randomized study of CPI-613 with modified FOLFIRINOX (mFFX) versus FOLFIRINOX (FFX) as first-line therapy for patients with metastatic adenocarcinoma of the pancreas (AVENGER500). J Clin Oncol. 2022;40(16_suppl):4023–4023. doi: 10.1200/JCO.2022.40.16_suppl.4023
- Bajor D. A Phase II/I Open-Label Clinical Trial of CPI-613 in Combination with Modified FOLFIRINOX in Patients with Locally Advanced Pancreatic Cancer and Good Performance Status. Report No: nCT03699319. [Internet]. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT03699319
- Kamgar M. A Phase I Dose-Escalation Study of CPI-613 (Devimistat) in Combination with Chemoradiation in Patients with Pancreatic Adenocarcinoma. Report No: nCT05325281. [Internet].clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT05325281
- Bajor DL, Mohamed A, Selfridge JE, et al. A phase II open-label study of cpi-613 in combination with modified (m)FOLFIRINOX in patients with locally advanced pancreatic cancer. J Clin Oncol. 2021;39(15_suppl):TPS4176–TPS4176. doi: 10.1200/JCO.2021.39.15_suppl.TPS4176
- Khan HY, Kamgar M, Aboukameel A, et al. Targeting Cellular Metabolism with CPI-613 Sensitizes Pancreatic Cancer Cells to Radiation Therapy. Adv Radiat Oncol. 2023;8(1):101122. doi: 10.1016/j.adro.2022.101122
- Majchrzak‐Stiller B, Buchholz M, Peters I, et al. GP -2250, a novel anticancer agent, inhibits the energy metabolism, activates AMP-Kinase and impairs the NF-kB pathway in pancreatic cancer cells. J Cell Mol Med. 2023;27(14):2082–2092. doi: 10.1111/jcmm.17825
- Geistlich Pharma AG. A Phase 1 Trial to Evaluate the Safety, Tolerability, Pharmacokinetics, and Preliminary Efficacy of GP-2250 in Combination with Gemcitabine in Subjects with Advanced Unresectable or Metastatic Pancreatic Adenocarcinoma Who Have Progressed on Prior Treatment with 5-Fluorouacil-based Chemotherapy. Report No: nCT03854110. [Internet]. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT03854110
- McDonald PC, Chia S, Bedard PL, et al. A phase 1 study of SLC-0111, a novel inhibitor of carbonic anhydrase IX, in patients with advanced solid tumors. Am J Clin Oncol. 2020;43(7):484–490. doi: 10.1097/COC.0000000000000691
- British Columbia Cancer Agency. An Open-label, Multi-center, Phase 1b Study to Investigate the Safety and Tolerability of SLC-0111 (WBI-5111) in Combination with Gemcitabine in Metastatic Pancreatic Ductal Adenocarcinoma Subjects Positive for Carbonic Anhydrase IX [Internet]. Report No: nCT03450018. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT03450018
- Wang W, Pan H, Ren F, et al. Targeting ASCT2-mediated glutamine metabolism inhibits proliferation and promotes apoptosis of pancreatic cancer cells. Biosci Rep. 2022;42(3):BSR20212171. doi: 10.1042/BSR20212171
- Schifferli KP, Monks NR, Tammali R, et al. Abstract LB-298: MEDI7247: A first in class antibody drug conjugate targeting ASCT2 in a range of solid tumors. Cancer Res. 2018;78(13_Supplement):LB–298. doi: 10.1158/1538-7445.AM2018-LB-298
- MedImmune LLC. A Phase 1/1b Multicenter, Open-label, Dose-escalation, and Dose-expansion Study to Evaluate the Safety, Tolerability, Pharmacokinetics, Immunogenicity, and Antitumor Activity of MEDI7247 in Patients with Advanced or Metastatic Disease in Selected Solid Tumors [Internet]. Report No: nCT03811652. clinicaltrials.gov; 2019 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT03811652
- Biancur DE, Paulo JA, Małachowska B, et al. Compensatory metabolic networks in pancreatic cancers upon perturbation of glutamine metabolism. Nat Commun. 2017;8(1):15965. doi: 10.1038/ncomms15965
- Gaglio D, Metallo CM, Gameiro PA, et al. Oncogenic K-Ras decouples glucose and glutamine metabolism to support cancer cell growth. Mol Syst Biol. 2011;7(1):523. doi: 10.1038/msb.2011.56
- Calithera Biosciences, Inc. A Phase 1b/2, Open Label, Dose Escalation and Expansion Study of the Glutaminase Inhibitor Telaglenastat (CB-839) in Combination with CDK4/6 Inhibitor Palbociclib in Patients with Advanced or Metastatic Solid Tumors [Internet]. Report No: nCT03965845. clinicaltrials.gov; 2022 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT03965845
- Bowles TL, Kim R, Galante J, et al. Pancreatic cancer cell lines deficient in argininosuccinate synthetase are sensitive to arginine deprivation by arginine deiminase. Int J Cancer. 2008;123(8):1950–1955. doi: 10.1002/ijc.23723
- Deiana C, Fabbri F, Tavolari S, et al. Improvements in systemic therapies for advanced malignant mesothelioma. Int J Mol Sci. 2023;24(13):10415. doi: 10.3390/ijms241310415
- Lowery MA, Yu KH, Kelsen DP, et al. A phase 1/1B trial of ADI-PEG 20 plus nab-paclitaxel and gemcitabine in patients with advanced pancreatic adenocarcinoma. Cancer. 2017;123(23):4556–4565. doi: 10.1002/cncr.30897
- Singh PK, Deorukhkar AA, Venkatesulu BP, et al. Exploiting arginine auxotrophy with pegylated arginine deiminase (ADI-PEG20) to sensitize pancreatic cancer to radiotherapy via metabolic dysregulation. Mol Cancer Ther. 2019;18(12):2381–2393. doi: 10.1158/1535-7163.MCT-18-0708
- Holbert CE, Foley JR, Murray Stewart T, et al. Expanded potential of the polyamine analogue SBP-101 (Diethyl Dihydroxyhomospermine) as a modulator of polyamine metabolism and cancer therapeutic. Int J Mol Sci. 2022;23(12):6798. doi: 10.3390/ijms23126798
- Singhal N, Sigal D, Tebbutt NC, et al. SBP-101, a polyamine metabolic inhibitor, administered in combination with gemcitabine and nab-paclitaxel, shows signals of efficacy as first-line treatment for subjects with metastatic pancreatic ductal adenocarcinoma. J Clin Oncol. 2021;39(15_suppl):4127–4127. doi: 10.1200/JCO.2021.39.15_suppl.4127
- Panbela Therapeutics, Inc. A Randomized, Double-Blind, Placebo-Controlled Study of Nab-Paclitaxel and Gemcitabine with or without SBP-101 in Subjects Previously Untreated for Metastatic Pancreatic Ductal Adenocarcinoma [Internet]. Report No: nCT05254171. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT05254171
- Kawakubo K, Castillo CF, Liss AS. Epigenetic regulation of pancreatic adenocarcinoma in the era of cancer immunotherapy. J Gastroenterol. 2022;57(11):819. doi: 10.1007/s00535-022-01915-2
- Li A, Omura N, Hong S-M, et al. Pancreatic cancer DNMT1 expression and sensitivity to DNMT1 inhibitors. Cancer Biol Ther. 2010;9(4):321–329. doi: 10.4161/cbt.9.4.10750
- Sohal D, Krishnamurthi S, Tohme R, et al. A pilot clinical trial of the cytidine deaminase inhibitor tetrahydrouridine combined with decitabine to target DNMT1 in advanced, chemorefractory pancreatic cancer. Am J Cancer Res. 2020;10:3047–3060.
- Cardone L. A Proof-of-concept, Biomarker-driven, Phase-II Clinical Trial to Explore the Activity of Decitabine Repurposing Against Advanced, Refractory, KRAS-dependent Pancreatic Ductal Adenocarcinoma (Pdac): the ORIENTATE Trial [Internet]. Report No: nCT05360264. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT05360264
- Von Hoff DD, Rasco DW, Heath EI, et al. Phase I study of CC-486 alone and in combination with Carboplatin or nab-paclitaxel in patients with relapsed or refractory solid tumors. Clin Cancer Res. 2018;24(17):4072–4080. doi: 10.1158/1078-0432.CCR-17-3716
- The First Hospital of Jilin University. Clinical Study of Low Dose of Decitabine Combined with Gemcitabine in First-line Treatment of Locally Advanced, Unresectable or Metastatic Pancreatic Cancer [Internet]. Report No: nCT02685228. clinicaltrials.gov; 2016 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT02685228
- Ebelt ND, Zuniga E, Johnson BL, et al. 5-Azacytidine Potentiates Anti-tumor Immunity in a Model of Pancreatic Ductal Adenocarcinoma. Front Immunol. 2020;11:538. doi: 10.3389/fimmu.2020.00538
- Safyan RA, Manji GA, Lee SM, et al. Phase 2 study of azacitidine (AZA) plus pembrolizumab (pembro) as second-line treatment in patients with advanced pancreatic ductal adenocarcinoma. J Clin Oncol. 2022;40(16_suppl):4158–4158. doi: 10.1200/JCO.2022.40.16_suppl.4158
- Algaze S, Hanna DL, Azad NS, et al. A phase Ib study of guadecitabine and durvalumab in patients with advanced hepatocellular carcinoma, pancreatic adenocarcinoma, and biliary cancers. J Clin Oncol. 2022;40(4_suppl):574–574. doi: 10.1200/JCO.2022.40.4_suppl.574
- GWT-TUD GmbH. A Multicenter, Phase I/II Study of Sequential Epigenetic and Immune Targeting in Combination with Nab-Paclitaxel/Gemcitabine in Patients with Advanced Pancreatic Ductal Adenocarcinoma. [Internet]. Report No: nCT04257448. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT04257448
- Siveke JT, Sinn M, Boeck S, et al. Abstract CT195: Interim safety results from SEPION/AIO-PAK-0118: A multicenter, Phase I/II study of sequential epigenetic and immune targeting in combination with nab-paclitaxel/gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Cancer Res. 2023;83(8_Supplement):CT195–CT195. CT195. doi: 10.1158/1538-7445.AM2023-CT195
- Klieser E, Swierczynski S, Mayr C, et al. Role of histone deacetylases in pancreas: implications for pathogenesis and therapy. World J Gastrointest Oncol. 2015;7(12):473–483. doi: 10.4251/wjgo.v7.i12.473
- Jo JH, Jung DE, Lee HS, et al. A phase I/II study of ivaltinostat combined with gemcitabine and erlotinib in patients with untreated locally advanced or metastatic pancreatic adenocarcinoma. Int J Cancer. 2022;151(9):1565–1577. doi: 10.1002/ijc.34144
- CG Pharmaceuticals, Inc. A Phase 1b/2, Dose-escalation, Randomized, Multicenter Study of Maintenance Ivaltinostat Plus Capecitabine or Capecitabine in Patients with Metastatic Pancreatic Adenocarcinoma Whose Disease Has Not Progressed on FOLFIRINOX [Internet]. Report No: nCT05249101. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT05249101
- M.D. Anderson Cancer Center. Phase I/II Trial of Vorinostat and Radiation Therapy in Patients with Locally Advanced Pancreatic Cancer [Internet]. Report No: nCT00831493. clinicaltrials.gov; 2012 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT00831493
- Chan E, Arlinghaus LR, Cardin DB, et al. Phase I trial of chemoradiation with capecitabine and vorinostat in pancreatic cancer. J Clin Oncol. 2013;31(4_suppl):225–225. doi: 10.1200/jco.2013.31.4_suppl.225
- Chan E. Phase I Trial of Chemoradiation with Capecitabine and Vorinostat in Pancreatic Cancer. [Internet]. Report No: nCT00983268. clinicaltrials.gov; 2015 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT00983268
- Poklepovic AS, Fields EC, Bandyopadhyay D, et al. A phase 1 study of neoadjuvant chemotherapy followed by concurrent chemoradiation with gemcitabine, sorafenib, and vorinostat in pancreatic cancer. J Clin Oncol. 2021;39(15_suppl):e16268–e16268. doi: 10.1200/JCO.2021.39.15_suppl.e16268
- Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins. A Phase 2 Clinical Trial of Entinostat in Combination with Nivolumab for Patients with Previously Treated Unresectable or Metastatic Cholangiocarcinoma and Pancreatic Adenocarcinoma [Internet]. Report No: nCT03250273. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT03250273
- National Cancer Institute (NCI). Phase Ib/II Study of ZEN003694 and Entinostat in Advanced and Refractory Solid Tumors and Lymphomas [Internet]. Report No.: nCT05053971. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT05053971
- Leal AS, Liu P, Krieger-Burke T, et al. The Bromodomain Inhibitor, INCB057643, Targets Both Cancer Cells and the Tumor Microenvironment in Two Preclinical Models of Pancreatic Cancer. Cancers (Basel). 2020;13(1):96. doi: 10.3390/cancers13010096
- Han T, Jiao F, Hu H, et al. EZH2 promotes cell migration and invasion but not alters cell proliferation by suppressing E-cadherin, partly through association with MALAT-1 in pancreatic cancer. Oncotarget. 2016;7(10):11194–11207. doi: 10.18632/oncotarget.7156
- Avan A, Crea F, Paolicchi E, et al. Molecular mechanisms involved in the synergistic interaction of the EZH2 inhibitor 3-deazaneplanocin a with gemcitabine in pancreatic cancer cells. Mol Cancer Ther. 2012;11(8):1735–1746. doi: 10.1158/1535-7163.MCT-12-0037
- Institut Bergonié. Combining Epigenetic and Immune Therapy to Beat Cancer. CAIRE Study [Internet]. Report No: nCT04705818. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT04705818
- Constellation Pharmaceuticals. A Phase 1 Study of CPI-1205 with Ipilimumab in Patients with Advanced Solid Tumors Followed by a Phase 2 Basket Study of CPI-1205 with Ipilimumab in Selected Tumor Types Previously Treated with PD-1 or PD-L1 Inhibitors [Internet]. Report No: nCT03525795. clinicaltrials.gov; 2022 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT03525795
- Wang C, Wu N, Pei B, et al. Claudin and pancreatic cancer. Front Oncol. 2023;13:1136227. doi: 10.3389/fonc.2023.1136227
- Wang X, Zhang C-S, Dong X-Y, et al. Claudin 18.2 is a potential therapeutic target for zolbetuximab in pancreatic ductal adenocarcinoma. World J Gastrointest Oncol. 2022;14(7):1252–1264. doi: 10.4251/wjgo.v14.i7.1252
- Park S, Shin K, Kim I-H, et al. Clinicopathological features and prognosis of resected pancreatic ductal adenocarcinoma patients with claudin-18 overexpression. J Clin Med. 2023;12(16):5394. doi: 10.3390/jcm12165394
- Sahin U, Türeci Ö, Manikhas G, et al. FAST: a randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann Oncol Off J Eur Soc Med Oncol. 2021;32(5):609–619. doi: 10.1016/j.annonc.2021.02.005
- Shitara K, Lordick F, Bang Y-J, et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): a multicentre, randomised, double-blind, phase 3 trial. Lancet Lond Engl. 2023;401:1655–1668. doi: 10.1016/S0140-6736(23)00620-7
- Astellas Pharma Global Development, Inc. A Phase 2, Open-Label, Randomized Study to Assess the Efficacy and Safety of Zolbetuximab (IMAB362) in Combination with Nab-Paclitaxel and Gemcitabine (Nab-P + GEM) as First Line Treatment in Subjects with Claudin 18.2 (CLDN18.2) Positive, Metastatic Pancreatic Adenocarcinoma [Internet]. Report No.: nCT03816163. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT03816163
- Suzhou Transcenta Therapeutics Co. Ltd. A Phase I/IIa Clinical Trial to Evaluate the Safety, Tolerability and Pharmacokinetics of TST001 Administered as Monotherapy or in Combination with Nivolumab or Standard of Care in Patients with Locally Advanced or Metastatic Solid Tumors [Internet]. Report No: nCT04396821. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT04396821
- Phanes Therapeutics. A Phase I, First-in-Human, Open-Label, Dose Escalation and Expansion Study of PT886 in Adult Patients with Advanced Gastric, Gastroesophageal Junction and Pancreatic Adenocarcinomas [Internet]. Report No: nCT05482893. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT05482893
- AstraZeneca. A Phase I/II Open-label Dose Escalation and Dose Expansion Study to Evaluate the Safety, Pharmacokinetics, Pharmacodynamics, and Efficacy of AZD5863, a T Cell-engaging Bispecific Antibody That Targets Claudin 18.2 (CLDN18.2) and CD3 in Adult Participants with Advanced or Metastatic Solid Tumors [Internet]. Report No: nCT06005493. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT06005493
- Spisek R. 2P SOT102, a novel CLDN18.2-targeting antibody-drug conjugate for gastric and pancreatic cancer with a wide range of the tumor target expression. ESMO Open. 2023 [cited 2023 Dec 14];8(1):101196. Available from: https://www.esmoopen.com/article/S2059-7029(23)00418-0/fulltext
- Sotio Biotech Inc. A Multicentric Phase 1/2 Trial to Evaluate the Safety and Efficacy of SOT102 as Monotherapy and in Combination with Standard of Care Treatment in Patients with Gastric and Pancreatic Adenocarcinoma [Internet]. Report No: nCT05525286. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT05525286
- Elevation Oncology. A Phase 1 Dose Escalation and Expansion Study of EO-3021, an Anti-claudin 18.2 (CLDN18.2) Antibody Drug Conjugate, in Patients with Solid Tumors Likely to Express CLDN18.2 [Internet]. Report No: nCT05980416. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT05980416
- Papadopoulos KP, Tehfe M, Abdul Razak AR, et al. A phase I/II dose escalation and expansion trial to evaluate safety and preliminary efficacy of BNT141 in patients with claudin-18.2-positive solid tumors. J Clin Oncol. 2023;41(16_suppl):TPS2670–TPS2670. doi: 10.1200/JCO.2023.41.16_suppl.TPS2670
- Voutsadakis IA. Mutations of p53 associated with pancreatic cancer and therapeutic implications. Ann Hepatobiliary Pancreat Surg. 2021;25(3):315–327. doi: 10.14701/ahbps.2021.25.3.315
- SynerGene Therapeutics, Inc. Phase II Study of Combined Targeted p53 Gene Therapy (SGT-53) Plus Gemcitabine/Nab-Paclitaxel for Treatment of Metastatic Pancreatic Cancer [Internet]. Report No: nCT02340117. clinicaltrials.gov; 2022 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT02340117
- Leung CP, Barve MA, Wu M-S, et al. A phase II trial combining tumor-targeting TP53 gene therapy with gemcitabine/nab-paclitaxel as a second-line treatment for metastatic pancreatic cancer. J Clin Oncol. 2021;39(15_suppl):4139–4139. doi: 10.1200/JCO.2021.39.15_suppl.4139
- Buscail L, Bournet B, Vernejoul F, et al. First-in-man phase 1 clinical trial of gene therapy for advanced pancreatic cancer: safety, biodistribution, and preliminary clinical findings. Mol Ther. 2015;23(4):779–789. doi: 10.1038/mt.2015.1
- University Hospital, Toulouse. Phase 2 Gene Therapy Trial of Locally Advanced Pancreatic Adenocarcinoma Using Intratumoral Injection of CYL-02 in Combination with Gemcitabine [Internet]. Report No: nCT02806687. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT02806687
- Perez SM, Brinton LT, Kelly KA. Plectin in Cancer: From Biomarker to Therapeutic Target. Cells. 2021;10(9):2246. doi: 10.3390/cells10092246
- Bausch D, Mino-Kenudson M, Fernández-Del Castillo C, et al. Plectin-1 is a biomarker of malignant pancreatic intraductal papillary mucinous neoplasms. J Gastrointest Surg Off J Soc Surg Aliment Tract. 2009;13(11):1948–1954; discussion 1954. doi: 10.1007/s11605-009-1001-9
- ZielBio, Inc. A Phase 1/2, First-in-Human, Open Label, Dose Escalation Study of a Cancer-Specific Plectin (CSP)-Targeting Functional Antibody in Solid Tumors That Are Likely to Express CSP. Report No: nCT05074472. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT05074472
- Sommerhalder D, Piha-Paul SA, Pelster M, et al. A phase 1/2, first-in-human trial of ZB131, a novel antibody targeting cancer-specific plectin (CSP) in advanced solid tumors. J Clin Oncol. 2023;41(16_suppl):3083–3083. doi: 10.1200/JCO.2023.41.16_suppl.3083
- Bardia A, Hurvitz SA, Tolaney SM, et al. Sacituzumab govitecan in Metastatic Triple-Negative breast cancer. N Engl J Med. 2021;384(16):1529–1541. doi: 10.1056/NEJMoa2028485
- Bardia A, Messersmith WA, Kio EA, et al. Sacituzumab govitecan, a trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann Oncol Off J Eur Soc Med Oncol. 2021;32(6):746–756. doi: 10.1016/j.annonc.2021.03.005
- Diab M. Sacituzumab Govitecan in Combination with Capecitabine for Advanced Gastrointestinal Cancers After Progression on Standard Therapy [Internet]. Report No: nCT06065371. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT06065371
- Azmi AS, Khan HY, Aboukameel A, et al. Preclinical assessment with clinical validation of Selinexor with gemcitabine and nab-paclitaxel for the treatment of pancreatic ductal adenocarcinoma. Clin Cancer Res Off J Am Assoc Cancer Res. 2020;26(6):1338–1348. doi: 10.1158/1078-0432.CCR-19-1728
- National University Hospital. Singapore. Phase I Dose Finding Study of Selinexor and Talazoparib in Patients with Advanced Refractory Solid Tumors, Followed by Phase II Expansion Cohort Study in Patients with Advanced/Metastatic Triple Negative Breast Cancers. (START) [Internet]. Report No: nCT05035745. clinicaltrials.gov; 2023 [cited 2024 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT05035745
- National University Hospital. Singapore. A Phase I Dose Escalation Study of Selinexor Plus Nivolumab and Ipilimumab in Advanced/Metastatic Solid Malignancies [Internet]. Report No: nCT04850755. clinicaltrials.gov; 2021 [cited 2024 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT04850755
- Wang J-Z, Barve MA, Chiorean EG, et al. Interim results from a phase 1 trial of SL-801, a novel XPO-1 inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2018;36(15_suppl):2560–2560. doi: 10.1200/JCO.2018.36.15_suppl.2560
- Mahdi H, Hafez N, Doroshow D, et al. Ceralasertib-Mediated ATR Inhibition Combined with Olaparib in Advanced Cancers Harboring DNA Damage Response and Repair Alterations (Olaparib Combinations). JCO Precis Oncol. 2021;(5):1432–1442. doi: 10.1200/PO.20.00439
- Prevo R, Fokas E, Reaper PM, et al. The novel ATR inhibitor VE-821 increases sensitivity of pancreatic cancer cells to radiation and chemotherapy. Cancer Biol Ther. 2012;13(11):1072–1081. doi: 10.4161/cbt.21093
- Aggarwal R. Phase II Trial of Ceralasertib (AZD6738) Alone and in Combination with Olaparib or Durvalumab in Patients with Selected Solid Tumor Malignancies [Internet]. Report No: nCT03682289. clinicaltrials.gov; 2023 [cited 2024 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT03682289
- IDEAYA Biosciences. A Study of PARG Inhibitor IDE161 in Participants with Advanced Solid Tumors [Internet]. Report No: nCT05787587. clinicaltrials.gov; 2024 [cited 2024 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT05787587
- Senhwa Biosciences, Inc. Phase Ib Expansion Study of CX-5461 in Patients with Solid Tumours and BRCA2 and/or PALB2 Mutation [Internet]. Report No: nCT04890613. clinicaltrials.gov; 2024 [cited 2024 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT04890613
- Hingorani SR, Zheng L, Bullock AJ, et al. HALO 202: randomized phase II study of PEGPH20 plus nab-Paclitaxel/Gemcitabine versus nab-Paclitaxel/Gemcitabine in patients with untreated, metastatic pancreatic ductal adenocarcinoma. J Clin Oncol. 2018;36(4):359–366. doi: 10.1200/JCO.2017.74.9564
- De Jesus-Acosta A, Sugar EA, O’Dwyer PJ, et al. Phase 2 study of vismodegib, a hedgehog inhibitor, combined with gemcitabine and nab-paclitaxel in patients with untreated metastatic pancreatic adenocarcinoma. Br J Cancer. 2020;122:498–505. doi: 10.1038/s41416-019-0683-3
- Hong D, Rasco D, Veeder M, et al. A phase 1b/2 study of the Bruton Tyrosine Kinase Inhibitor Ibrutinib and the PD-L1 Inhibitor durvalumab in patients with pretreated solid tumors. Oncology. 2019;97(2):102–111. doi: 10.1159/000500571
- Dean A, Gill S, McGregor M, et al. Dual αV-integrin and neuropilin-1 targeting peptide CEND-1 plus nab-paclitaxel and gemcitabine for the treatment of metastatic pancreatic ductal adenocarcinoma: a first-in-human, open-label, multicentre, phase 1 study. Lancet Gastroenterol Hepatol. 2022;7(10):943–951. doi: 10.1016/S2468-1253(22)00167-4
- Kocher HM, Basu B, Froeling FEM, et al. Phase I clinical trial repurposing all-trans retinoic acid as a stromal targeting agent for pancreatic cancer. Nat Commun. 2020;11(1):4841. doi: 10.1038/s41467-020-18636-w
- Hu ZI, Bendell JC, Bullock A, et al. A randomized phase II trial of nab-paclitaxel and gemcitabine with tarextumab or placebo in patients with untreated metastatic pancreatic cancer. Cancer Med. 2019;8(11):5148–5157. doi: 10.1002/cam4.2425
- Pedersen KS, Kim GP, Foster NR, et al. Phase II trial of gemcitabine and tanespimycin (17AAG) in metastatic pancreatic cancer: a mayo clinic phase II consortium study. Invest New Drugs. 2015;33(4):963–968. doi: 10.1007/s10637-015-0246-2
- Laquente B, Lopez-Martin J, Richards D, et al. A phase II study to evaluate LY2603618 in combination with gemcitabine in pancreatic cancer patients. BMC Cancer. 2017;17(1):137. doi: 10.1186/s12885-017-3131-x
- Heinemann V, Ebert MP, Laubender RP, et al. Phase II randomised proof-of-concept study of the urokinase inhibitor upamostat (WX-671) in combination with gemcitabine compared with gemcitabine alone in patients with non-resectable, locally advanced pancreatic cancer. Br J Cancer. 2013;108(4):766–770. doi: 10.1038/bjc.2013.62
- Chee CE, Krishnamurthi S, Nock CJ, et al. Phase II study of dasatinib (BMS-354825) in patients with metastatic adenocarcinoma of the pancreas. Oncology. 2013;18(10):1091–1092. doi: 10.1634/theoncologist.2013-0255
- Chou A, Waddell N, Cowley MJ, et al. Clinical and molecular characterization of HER2 amplified-pancreatic cancer. Genome Med. 2013;5(8):78. doi: 10.1186/gm482
- Akhave NS, Biter AB, Hong DS. Mechanisms of resistance to KRASG12C-targeted therapy. Cancer Discov. 2021;11(6):1345–1352. doi: 10.1158/2159-8290.CD-20-1616
- Desai J, Alonso G, Kim SH, et al. Divarasib plus cetuximab in KRAS G12C-positive colorectal cancer: a phase 1b trial. Nat Med. 2024;30(1):271–278. doi: 10.1038/s41591-023-02696-8
- Lu C, Talukder A, Savage NM, et al. JAK-STAT-mediated chronic inflammation impairs cytotoxic T lymphocyte activation to decrease anti-PD-1 immunotherapy efficacy in pancreatic cancer. Oncoimmunology. 2017;6(3):e1291106. doi: 10.1080/2162402X.2017.1291106
- Davis L. Serial Measurements of Molecular and Architectural Responses to Therapy (SMMART) Trial: Adaptive Clinical Treatment (ACT) [Internet]. Report No: nCT05238831. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT05238831
- Lin S. A Real-world Study to Explore and Evaluate Individualized Targeted Agents for Patients of Digestive Cancers Based on Molecular Characteristics After Standard Therapy Failure in China [Internet]. Report No.: nCT04584008. clinicaltrials.gov; 2020 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT04584008
- National Cancer Institute (NCI). Molecular Analysis for Therapy Choice (MATCH) [Internet]. Report No: nCT02465060. clinicaltrials.gov; 2023 [cited 2023 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT02465060
- Zeng S, Pöttler M, Lan B, et al. Chemoresistance in Pancreatic Cancer. Int J Mol Sci. 2019;20(18):4504. doi: 10.3390/ijms20184504
- Brown TJ, Reiss KA, O’Hara MH. Advancements in Systemic Therapy for Pancreatic Cancer. Am Soc Clin Oncol Educ Book. 2023;(43):e397082. doi: 10.1200/EDBK_397082
- Philip PA, Azar I, Xiu J, et al. Molecular characterization of KRAS wild-type tumors in patients with pancreatic adenocarcinoma. Clin Cancer Res Off J Am Assoc Cancer Res. 2022;28(12):2704–2714. doi: 10.1158/1078-0432.CCR-21-3581
- Singh H, Keller RB, Kapner KS, et al. Oncogenic drivers and therapeutic vulnerabilities in KRAS wild-type pancreatic cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2023;29(22):4627–4643. doi: 10.1158/1078-0432.CCR-22-3930
- Ben-Ammar I, Rousseau A, Nicolle R, et al. Precision medicine for KRAS Wild-Type pancreatic adenocarcinomas. Eur J Cancer. 2023;197:113497. doi: 10.1016/j.ejca.2023.113497
- Espiau-Romera P, Courtois S, Parejo-Alonso B, et al. Molecular and metabolic subtypes correspondence for pancreatic ductal adenocarcinoma classification. J Clin Med. 2020;9(12):4128. doi: 10.3390/jcm9124128
- O’Kane GM, Grünwald BT, Jang G-H, et al. GATA6 Expression Distinguishes Classical and Basal-like Subtypes in Advanced pancreatic cancer. Clin Cancer Res. 2020;26(18):4901–4910. doi: 10.1158/1078-0432.CCR-19-3724
- Zhou X, Hu K, Bailey P, et al. Clinical Impact of Molecular Subtyping of Pancreatic Cancer. Front Cell Dev Biol. 2021 [cited 2024 Apr 3];9. doi: 10.3389/fcell.2021.743908
- Collisson EA, Sadanandam A, Olson P, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med. 2011;17(4):500–503. doi: 10.1038/nm.2344
- Vallés-Martí A, Mantini G, Manoukian P, et al. Phosphoproteomics guides effective low-dose drug combinations against pancreatic ductal adenocarcinoma. Cell Rep. 2023 Dec 19;42(6):112581. doi: 10.1016/j.celrep.2023.112581
- Sivapalan L, Kocher HM, Ross-Adams H, et al. Molecular profiling of ctDNA in pancreatic cancer: Opportunities and challenges for clinical application. Pancreatol Off J Int Assoc Pancreatol IAP Al. 2021;21(2):363–378. doi: 10.1016/j.pan.2020.12.017