ABSTRACT
Introduction
Combinations of immune checkpoint inhibitors (ICIs) and tyrosine kinase inhibitors (TKIs) can be responsible for major adverse cardiovascular events (MACEs). We performed a meta-analysis to assess the relative risk (RR) of MACEs and hypertension in cancer patients treated with ICI+TKI combinations.
Research design and methods
We selected prospective trials through MEDLINE/PubMed, Cochrane Library, and ASCOMeeting abstracts. We calculated combined ORs, RRs, and 95% CIs using RevMansoftware for meta-analysis (v.5.2.3).
Results
Seven studies were selected for the analysis of MACEs (3849 patients). The incidence MACEs were 0.8% with ICI+TKI combinations, compared to 0.2% in the control arms for both any- and high-grade. ICI+TKI combinations significantly increased the risk of any- (OR = 3.21; p = 0.01) and high-grade MACEs (OR = 2.72; p = 0.05). Ten studies were selected for the analysis of hypertension (5744 patients). The incidence of treatment-related hypertension of any-grade and high-grade was41.3% (vs. 20.8%) and 26.1% (vs. 12.3%) with ICI+TKI combinations, respectively. ICI+TKI combinations significantly increased the risk of treatment-related hypertension of any-grade (RR = 2.10; p = 0.001), but not of high-grade (p = 0.11).
Conclusions
ICI+TKI combinations increase the risk of MACEs compared to controls, although the absolute incidence is eventually low. Routine cardiovascular monitoring in asymptomatic patients is therefore not recommended.
1. Introduction
Combinations of immune checkpoint inhibitors (ICIs) targeting PD-1 or PD-L1 with tyrosine kinase inhibitors (TKIs) have a synergistic anti-cancer activity, leading to a paradigmatic change in the treatment of several cancer types [Citation1–3]. Actually, TKIs targeted therapy that recognizes the receptor of the vascular endothelial growth factor (VEGFR) as the major target for inhibition is the cornerstone of treatment of solid tumors particularly dependent on angiogenesis (i.e. metastatic renal cell carcinoma, mRCC). In the last years, it has become evident that combining an ICI plus a VEGFR-TKI significantly improves outcomes of mRCC patients, given the ability of these combinations to circumvent immune-resistance and trigger vascular normalization [Citation4]. This therapeutic strategy is under development for other solid tumors, with the use of TKIs targeting the main protein kinase involved in the carcinogenesis of a particular cancer type (i.e. MEK for melanoma).
Certainly, ICI+TKI combinations have an arguably manageable safety profile, yet overlapping adverse events (AEs) and immune-related AEs can make treatment less tolerable [Citation5]. It is well established that TKIs, mainly those directed against the VEGFR, can be responsible for cardiovascular effects, ranging from asymptomatic subclinical abnormalities up to severe left ventricular dysfunction and congestive heart failure [Citation6–9]. To further complicate this scenario, immunotherapy comes with side effects too, being responsible for potential immune-mediated cardiovascular events, which can be potentially life threatening [Citation10]. Most cardiotoxic events appear to be inflammatory in nature (i.e. myocarditis, pericarditis, and vasculitis) [Citation11]. It is likely that the spectrum of cardiovascular adverse events observed with ICI+TKI combinations is not only the mere sum of cardiovascular damage caused by the single drugs but rather the result of an enhanced toxicity mediated by different mechanisms of action that are potentiated. The risk of developing cardiovascular toxicity and hypertension related to ICI+TKI combinations and the proper management of these side effects have yet to be definitely stated. Therefore, we performed a systematic review and meta-analyzed phase II and III randomized studies to comprehensively evaluate the incidence and relative risk (RR) of developing cardiovascular AEs (Major Adverse Cardiovascular Events, MACEs) and hypertension after combining ICIs with TKIs, with a particular focus on those combinations including a VEGFR-TKI.
2. Patients and methods
2.1. Definition of outcomes
The objective of this analysis was to assess the incidence and RR of MACEs and hypertension in patients with solid tumors treated with the combinations of an ICI targeting PD-1/PD-L1 or CTLA4 and a TKI. MACEs considered included acute myocardial infarction, cardiac arrest, and myocarditis. We considered together these cardiovascular events, given their rarity and the lack of cumulative data reported in the studies. For each trial, ICI+TKI combination therapy was considered as the experimental arm and the comparator as the control. The severity of AEs was graduated according to Common Terminology Criteria for Adverse Events (CTCAE) v4.0 ( Suppl). Both all-grades (grades 1–4) and high-grade (grades 3–5) events were considered as the main outcomes; the analysis was conducted in order to identify a significant difference between the two treatment arms. The secondary endpoint was to assess the RR of MACEs and hypertension in the subgroup of patients treated with an ICI plus a TKI specifically directed against the VEGFR compared to monotherapy with VEGFR-TKIs. Moreover, for hypertension, a sub-group analysis was made to highlight the RR of treatment-related hypertension with both ICI+TKI and ICI+VEGFR-TKI combinations.
2.2. Selection of studies
We reviewed MEDLINE/PubMed, the Cochrane Library, and ASCO University Meeting abstracts for citations up to 31 March 2022. The search criteria were limited to articles published in the English language and phase III or phase II RCTs in patients with solid tumors treated with a combination of ICI+TKI in the experimental arm and with available data about treatment-related cardiovascular events. The MeSH terms used for the search of PubMed and the Cochrane Library were ‘immune checkpoint inhibitor,’ ‘anti-PD-1,’ ‘anti-PD-L1,’ ‘anti-CTLA4,’ ‘tyrosine kinase inhibitors,’ ‘VEGFR-TKI,’ or the name of the drugs (i.e. atezolizumab, axitinib, nivolumab, pembrolizumab, etc.). For the search in the ASCO University abstracts, we used the name of the drugs and the terms ‘phase II’ or ‘phase III.’ The summaries for the product characteristics were searched for at http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm. If more than one publication was found for the same trial, the most recent, complete, and updated version was included in the final analysis.
The quality of the selected studies was assessed using the Jadad 5-itemscale, taking into account randomization, double blinding, and withdrawals. The final score ranged from 0 to 5 [Citation12].
2.3. Data extraction
Data extraction was performed by three authors (CC, RI, and AS) independently, according to the Preferred Reporting Items for Systematic review and Meta-Analysis (PRISMA) statement ( Suppl.). Any kind of discrepancies were resolved by consensus among the three authors [Citation13]. The data extracted for each study were: first author’s name, year of publication, phase of the study, number of enrolled patients, type of therapy in each study arm, type of primary solid tumor, and number of MACEs and hypertension events of both all-grades and high grades in the experimental and control arms.
2.4. Statistical methodology
The incidence of cardiovascular adverse events was calculated from the data available in each study. The proportion of patients with MACEs and hypertension events and the derived 95% confidence intervals (CIs) were calculated for each study. The risk ratio (RR) and CIs of these events in the experimental arm (ICI+TKI combination therapies) compared to the controls were performed in the same trial. The variance of a log-transformed study-specific RR for calculating the 95% CIs was obtained using the delta method [Citation14]. Notably, considering in each study the paucity of the different types of MACE events when considered individually, we considered them together merged into the definition of MACEs as aggregate data, and we used the Peto method for calculating the odds ratio (OR). The Mantel–Haenszel method was used for assessing the RR of hypertension. Cochrane’s Q statistic was used to evaluate statistical heterogeneity between the studies included in the meta-analysis; inconsistency was quantified with an I2 statistic (100% x [Q-df)/Q]) [Citation15]. The assumption of homogeneity was considered to be invalid for p values less than 0.1. Random- or fixed-effects models were used to calculate summary incidence and RRs, depending on the heterogeneity of the included trials. In case of no substantial heterogeneity, the pooled estimate – calculated with the fixed-effects model – was reported using the inverse variance method. In case of substantial heterogeneity, the pooled estimate – assessed with the random-effects model – was stated using the DerSimonian et al. method [Citation16], which takes into account both within- and between-study variations [Citation15]. We used the chi-square test for analyzing indirect comparisons between the groups. A two-tailed p-value of less than 0.05 was considered statistically significant. All the data was collated using Microsoft Office Excel 2007. We used RevMan software for meta-analysis (v.5.2.3) for performing statistical analyses [Citation17].
3. Results
3.1. Search results
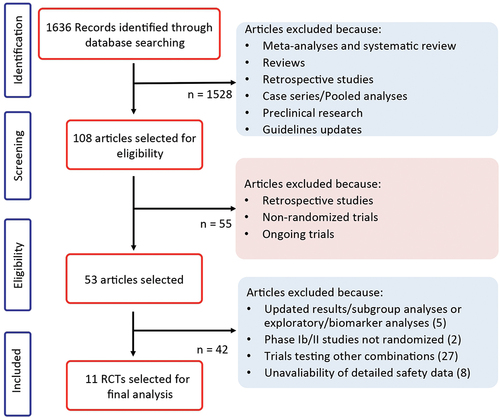
The electronic search revealed 1635 citations; 52 full-text articles were reviewed for further assessment and 42 citations were excluded because they did not meet the inclusion criteria. This reviewed process () led to the selection of 10 articles considered for the final analysis of hypertension and 7 articles were considered for MACEs based on their adequate quality and relevance for inclusion in this meta-analysis [Citation18–27].
Nine out of ten were randomized phase III trials; one was a randomized phase II trial; and one was a randomized, open-label, phase 2/3 study.
The experimental arms included combinations of an ICI targeting PD-1 or PD-L1 (i.e. atezolizumab, avelumab, nivolumab, or pembrolizumab) plus a TKI. In particular, the TKIs were specific inhibitors of the VEGF receptor (VEGFR) in 7 out of the 10 selected studies; in the remaining 3 trials the TKI was cobimetinib (a MEK inhibitor). The comparator was cytotoxic chemotherapy in one study, VEGFR-TKIs in six trials, and an ICI directed against PD-1/PD-L1 in the remaining three studies. As concerning the tumor type, four studies enrolled patients affected by RCC, the remaining trials included patients affected by colorectal cancer (1 study), urothelial carcinoma (1 study), melanoma (1 study), endometrial carcinoma (1 study), hepatocellular carcinoma (1 study), and biliary tract tumors (1 study). A total of 5744 patients were available for meta-analysis: 3059 had RCC, and the remaining 2685 patients had the other cancer types. The characteristics of each trial included in this study are shown in .
Table 1. Main characteristic of the included studies.
3.2. Incidence and relative risk of MACEs
For incidence of MACEs of any-grade, seven studies were selected for the analysis, with a total of 3849 patients. Among them, 1937 were treated in the experimental arms with a combination of ICI+TKI, while 1912 received standard therapy in the control arms. As reported above, we considered together the diverse cardiovascular events (acute myocardial infarction, cardiac arrest, and myocarditis) given their very low incidence rate. In the overall cohort, MACEs of any-grade were reported in 15 of 1937 patients treated with ICI+TKI combinations, corresponding to an incidence of 0.8%, compared to 0.2% in the control arms (4 events among 1912 patients) (). Treatment with ICI+TKI combinations significantly increased the risk of MACEs of any-grade compared to controls (Peto, fixed, Peto OR = 3.21, 95% CI, 1.30–7.95; p = 0.01). No significant heterogeneity was observed in this analysis (Chi2 = 2.77, p = 0.84; I2 = 0%) ().
Figure 2. Relative risk for major adverse cardiovascular events (MACEs) of any-grade (a) and high-grade (b) in patients treated with ICI plus TKI combinations for solid tumors.
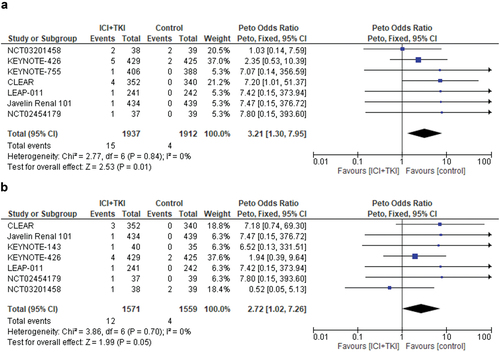
Table 2. Overall incidence of any- and high-grade MACEs and hypertension from all included trials.
As a concern for MACEs of high-grade, data were available from seven studies and 3130 patients. MACEs events of high grade were reported in 12 of 1571 patients treated with ICI+TKI combinations, corresponding to an incidence of 0.8%, compared to 0.2% in the control arms (4 events among 1559 patients). Treatment with ICI+TKI combinations significantly increased the risk of high-grade MACEs compared to controls (Peto fixed, Peto OR = 2.72, 95% CI, 1.02–7.26; p = 0.05). No significant heterogeneity was observed in this analysis (Chi2 = 3.86, p = 0.70; I2 = 0%) ().
3.3. Incidence and relative risk of hypertension
As concern hypertension, ten studies were selected for the analysis, with a total of 5744 patients. Among them, 3048 were treated in the experimental arms with a combination of ICI+TKI, while 2696 received standard therapies in the control arms.
In the overall cohort, hypertension of any-grade was reported in 1209 of 3048 patients treated with ICI+TKI combinations, corresponding to an incidence of 39.7%, compared to 26.5% in the control arms (715 cases of hypertension among 2696 patients).
Treatment with ICI+TKI combinations significantly increased the risk of hypertension of any-grade compared to controls (random-effects, RR = 1.62, 95% CI, 1.08–2.44; p = 0.02). Significant heterogeneity was observed in this analysis (Chi2 = 202, p < 0.00001; I2 = 96%) (Figure S1).
Since the high prevalence of hypertension in the normal population could over-estimate the real incidence of hypertension as a side effect of anti-cancer drugs, we performed a separate analysis taking into account only the treatment-related hypertension events. When the analysis was restricted to treatment-related hypertension of any grade, data from eight studies were available. The incidence of treatment-related hypertension of any-grade was 41.3% with ICI+TKI combinations compared to 26.1% in the control arms (). Treatment with ICI+TKI combinations significantly increased the risk of treatment-related hypertension of any-grade compared to controls (random-effects, RR = 2.10, 95% CI, 1.34–3.29; p = 0.001). Significant heterogeneity was observed in this analysis (Chi2 = 156.02, p < 0.00001; I2 = 96%) ().
Figure 3. Relative risk for treatment-related hypertension of any-grade (a) and high-grade (b) in patients treated with ICI plus TKI combinations for solid tumors.
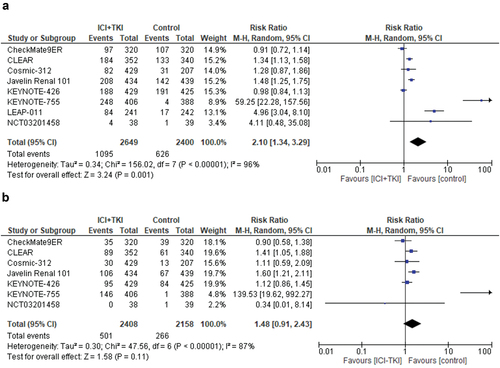
We evaluated the incidence and relative risk of developing hypertension of high-grade; nine studies were selected with a total of 5261 patients. Hypertension of high-grade was reported in 543 of 2807 patients treated with ICI+TKI combinations, corresponding to an incidence of 19.3%, compared to 12.5% in the control arms (306 cases of hypertension among 2454 patients). Treatment with ICI+TKI combinations did not significantly increase the risk of high-grade hypertension compared to controls (random-effects, RR = 1.41, 95% CI, 0.86–2.34; p = 0.18). Significant heterogeneity was seen in this analysis (Chi2 = 81.33, p < 0.00001; I2 = 90%) (Figure S1). When the analysis was restricted to treatment-related hypertension of high-grade, data were reported for seven trials for a total of 4566 patients. The incidence of treatment-related hypertension of high grade was 20.8% with ICI+TKI combinations compared to 12.3% in the control arms. Treatment with ICI+TKI combinations did not significantly increase the risk of treatment-related hypertension of high-grade compared to controls (random-effects, RR = 1.48, 95% CI, 0.91–2.43; p = 0.11). Significant heterogeneity was observed (Chi2 = 47.56, p < 0.00001; I2 = 87%) ().
3.4. Incidence and relative risk of MACEs with ICI+VEGFR-TKI combinations compared to VEGFR-TKIs monotherapy
We analyzed the incidence and relative risk of developing MACEs of any grade with combinations of an ICI plus a TKI that specifically inhibits the VEGF receptor compared to a VEGFR-TKI monotherapy. Three studies were selected with a total of 2419 patients. Cardiovascular events of any-grade were reported in 10 of 1215 patients treated with ICI+VEGFR-TKI combinations, corresponding to an incidence of 0.8%, compared to 0.2% in the control arms (2 cases among 1204 patients). ICI+VEGFR-TKI combinations significantly increase the risk of MACEs of any-grade compared to VEGFR-TKI monotherapy (Peto fixed, Peto OR = 3.76, 95% CI, 1.21–11.70; p = 0.02). No significant heterogeneity was found in this analysis (Chi2 = 0.92, p = 0.63; I2 = 0%) ().
Figure 4. Relative risk for major adverse cardiovascular events (MACEs) of any-grade (a) and high-grade (b) in patients treated with VEGFR-TKI+ICI combinations compared to VEGFR-TKI monotherapy for solid tumors.
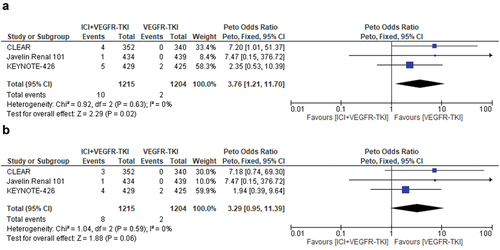
The incidence of MACEs of high-grade with ICI+VEGFR-TKI combinations was 0.6% (corresponding to 8 cases among 1215 patients), compared to 0.2% in the control arms (2 cases of high-grade MACEs among 1204 patients). ICI+VEGFR-TKI combinations were associated with an increased risk of MACEs compared to VEGFR-TKI monotherapy, with the caveat of the small number of events observed (Peto fixed, Peto OR = 3.29, 95% CI, 0.95–11.39; p = 0.06). No significant heterogeneity was observed (Chi2 = 1.04, p = 0.59; I2 = 0%) ().
3.5. Incidence and relative risk of treatment-related hypertension with ICI+VEGFR-TKI combinations compared to VEGFR-TKIs monotherapy
We analyzed the incidence and relative risk of developing treatment-related hypertension of any-grade with combinations of an ICI plus a TKI that specifically inhibits the VEGF receptor compared to a VEGFR-TKI monotherapy.
Five studies were selected with a total of 3695 patients. Treatment-related hypertension of any-grade was reported in 759 of 1964 patients treated with ICI+VEGFR-TKI combinations, corresponding to an incidence of 38.6%, compared to 34.9% in the control arms (604 cases of hypertension among 1731 patients). ICI+VEGFR-TKI combinations did not significantly increase the risk of hypertension of any-grade compared to VEGFR-TKI monotherapy (random-effects, RR = 1.17, 95% CI, 0.96–1.43; p = 0.12). Significant heterogeneity was found in this analysis (Chi2 = 20.83, p < 0.0003; I2 = 81%) ().
Figure 5. Relative risk for hypertension of any-grade (a) and high-grade (b) in patients treated with VEGFR-TKI+ICI combinations compared to VEGFR-TKI monotherapy for solid tumors.
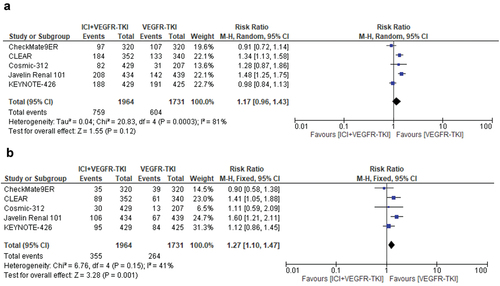
The incidence of treatment-related hypertension of high-grade with ICI+VEGFR-TKI combinations was 18.0% (corresponding to 355 cases among 1964 patients), compared to 15.2% in the control arms (264 cases of high-grade hypertension among 1731 patients). ICI+VEGFR-TKI combinations significantly increased the risk of treatment-related high-grade hypertension compared to VEGFR-TKI monotherapy (fixed-effects, RR = 1.27, 95% CI, 1.10–1.47; p = 0.001). No significant heterogeneity was observed (Chi2 = 6.76, p = 0.15; I2 = 41%) ().
3.6. Quality of the studies
All the studies were randomized clinical trials, and all of them were of good quality according to the Jadad’ scale (scores ≥3) (). Risk of bias in each study is reported in Figure S2.
4. Discussion
Targeted agents and immunotherapy, either alone or in several combined schedules, represent the main breakthrough of anticancer therapy leading to profound improvements in patients’ prognosis and crucial changes in the treatment landscape of several cancer types. Despite the remarkable efficacy of ICI+TKI combinations, concerns have been raised about their safety profile. We have focused our attention on two different types of potential adverse events, hypertension and MACEs, both of which can be related to these combinations even though with a diverse frequency, severity, and consequences for the patients [Citation28–31].
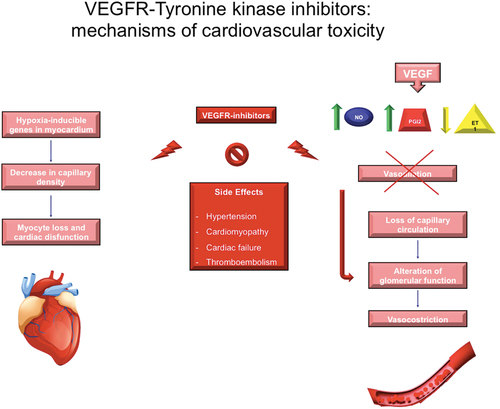
Targeted therapy with TKIs can be responsible for hypertension and cardiotoxicity with different mechanisms, depending on the target of inhibition. Most of the data concern monotherapy with TKIs targeting VEGFR (and conducted mainly in RCC tumors); hypertension, cardiomyopathy, cardiac failure, and thromboembolic events, are a well-characterized adverse event during VEGFR-TKIs [Citation28]. Many mechanisms for the development of hypertension with VEGFR-inhibitors therapy have been hypothesized (). Both functional and anatomic changes in the endothelium appear to promote increased vascular resistance leading to hypertension. In particular, VEGF induces the production of two vasodilators, nitric oxide (NO) and prostacyclin (PGI2) and decreases the production of endothelin-1 (ET1), a potent vasoconstrictor. Furthermore, given the important role of VEGF in mediating endothelial and kidney cells cellular proliferation, VEGFR-inhibitors may lead to an imbalance between vasodilators and vasoconstrictors, loss of capillary circulation, and alteration in glomerular function, all of which contribute to hypertension [Citation29,Citation32–34]. The pathogenic mechanisms responsible for cardiac damage related to VEGFR-TKIs are not yet fully known; multiple cofactors could play a role. The drug-induced inhibition of tyrosine kinases normally expressed in the myocardium and blood vessels might be responsible for this toxicity. In particular, the inhibition of those pathways (VEGFR first of all, then RSK, AMPK, PDGFR, MEK) with a key role in cardiomyocyte survival/apoptosis homeostasis could be at least partially responsible for myocyte loss and cardiac dysfunction [Citation35]. Moreover, the inhibition of VEGFR can lead to decrease in capillary density, induction of hypoxia e hypoxia-inducible genes in the myocardium, ultimately resulting in cardiac dysfunction [Citation32,Citation36]. Furthermore, it is important to notice that other TKIs-related adverse events – including thyroid dysfunctions and hypertension (the latter leading to a reduced vasodilatory nitric oxide production and to capillary density rarefaction) – could indirectly contribute to the damage to myocardial function [Citation37]. Besides VEGFR-TKIs, the other TKIs can cause myocardial damage by targeting different signaling pathways. For example, several studies showed the cardioprotective role of Ras-Raf-MEK-ERK pathway against oxidative stress, myocardial ischemia-perfusion injury, and adaptive hypertrophy [Citation38–41]. Therefore, BRAF/MEK inhibitors – which represent a treatment option for melanoma – can induce heart failure and may increase the susceptibility of cardiomyocytes to ICI-mediated inflammation [Citation42,Citation43].
ICIs can elicit immune-mediated cardiovascular events, involving the cardiovascular system on multiple different levels and being therefore responsible for a wide spectrum of clinical events, including myocarditis, acute coronary syndromes, congestive heart failure, nonmalignant pericardial disorders, dysrhythmias, and cardiac arrest. Immune-mediated MACEs are rare but potentially life-threatening. In the vast majority of cases (about 80%), immune-related MACEs occur within the first six weeks from the start of treatment, underlying the necessity of an early monitoring of patients [Citation10,Citation11,Citation31,Citation44,Citation45]. Preclinical studies supported the crucial role of CTLA-4 and PD-1/PD-L1 pathways in suppressing T cell responses in the heart, thus playing a protective role against myocardial inflammation and myocyte damage in T cell-mediated myocarditis [Citation46–48]. Inhibition of CTLA-4, PD-1, or PD-L1 can therefore result in autoimmune T-cell-mediated cardiovascular events [Citation10,Citation30,Citation49]. In particular, clinicians should promptly recognize the development of ICI-related myocarditis, which is an adverse event uncommon (approximately 0.5–1% of ICI-treated patients) but with a high fatality rate (46%) [Citation50,Citation51].
Until now, it was unclear whether the combinations of a [VEGFR]-TKI and an ICI could result in increased incidence of MACEs and hypertension compared to the standard of care. A recent meta-analysis suggested a higher incidence (more than doubled) of immune-related MACEs with the combinations of anti-PD-(L)1 plus targeted therapies (anti-VEGF or VEGFR agents) compared with dual ICIs (anti – PD-1 plus anti – CTLA-4) and a higher incidence of myocarditis with anti – PD-(L)1-based combinations versus single-agent anti – PD-(L)1 [Citation44]. These results suggested the potential negative role of anti-angiogenic targeted therapies in the development of cardiovascular events, as extensively already demonstrated.
As far as we know, this is the largest and most updated meta-analysis investigating the incidence and relative risk of MACEs and hypertension in patients treated with ICI+TKI combinations for solid tumors, with more than 5000 patients included in the final analysis. Our analysis demonstrated, indeed, that the combinations of ICI+TKI resulted in a significantly higher risk of both any- (RR 3.21, p = 0.001) and high-grade MACEs (RR 2.72, p = 0.05) compared to controls, although the absolute incidence of these events was extremely low (less than 1%) in both cases. Restricting the analysis to VEGFR-TKIs, the results confirmed what already found in the overall population; ICI+VEGFR-TKI combinations increased the risk of MACEs of high-grade, even if the small number of events does not allow reaching definitive conclusions. It is important to highlight that the population enrolled in clinical trials is usually highly selected and undergoes basal cardiac function screening tests; this can lead to a potential underestimation of the risk of MACEs in the ‘real word’ patient population, commonly with more medical comorbidities. To further support this hypothesis, the incidence of cardiovascular events in a real-world ICI-treated cohort of patients exceeded 10%, a higher rate than reported in clinical trials [Citation45].
With regards to hypertension, the combinations of ICI+TKI resulted in a more than two-fold increased risk of developing treatment-related hypertension of any-grade (RR 2.10, p = 0.001), but not of high-grade (p = 0.11), reassuring about the safety profile of these combinations. Of note, the subgroup analysis of ICI+VEGFR-TKI was responsible for a significantly increased risk of high-grade hypertension compared to control arms (RR 1.27; p = 0.001), thus confirming the well-known peripheral circulatory system-damaging effects of the VEGFR-TKIs and the theoretical potential for an augmented risk of cardiovascular events with combination therapies.
Considering the low overall incidence of MACEs, our results suggest that asymptomatic patients treated with ICI+TKI combinations do not need strict routine cardiac monitoring. These data are in line with those emerging from the prospective cardiovascular surveillance conducted in the JAVELIN Renal 101 trial, which showed how periodic control of LVEF, and serum cardiac biomarkers did not predict myocarditis in asymptomatic patients [Citation52]. However, a baseline evaluation of cardiac function and risk factors is advisable to identify those patients with higher risk of developing MACEs, reinforcing the necessity of a prompt recognition of cardiovascular symptoms so as to quickly set up an adequate diagnosis, monitoring, and treatment. On the contrary, continuous monitoring of blood pressure is mandatory for patients treated with VEGFR-TKIs. Therefore, a multidisciplinary team involving oncologists and cardiologists should actively collaborate for better management of cardiovascular side effects so as to improve patient outcomes. Currently, there are no standardized guidelines for medical management of ICI+[VEGFR]TKI related MACEs. High-dose prednisolone (1–2 mg/kg) has been recommended, together with an appropriate cardiac therapy with diuretics and inotropes, depending on the spectrum of the MACEs clinical presentations, as per guidelines from the American College of Cardiology/American Heart Association [Citation44]. In the case of glucocorticoid-refractory cases, the benefit of intensified immunosuppressive therapies (i.e. intravenous immunoglobulins, infliximab, mycophenolate) is anecdotal [Citation53].
Our study has several limitations. First of all, the definition of MACEs included various medical conditions that could not be standardized across the trials included. The major limit is the impossibility of access to raw data and the unavailability of public data about single cardiovascular events for each individual patient. This could lead to a potential overestimation of the incidence of MACEs in case of a concomitant occurrence of two or more cardiovascular adverse events in a single patient. However, given the very low incidence of each single cardiovascular event, we considered the sum of all the adverse events, and we used the Peto’s method for limiting this potential bias. Furthermore, the lack of individual data did not allow performing a specific sub-analysis for each treatment-related MACEs, as we did for hypertension. To further complicate this scenario, it is important to consider that some clinical information that might be associated with an increased risk of cardiovascular toxicity (including patient’s cardiovascular preexistent comorbidities) was not available, but they have to be taken into consideration in an unselected population such as that of clinical practice.
5. Conclusions
In conclusion, although ICI-TKI combinations significantly increase the risk of MACEs and hypertension compared to controls, the low overall incidence of MACEs (<1%) reassures the possibility that these events could be a limit for the therapeutic choice, even considering the consistently higher rate of other systemic treatment-related common adverse events. Therefore, a routine periodic assessment of the cardiac function is not recommended in asymptomatic patients but has to be done straightaway at the occurrence of clinical symptoms in order to avoid severe and potentially life-threatening consequences. Further efforts are strongly encouraged for a better comprehension of the physiopathological mechanisms of ICI+TKI-related cardiac damage and for establishing proper routes for prevent, early diagnose, reduce severity, and effectively treat these adverse events.
6. Expert opinion
The management of cancer patients undergoing innovative therapies can be challenging for clinicians. Actually, the advent of immunotherapy with ICIs is one of the most impressive breakthroughs in the treatment of tumors of the last decade. The next step was aimed at further enhancing the anti-tumor activity of immunotherapy by combining ICIs with molecular targeted therapies. These combinations certainly resulted in an increased efficacy; however, at the cost of increased toxicity, thus requiring clinicians to learn how to recognize and manage treatment-related adverse events. Combinations of ICIs plus TKI (in particular VEGFR-TKI) are associated, among others, with an increased risk of cardiovascular adverse events (i.e. MACEs and hypertension). Although rare, the occurrence of a severe cardiac adverse event can be potentially fatal and requires rapid, accurate, and joint management between oncologists and cardiologists. As a concern treatment-related hypertension – more frequent, although less dangerous – it impacts the chronic management of the patient, often forming part of the context of other comorbidities (i.e. diabetes, metabolic syndrome) whose management cannot be overlooked.
Therefore, a multidisciplinary team involving oncologists and cardiologists should actively collaborate for identifying those patients at higher risk of developing MACEs because of preexisting clinical conditions that require closer monitoring during treatment. In this context, the identification of baseline clinical/laboratoristic features and the definition of a risk model that could categorize patients who deserve intensive follow-up would be highly desirable. Furthermore, timely recognition of cardiovascular symptoms that leads to prompt diagnosis, monitoring, and treatment is of utmost importance to improve patient outcomes and requires awareness among clinicians. Studies are recommended to define the best therapy for both acute and severe events, but also for the prevention and the correct chronic management of mild-moderate severity cases.
In conclusion, further efforts are urgently needed for a better comprehension of the physiopathological mechanisms of ICI+TKI-related cardiac damage so as to establish proper routes for prevent, early diagnose, reduce severity, and effectively treat these adverse events.
Declaration of interests
C Ciccarese: occasional consultant or advisory board member for Ipsen, MSD, AstraZeneca, Pfizer, Janssen; all unrelated to the present paper. E Bria supported for the Italian Association for Cancer Research AIRC_IG 20583. Supported by IASLC, LILT, and Fondazione CARIVERONA. He received fees for speakers and travels from Helsinn, Eli-Lilly, MSD, AstraZeneca, Pfizer, BMS, Novartis, and Roche. He received institutional grants from Roche and AstraZeneca, all unrelated to the present paper. R Iacovelli advisory board member for Ipsen, BMS, AstraZeneca, Pfizer, Janssen, Sanofi; all unrelated to the present paper. G Tortora advisory board member for Novartis and BMS; all unrelated to the present paper. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Authors contributions
Conceptualization, Methodology, Supervision, Validation: C Ciccarese, A Anghelone; R Iacovelli, E Bria, and G Tortora. Methodology, Data curating, Writing- Original draft preparation, Supervision: A Stefani, A Cigliola, A Strusi, and F D’Agostino. Resources, Investigation: C Ciccarese, A Anghelone, A Stefani, A Cigliola, A Strusi, and F D’Agostino. Project administration, validation: C Ciccarese and R Iacovelli. Data curating, Writing – Review & Editing: A Anghelone and R Iacovelli. Writing- Reviewing and Editing: R Iacovelli, E Bria and G Tortora. Resources, Writing – Review & Editing: C Ciccarese, R Iacovelli, G Tortora. Software, Validation, Supervision: R Iacovelli, G Tortora. All authors have substantially contributed to the conception and design of the article, interpreting the relevant literature, writing the article, and revising it for intellectual content.
All the authors have agreed on the journal to which the article will be submitted; reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage. Finally, all the authors have agreed to take responsibility and be accountable for the contents of the article and to share responsibility to resolve any questions raised about the accuracy or integrity of the published work.
Ethics statement
Ethical approval was not needed for this paper
Supplemental Material
Download Zip (338.6 KB)Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14737140.2024.2357814.
Additional information
Funding
References
- Dirkx AEM, Egbrink MGA, Castermans K, et al. Anti-angiogenesis therapy can overcome endothelial cell anergy and promote leukocyte-endothelium interactions and infiltration in tumors. FASEB J. 2006;20(6):621–630. doi: 10.1096/fj.05-4493com
- Yasuda S, Sho M, Yamato I, et al. Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 (VEGFR2) induces synergistic anti-tumour effect in vivo. Clin Exp Immunol. 2013;172(3):500–506. doi: 10.1111/cei.12069
- Przybylski DJ, Bergsbaken JJ, Piccolo JK. Unleashing the power of immunotherapy and targeted therapy combinations: advancing cancer care or discovering unknown toxicities? J Oncol Pharm Pract. 2021;27(4):930–938. doi: 10.1177/1078155220984235
- Lombardi P, Filetti M, Falcone R, et al. New first-line immunotherapy-based combinations for metastatic renal cell carcinoma: a systematic review and a network meta-analysis. Cancer Treat Rev. 2022;106:102377. doi: 10.1016/j.ctrv.2022.102377
- Chaar M, Kamta J, Ait-Oudhia S. Mechanisms, monitoring, and management of tyrosine kinase inhibitors-associated cardiovascular toxicities. Onco Targets Ther. 2018;11:6227–6237. doi: 10.2147/OTT.S170138
- Chu TF, Rupnick MA, Kerkela R, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370(9604):2011–2019. doi: 10.1016/S0140-6736(07)61865-0
- Schmidinger M, Zielinski CC, Vogl UM, et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2008;26(32):5204–5212. doi: 10.1200/JCO.2007.15.6331
- Richards CJ, Je Y, Schutz FA, et al. Incidence and risk of congestive heart failure in patients with renal and nonrenal cell carcinoma treated with sunitinib. J Clin Oncol. 2011;29(25):3450–3456. doi: 10.1200/JCO.2010.34.4309
- Iacovelli R, Ciccarese C, Fornarini G, et al. Cabozantinib-related cardiotoxicity: a prospective analysis in a real-world cohort of metastatic renal cell carcinoma patients. Br J Clin Pharmacol. 2019;85(6):1283–1289. doi: 10.1111/bcp.13895
- Lyon AR, Yousaf N, Battisti NML, et al. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018;19(9):e447–e458. doi: 10.1016/S1470-2045(18)30457-1
- Salem J, Manouchehri A, Moey M, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19(12):1579–1589. doi: 10.1016/S1470-2045(18)30608-9
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339(jul21 1):b2535. doi: 10.1136/bmj.b2535
- Morris JA, Gardner MJ. Statistics in medicine: calculating confidence intervals for relative risks (odds ratios) and standardised ratios and rates. Br Med J (Clin Res Ed). 1988;296(6632):1313–1316. doi: 10.1136/bmj.296.6632.1313
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2
- Review manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014. http://ims.cochrane.org/revman/download
- Powles T, Plimack ER, Soulières D, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21(12):1563–1573. doi: 10.1016/S1470-2045(20)30436-8
- Motzer R, Alekseev B, Rha SY, et al. CLEAR trial investigators. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289–1300. doi: 10.1056/NEJMoa2035716
- Choueiri TK, Powles T, Burotto M, et al. CheckMate 9ER investigators. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829–841. doi: 10.1056/NEJMoa2026982
- Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103–1115. doi: 10.1056/NEJMoa1816047
- Gutzmer R, Stroyakovskiy D, Gogas H, et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;395(10240):1835–1844. doi: 10.1016/S0140-6736(20)30934-X
- Makker V, Colombo N, Casado Herráez A, et al. Study 309–KEYNOTE-775 investigators. Lenvatinib plus pembrolizumab for advanced endometrial cancer. N Engl J Med. 2022;386(5):437–448. doi: 10.1056/NEJMoa2108330
- Kelley Rk T, Yau T, Cheng AL, et al. Cabozantinib plus atezolizumab versus sorafenib as first-line systemic treatment for advanced hepatocellular carcinoma: Results from the randomized phase III COSMIC-312 trial. 2021 ESMO virtual plenary. Abstract VP10- 2021. cited 2021 Nov 20.
- Yarchoan M, Cope L, Ruggieri AN, et al. Multicenter randomized phase II trial of atezolizumab with or without cobimetinib in biliary tract cancers. J Clin Invest. 2021;131(24):e152670. doi: 10.1172/JCI152670
- Loriot Y, Grivas P, de Wit R, et al. First-line pembrolizumab with or without lenvatinib in patients with advanced urothelial carcinoma (LEAP-011): a phase 3, randomized, double-blind study. J Clin Oncol. 2022;40(suppl 6):432. doi: 10.1200/JCO.2022.40.6_suppl.432
- Eng C, Kim TW, Bendell J, et al. For the IMblaze370 investigators. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2019;20(6):849–861. doi: 10.1016/S1470-2045(19)30027-0
- Moslehi JJ, Longo DL. Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med. 2016;375(15):1457–1467. doi: 10.1056/NEJMra1100265
- Touyz RM, Herrmann SMS, Herrmann J. Vascular toxicities with VEGF inhibitor therapies–focus on hypertension and arterial thrombotic events. J Am Soc Hypertens. 2018;12(6):409–425. doi: 10.1016/j.jash.2018.03.008
- Hu JR, Florido R, Lipson EJ, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc Res. 2019;115(5):854–868. doi: 10.1093/cvr/cvz026
- Wang DY, Okoye GD, Neilan TG, et al. Cardiovascular toxicities associated with cancer immunotherapies. Curr Cardiol Rep. 2017;19(3):21. doi: 10.1007/s11886-017-0835-0
- Bair SM, Choueiri TK, Moslehi J. Cardiovascular complications associated with novel angiogenesis inhibitors: emerging evidence and evolving perspectives. Trends Cardiovasc Med. 2013;23(4):104–113. doi: 10.1016/j.tcm.2012.09.008
- Lankhorst S, Saleh L, Danser AJ, et al. Etiology of angiogenesis inhibition-related hypertension. Curr Opin Pharmacol. 2015;21:7–13. doi: 10.1016/j.coph.2014.11.010
- Dalbeni A, Ciccarese C, Bevilacqua M, et al. Effects of antiangiogenetic drugs on microcirculation and macrocirculation in patients with advanced-stage renal cancer. Cancers (Basel). 2018;11(1):30. doi: 10.3390/cancers11010030
- Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer. 2007;7(5):332–344. doi: 10.1038/nrc2106
- May D, Gilon D, Djonov V, et al. Transgenic system for conditional induction and rescue of chronic myocardial hibernation provides insights into genomic programs of hibernation. Proc Natl Acad Sci. 2008;105:282–287.
- Izumiya Y, Shiojima I, Sato K, et al. Vascular endothelial growth factor blockade promotes the transition from compensatory cardiac hypertrophy to failure in response to pressure overload. Hypertension. 2006;47(5):887–893. doi: 10.1161/01.HYP.0000215207.54689.31
- Iijima Y, Laser M, Shiraishi H, et al. C-Raf/MEK/ERK pathway controls protein kinase C-mediated p70S6K activation in adult cardiac muscle cells. J Biol Chem. 2002;277(25):23065–23075. doi: 10.1074/jbc.M200328200
- Sheng Z, Knowlton K, Chen J, et al. Cardiotrophin 1 (CT-1) inhibition of cardiac myocyte apoptosis via a mitogen-activated protein kinase-dependent pathway. Divergence from downstream CT-1 signals for myocardial cell hypertrophy. J Biol Chem. 1997;272(9):5783–5791. doi: 10.1074/jbc.272.9.5783
- Ramirez MT, Sah VP, Zhao XL, et al. The MEKK-JNK pathway is stimulated by α1-adrenergic receptor and ras activation and is associated with in vitro and in vivo cardiac hypertrophy. J Biol Chem. 1997;272(22):14057–14061. doi: 10.1074/jbc.272.22.14057
- Lorenz K, Schmitt JP, Schmitteckert EM, et al. A new type of ERK1/2 autophosphorylation causes cardiac hypertrophy. Nat Med. 2009;15(1):75–83. doi: 10.1038/nm.1893
- Guo CW, Alexander M, Dib Y, et al. A closer look at immune-mediated myocarditis in the era of combined checkpoint blockade and targeted therapies. Eur J Cancer. 2020;124:15–24. doi: 10.1016/j.ejca.2019.09.009
- Shah RR, Morganroth J, Shah DR. Cardiovascular safety of tyrosine kinase inhibitors: with a special focus on cardiac repolarization (QT interval). Drug Saf. 2013;36(5):295–316. doi: 10.1007/s40264-013-0047-5
- Naqash AR, Moey M, Tan XWC, et al. Major adverse cardiac events with immune checkpoint inhibitors: a pooled analysis of trials sponsored by the national cancer institute—cancer therapy evaluation program. J Clin Oncol. 2022;40(29):3439–3452. doi: 10.1200/JCO.22.00369
- Laenens D, Yu Y, Santes B, et al. Incidence of cardiovascular events in patients treated with immune checkpoint inhibitors. J Clin Oncol. 2022;40(29):3430–3438. doi: 10.1200/JCO.21.01808.
- Lichtman AH. The heart of the matter: protection of the myocardium from T cells. J Autoimmun. 2013;45:90–96. doi: 10.1016/j.jaut.2013.05.004
- Tarrio ML, Grabie N, Bu DX, et al. PD-1 protects against inflammation and myocyte damage in T cell-mediated myocarditis. J Immunol. 2012;188(10):4876–4884. doi: 10.4049/jimmunol.1200389
- Groschel C, Sasse A, Rohrborn C, et al. T helper cells with specificity for an antigen in cardiomyocytes promote pressure overload-induced progression from hypertrophy to heart failure. Sci Rep. 2017;7(1):15998. doi: 10.1038/s41598-017-16147-1
- Lutgens E, Seijkens TTP. Cancer patients receiving immune checkpoint inhibitor therapy are at an increased risk for atherosclerotic cardiovascular disease. J Immunother Cancer. 2020;8(1):e000300. doi: 10.1136/jitc-2019-000300
- Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71(16):1755–1764. doi: 10.1016/j.jacc.2018.02.037
- Moslehi JJ, Salem JE, Sosman JA, et al. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;391(10124):933. doi: 10.1016/S0140-6736(18)30533-6
- Rini BI, Moslehi JJ, Bonaca M, et al. Prospective cardiovascular surveillance of immune checkpoint inhibitor-based combination therapy in patients with advanced renal cell cancer: data from the phase III JAVELIN renal 101 trial. J Clin Oncol. 2022;40(17):1929–1938. doi: 10.1200/JCO.21.01806
- Cautela J, Zeriouh S, Gaubert M, et al. Intensified immunosuppressive therapy in patients with immune checkpoint inhibitor-induced myocarditis. J Immunother Cancer. 2020;8(2):e001887. doi: 10.1136/jitc-2020-001887