ABSTRACT
Introduction
Relacorilant (CORT125134, Corcept Therapeutics) is a selective glucocorticoid receptor modulator, which reverses the glucocorticoid-mediated anti-apoptotic effects and restores the taxane chemosensitivity in epithelial ovarian cancer cells. Given those preclinical findings, relacorilant is currently under investigation in clinical trials in combination with nab-paclitaxel for the platinum-resistant ovarian cancer setting.
Areas covered
Already published preclinical and clinical evidence of relacorilant antitumor activity was analyzed and discussed. Ongoing clinical trials registered on clincaltrials.gov were also reported. The review aimed to summarize the status of relacorilant, the mechanism of action, the published and ongoing trials, and its safety and efficacy.
Expert opinion
Relacorilant combined with nab-paclitaxel, may represent a promising strategy for the treatment of platinum-resistant ovarian cancer patients. After preliminary positive results in terms of clinical efficacy, a randomized phase III trial is ongoing to confirm the findings from the published phase II study.
1. Introduction
The platinum-ineligible setting – represented by patients progressing under platinum, with early symptomatic progression, or with platinum intolerance – remains a huge clinical unmet need with limited therapeutic options for ovarian cancer patients.
Indeed, the median overall survival (OS) for patients who progressed on or within 6 months from the last cycle of platinum-based chemotherapy is approximately 12–13 months with a median progression-free survival (PFS) of 3–4 months in those treated with single, non-platinum agent [Citation1]. In this context, it is crucial to unravel clinically relevant resistance mechanisms to standard treatment options available in this setting and develop new therapeutic strategies potentially targeted to overcome or bypass the resistance. Beyond DNA and RNA sequencing strategies, the use of proteomics technologies has highlighted the intrinsic genomic and epigenomic heterogeneity and complexity of this disease and improved our knowledge of the potential molecular signaling events leading to ovarian cancer development and drug resistance [Citation2]. As such, several mechanisms of drug resistance have been studied, e.g. P-gp mediated efflux, upregulation of survival pathways, and increased anti-apoptotic factors [Citation3]. Interestingly, in preclinical tumor models, glucocorticoid receptor (GR) was shown to be involved in chemotherapy resistance, with stimulation of GR shown to reduce chemotherapy sensitivity while blockade of GR shown to enhance chemotherapy sensitivity [Citation4–6]. Based on those findings, the selective GR modulator relacorilant (CORT125134, Corcept Therapeutics) has entered the clinical setting to investigate the potential antitumor activity based on its ability to reverse glucocorticoid-mediated anti-apoptotic effects. Furthermore, given the evidence of taxane-induced apoptotic suppression mediated by glucocorticoids as a mechanism of taxane resistance [Citation7], the combination of relacorilant and nab-paclitaxel has recently gained increasing interest and has been investigated in clinical trials, with the aim of restoring taxane-chemosensitivity and enhancing antitumor efficacy.
This review aims to present clinical evidence and future perspectives regarding the role of relacorilant in recurrent platinum-ineligible ovarian cancer, providing a critical point of view.
2. Overview of the market
Beyond the well-known anti-inflammatory and immunosuppressive properties, glucocorticoids exert different effects in several cell types through the binding of the GR, which translocates to the nucleus, thus inducing translational and post-translational changes and finally leads to the activation of pro- or anti-apoptotic pathways, depending on the cell type. GR signaling was shown to activate the intrinsic pro-apoptotic cascade in cells belonging to the muscular, nervous, endocrine, reproductive, immune, circulatory, skeletal systems [Citation8], as well as in hematological tumor cells [Citation9], while a GR-induced anti-apoptotic effect was observed in distinct epithelial tumor cells, including ovarian cancer [Citation4,Citation7,Citation10,Citation12]. Specifically, the activation of GR in ovarian carcinoma cell lines leads to the upregulation of glucocorticoid-regulated kinase 1 (SGK1) and map kinase phosphatase 1 (MKP1)/dual-specificity phosphatase 1 (DUSP1) genes [Citation11,Citation12]. Interestingly, a BRCA1-GR interplay has been studied in vitro both in triple negative breast cancer and ovarian cancer models, with the evidence of an interaction between BRCA1 and the nuclear steroid receptor, which leads to transcriptional changes, including the repression of the estrogen receptor alpha (ERα), the co-activation of the androgen receptor (AR), and the modulation of the MAPK signaling [Citation13].
Given the preclinically promising anti-tumorigenic effect of the GR antagonism, the nonselective GR antagonist mifepristone was initially tested as a single agent in the clinical setting. However, it demonstrated controversial clinical efficacy with an objective response rate (ORR) of 26.5% in an ovarian cancer platinum-resistant population [Citation14]. Those findings were not further confirmed by a more recent multicenter phase II trial, which showed only an ORR of 4.5% [Citation15].
Subsequently, the GR-induced anti-apoptotic cascade activation was demonstrated to interfere with different drugs widely used in the treatment of platinum-resistant ovarian cancer, including paclitaxel [Citation16]; moreover, high GR expression in ovarian cancer has been correlated with shorter PFS [Citation5,Citation17]. Building on this rationale, GR antagonists were tested in the clinical setting in combination with nab-paclitaxel. This nanoparticle albumin-coated paclitaxel does not require pre-medication with corticosteroids to avoid hypersensitivity reactions.
While the GR-antagonists mifepristone [Citation18] and ORIC-101 [Citation19] did not demonstrate an improvement in the clinical efficacy of nab-paclitaxel in solid tumors, the relacorilant/nab-paclitaxel combination therapy showed promising results in platinum-resistant ovarian cancer patients [Citation20].
The failure of a potential clinical use of mifepristone in the clinical setting, both as a single-agent and in combination with other drugs, might be explained by its non-selectivity for GR, which can be overcome with the selective GR modulator, relacorilant. On the other hand, nab paclitaxel, acting as a microtubule inhibitor, induces tumor cell apoptosis via BCL2 and FOXO3a activities; these pathways are weakened by the effect of cortisol on GR. Interestingly, relacorilant antagonizes cortisol effects, and nab-paclitaxel – induced tumor cell apoptosis is restored, with increasing chemotherapy’s sensitivity. Furthermore, relacorilant is under investigation in combination with enzalutamide in a phase 2 study enrolling high-risk localized prostate cancer patients (NCT05726292) [Citation21].
3. Introduction to the compound
Relacorilant is a potent and selective modulator of the GR. Given the inhibition constant (Ki) for the receptor binding of 0.15 nM, it displays a high affinity to the GR. Furthermore, no measurable binding was observed with the use of other steroid receptors (i.e., progesterone, estrogen, and AR) [Citation22]. Relacorilant is an orally active drug, which was initially administered with two different schedules: continuously, with a dosage of 100 mg once daily for 28 days in combination with nab-paclitaxel 80 mg/m2 intravenously (IV) on Days 1, 8, and 15 of each 28-day cycle; or intermittently, with a dosage of 150 mg once daily three days of each 28-day cycle (the day before/the day of/the day after nab-paclitaxel infusion) in combination with nab-paclitaxel 80 mg/m2 IV on Days 1, 8, and 15 of each 28-day cycle [Citation20]. However, the intermittent schedule was the recommended regimen currently being investigated in the phase III ongoing trial [Citation23].
Relacorilant is an optimized selective GR antagonist based on a fused azadecalin template. The exact chemical molecule is (R)‐(1-(4-Fluorophenyl)-6-((1-methyl‐1 H‐pyrazol-4-yl) sulfonyl)-4,4a,5,6,7,8-hexahydro‐1 H‐pyrazolo[3,4‐g] isoquinolin-4a-yl) (4-(trifluoromethyl) pyridin-2-yl) methanone [Citation22].
Relacorilant antagonizes the effects of cortisol activity by selectively binding the GR receptor without interacting with estrogen, progesterone, AR, and mineralocorticoid receptors [Citation22]. In a phase I trial on healthy volunteers, relacorilant 500 mg (single dose) and 250 mg daily for 14 days was demonstrated to reverse all the effects of prednisone 25 mg [Citation24]. GR antagonism was also confirmed in a phase I study evaluating the pharmacodynamics of the relacorilant + nab-paclitaxel combination therapy. RNA profiling after 15 days of treatment revealed on-target suppression of GR-dependent genes, such as PTGS2, CXCL8, PTGER4, AND IDO1 [Citation25]. Gene expression changes after 15 days of treatment, particularly in prednisone-induced genes such as CD163 and IGF2R, correlated with response to relacorilant + nab-paclitaxel therapy [Citation25].
Absorption of relacorilant with ranging dosages from 5 to 500 mg is very rapid, given 0.75 to 2.0 h as the median time to peak concentration. Dose increment leads to a maximum plasma concentration increase in a greater than proportional manner, with the following mean percentage variation peak concentration (Cmax) values of 7.2 (42%, relacorilant dosage: 5 mg), 25 (59%, relacorilant dosage: 15 mg), 167 (48%, relacorilant dosage: 50 mg), 672 (42%, relacorilant dosage: 150 mg), 802 (46%, relacorilant dosage: 300 mg), and 1567 (20%, relacorilant dosage: 500 mg) ng/mL. A dose-dependent clearance was observed. A mean elimination half‐life of 17–19 h was recorded at a dosage of 150 mg or greater, and achievement of a steady state was observed by day 7 with a daily dosing administration [Citation24].
4. Clinical efficacy
4.1. Methods
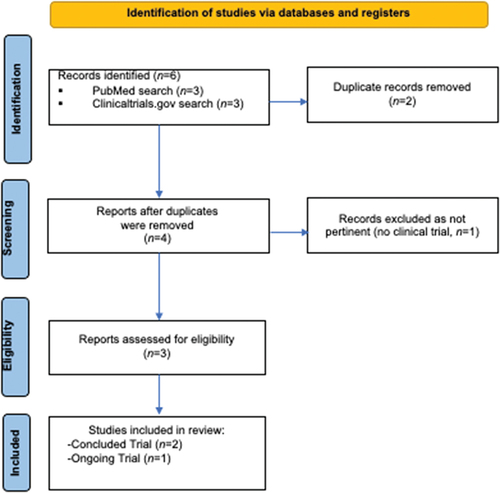
A search in PubMed and Clinicaltrials.gov up to February 2024 was performed, combining the following terms: ‘Ovarian cancer’ AND ‘Relacorilant’ following the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines ().
Inclusion criteria were as follows: 1) Phase I-II-III trial with already published efficacy and/or safety data or 2) Ongoing phase I-II-III trial (not yet published results, irrespective of the recruiting status), investigating the role of relacorilant in recurrent ovarian cancer patients, alone or in combination with other agents. Non-English language publications were excluded.
4.2. Phase I
A phase I/II clinical trial was designed to assess the safety and preliminary efficacy of relacorilant given in combination with nab-paclitaxel in patients with advanced/metastatic solid tumors progressing after available therapeutic options (ClinicalTrials.gov identifier: NCT02762981) [Citation25]. In this non-randomized, single-arm, open-label, multicenter study, alternative dosing schedules (continuous-dosing and intermittent-dosing cohorts) of relacorilant at escalating dose levels were tested in combination with nab-paclitaxel. Overall, 75 participants were enrolled. Sixty-four patients underwent the continuous-dosing regimen, receiving nab-paclitaxel infusions on days 1, 8, and 15 of each 28-day cycle and oral relacorilant once daily. Twenty-one patients received nab-paclitaxel infusions on days 1, 8, and 15 of each 28-day cycle in combination with oral relacorilant administered the day before, the day of, and the day after nab-paclitaxel (intermittent-dosing regimen). Seventy-three patients were able to receive at least one dose of relacorilant. Of these, 14 (19.2%) had ovarian cancer, and 13 were RECIST-evaluable, with observed 1 complete response (CR), 2 partial responses (PRs), and 7 stable diseases (SDs). Sustained clinical benefit (CR, PR, or SD ≥16 weeks) was observed in 38.5% of ovarian cancer patients. Median PFS in ovarian cancer participants was 4.6 months (95% CI, 3.7–N/A) [Citation25].
4.3. Phase II study
Building on the success of relacorilant/nab-paclitaxel combination therapy in ovarian cancer patients enrolled in phase I/II clinical trial [Citation25], the phase II, randomized, open-label, three-arm study CORT125134–552 (ClinicalTrials.gov identifier: NCT03776812) was designed to further assess the clinical efficacy (PFS as primary endpoint) of nab-paclitaxel and relacorilant in the two different dosing-regimens (intermittent and continuous) compared to nab-paclitaxel monotherapy. One hundred seventy-eight patients with recurrent, high-grade serous/endometrioid ovarian cancer or carcinosarcoma with platinum-resistant or refractory disease were randomly assigned to receive 1:1:1: oral relacorilant 100 mg once daily in combination with nab-paclitaxel 80 mg/m2 administered intravenously on days 1, 8, and 15 of each 28-day cycle (Arm A, continuous arm) versus oral relacorilant 150 mg on the day before, the day of, and the day after nab-paclitaxel 80 mg/m2 administered on days 1, 8, and 15 of each 28-day cycle (Arm B, intermittent arm) versus nab-paclitaxel monotherapy 100 mg/m2 on days 1, 8, and 15 of each 28-day cycle (Arm C, comparator arm). After a median follow-up of 11.1 months, the intermittent relacorilant/nab-paclitaxel combination regimen (Arm B) showed the longest PFS (5.6 months). The primary endpoint of the study was not met, since the ORR after the protocol-prespecified Hochberg step-up multiplicity adjustment did not show any statistically differences in terms of PFS (p < 0.025) between the two study groups at 1-sided 0.05 level of significance (nab-paclitaxel monotherapy, Arm C, versus simultaneous testing of each relacorilant-containing arms Arm A and B). However, a significant improvement in PFS with the addition of intermittent relacorilant to nab-paclitaxel monotherapy was observed by comparing only the intermittent relacorilant arm, Arm B (PFS of 5.6 months) with the nab-paclitaxel monotherapy, Arm C (3.8 months), with an HR of 0.66; 95% CI, 0.44 to 0.98; p = 0.038). In line with those results, despite a similar ORR across all arms (Arm A: 35.2%; Arm B: 35.7%; Arm C: 35.8%), median Duration of Response (DoR) observed in patients treated with intermittent relacorilant/nab-paclitaxel combination therapy was significantly longer than DoR observed with nab-paclitaxel monotherapy (Arm B: 5.55 months versus Arm C: 3.65 months, HR 0.36; 95% CI, 0.16 to 0.77; p = 0.006). Additionally, a subgroup ad-hoc analysis including patients previously treated with up to 3 prior lines of anticancer therapy, with at least one regimen containing bevacizumab, and excluding patients with primary platinum-refractory disease showed significant improvement in OS with the use of intermittent relacorilant/nab-paclitaxel combination therapy compared to nab-paclitaxel monotherapy (median OS, 17.9 months; 95% CI, 12.8 to not reached, versus 12.6 months; 95% CI, 6.4 to 15.3; HR 0.38; 95% CI, 0.17 to 0.82). Furthermore, while the decrease in Patient Reported Outcomes (PROs) from baseline registered in the intermittent-relacorilant arm (Arm B) and in the nab-paclitaxel monotherapy (Arm C) were similar, a statistically significant decrease was reported by patients enrolled in the continuous-relacorilant (Arm A) [Citation20].
4.4. Ongoing trials
The promising results of the ad-hoc analysis from the phase II CORT125134–552 trial led to the randomized, phase III, two-arms, open-label ROSELLA study (GOG-3073, ENGOT-Ov72/MITO, ClinicalTrials.gov identifier: NCT05257408) [Citation23] to further confirm whether the addition of intermittent relacorilant to nab-paclitaxel might improve the clinical efficacy compared to nab-paclitaxel monotherapy in a larger population. This trial is currently ongoing, with a target population of 360 participants (platinum-resistant but not primary platinum-refractory ovarian cancer patients, 1–3 prior lines of chemotherapy including bevacizumab) randomized 1:1 to receive intermittent oral relacorilant 150 mg the day before, the day of and the day after nab-paclitaxel 80 mg/m2 infusions on days 1, 8, and 15 of each 28-day cycle versus nab-paclitaxel monotherapy 100 mg/m2 on days 1, 8, and 15 of each 28-day cycle. The primary endpoint of the study is PFS assessed by blinded independent central review [Citation23].
To date, the previously described phase II and phase III trials (NCT03776812 and NCT05257408) are the only two active studies investigating relacorilant in the platinum-resistant ovarian cancer setting as reported in . The primary analysis for PFS and the final analysis for OS of the phase II trial (NCT03776812) were previously published as reported above in this review [Citation20]. The phase III trial is actually recruiting, and the estimated completion date of the study is June 2024 [Citation23].
Table 1. Ongoing trials registered in the Clinicaltrials.Gov database.
5. Safety and tolerability
The most common adverse events (AEs) of grade ≥3 were neutropenia (21.9%), followed by anemia (9.6%) in the phase I/II trial [Citation25]. Interestingly, different dermatological AEs (any grade) were observed, including skin disorders and hyperpigmentation (maculopapular or pruritic rash, acne/acneiform dermatitis, dry skin, cellulitis, skin abrasions) leading to permanent drug discontinuation and dose interruption in 2.7% and 9.6% of patients, respectively. About 15.1% of patients experienced acne, which was treated with topical corticosteroids, leading to dose reduction in 1.4% of patients. Compared to nab-paclitaxel monotherapy, safety findings from the phase II trials showed a numerically higher incidence of grade ≥3 fatigue and anemia in both the intermittent (Arm B, 11.7% and 13.3%, respectively) and continuous (Arm A, 8.8% and 19.3%, respectively) relacorilant arms compared to nab-paclitaxel monotherapy arm (Arm C, 1.7% and 11.7%). Interestingly, incidence of grade ≥3 neutropenia and peripheral neuropathy were lower in the intermittent relacorilant arm (Arm B, 6.7% and 0%, respectively) compared with nab-paclitaxel monotherapy (Arm C, 15% and 5%, respectively). Overall, the toxicity profile observed in the intermittent relacorilant arm (Arm B) was more manageable than the continuous relacorilant arm (Arm A) [Citation20].
6. Conclusion
Data from the single-arm phase I/II study evaluating relacorilant given both intermittently or continuously and in combination with nab-paclitaxel showed a manageable toxicity profile and promising clinical activity in the ovarian cancer population [Citation25]. The phase II study confirmed the tolerability of the relacorilant/nab-paclitaxel combination, suggesting a more favorable safety profile with the use of the intermittent regimen. Despite a similar ORR was observed in the all comers population treated with the intermittent and continuous arms, and no statistically significant differences were found in terms of PFS in the three arms of the study protocol (intermittent, relacorilant arm – continuous, relacorilant arm – nab-paclitaxel alone arm), the subgroup analysis of the phase II trial might have identified a subpopulation of patients who will probably benefit from the addition of intermittent relacorilant to nab-paclitaxel. However, the underlying molecular explanation of the better performance of the intermittent dosing regimen over the continuous administration has not been investigated and remains unclear. Indeed, a promising efficacy was found in platinum-resistant but not platinum-refractory high-grade serous/endometrioid ovarian cancer or carcinosarcoma patients, multiple pre-treated (up to 3 prior lines), with at least one bevacizumab-containing regimen [Citation20]. Consequently, the two-arm, randomized (1:1) phase III trial further assesses the clinical efficacy of the oral intermittent relacorilant in combination with nab-paclitaxel compared with nab-paclitaxel alone in this specific population [Citation23].
7. Expert opinion
New treatment options for the ovarian cancer platinum-resistant setting are urgently needed because of the short survival rates observed in this hard-to-treat population. Beyond the single-agent chemotherapy options currently available in this setting (paclitaxel, pegylated liposomal doxorubicin, topotecan, and gemcitabine), showing PFS of 3–4 months, AURELIA trial was the first study to identify a combination strategy to enhance the efficacy of a non-platinum monotherapy for the platinum-resistant setting, using the monoclonal antibody bevacizumab in addition to standard chemotherapy. Albeit showing a significant PFS and ORR benefit, this study failed to demonstrate an improvement in terms of OS [Citation26]. In addition, in the recently published MIRASOL trial, the new antibody-drug conjugated agent Mirvetuximab-Soravtansine yielded 5.62 months of PFS and was the first agent to demonstrate improvements of OS in the platinum-resistant setting over standard chemotherapy [Citation1]. However, the use of Mirvetuximab-Soravtansine is limited to around 35–40% of the ovarian cancer population with ‘high’ folate-receptor α (FRα) tumor expression [Citation27]. Therefore, alternative therapeutic options are needed for the ‘non-high’ FRα expression population and in the patient population previously treated with bevacizumab. In this context, selective GR modulation with orally active relacorilant is a promising strategy to overcome paclitaxel resistance and to enhance nab-paclitaxel efficacy by restoring taxane chemosensitivity through the suppression of anti-apoptotic GR target genes, such as SGK1 and DUSP1, without showing cumulative toxicity.
Of note, while the effect of previous bevacizumab maintenance strategy on nab-paclitaxel/relacorilant combination therapy has been evaluated in the phase II trial, the impact of PARP inhibitors (PARPi) in this population is not already evaluable, since only 36.5% of patients enrolled in the study previously received this maintenance treatment strategy [Citation20]. The ongoing phase III will probably clarify whether the previous treatment with PARPi might influence the subsequent nab-paclitaxel/relacorilant combination therapy. Additionally, preclinical evidence of a crosstalk between BRCA1 and GR was not investigated in the clinical setting, since the results of the two published studies were not stratified by BRCA status, thus deserving future investigation.
Finally, among the different regimens tested, the intermittent-dosing regimen was demonstrated to be the most promising in enhancing the efficacy of nab-paclitaxel. Results from the ongoing confirmatory phase III trial ROSELLA (GOG-3073, ENGOT-Ov72/MITO) are awaited to finally demonstrate the efficacy of this combination strategy.
8. Information resources section
Glucocorticoid-receptor selective modulator named relacorilant is a promising new drug under investigation in clinical trials to overcome taxane resistance in the ovarian cancer platinum-resistant setting. The single-arm, phase I/II study (NCT02762981) was the first trial investigating the role of relacorilant in combination with nab-paclitaxel in patients with ovarian cancer, showing an acceptable safety profile and promising preliminary efficacy [Citation1]. Based on those data, a phase II, three-arm study (NCT03776812) for platinum-resistant ovarian cancer patients was designed to test the efficacy of the nab-paclitaxel/relacorilant combination therapy, with the intermittent-relacorilant schedule demonstrating prolonged survival outcomes over the nab-paclitaxel control arm [Citation2]. Based on those findings, the ongoing confirmatory phase III, two-arm trial ROSELLA was designed to demonstrate the superiority of the combination therapy (nab-paclitaxel plus intermittent dosing relacorilant) over the nab-paclitaxel monotherapy [Citation3].
Munster PN, Greenstein AE, Fleming GF, et al. Overcoming Taxane Resistance: Preclinical and Phase 1 Studies of Relacorilant, a Selective Glucocorticoid Receptor Modulator, with Nab-Paclitaxel in Solid Tumors. Clin Cancer Res. 2022;28:3214–3224.
Colombo N, Van Gorp T, Matulonis UA, et al. Relacorilant + Nab-Paclitaxel in Patients With Recurrent, Platinum-Resistant Ovarian Cancer: A Three-Arm, Randomized, Controlled, Open-Label Phase II Study. J Clin Oncol. 2023;41:4779–4789.
Lorusso D, Bagameri A, Bishop E, et al. #205 ROSELLA (GOG-3073, ENGOT-Ov72/MITO): a phase 3 study of relacorilant + nab-paclitaxel vs. nab-paclitaxel in advanced, platinum-resistant ovarian cancer. International Journal of Gynecologic Cancer [Internet]. 2023 [cited 2024 Jan 21];33. Available from: https://ijgc.bmj.com/content/33/Suppl_3/A411.2
Article highlights
The selective glucocorticoid receptor (GR) modulator relacorilant represents a new promising agent to overcome drug resistance for ovarian cancer patients.
Relacorilant in combination with nab-paclitaxel might be a new therapeutic option for ovarian cancer patients.
Relacorilant antitumor activity is attributable to the capacity to restore taxane-chemosensitivity.
A phase II trial showed promising results with the combination of oral intermittent relacorilant and nab-paclitaxel in the platinum-resistant setting.
The confirmatory phase III trial is ongoing to further assess the benefit of adding relacorilant to improve nab-paclitaxel clinical efficacy.
Declaration of interest
V. Salutari has received honoraria/consultation fees from AstraZeneca, MSD, GSK, Pharmamar, and Novocure. A. Fagotti has received grants/research support by AstraZeneca, MSD; honoraria/consultation fees by Johnson&Johnson; she participated in a company sponsored speaker’s bureau: Fondazione Internazionale Menarini. G. Scambia has received honoraria/consultation fees from Covidien A.G., AstraZeneca, MSD, Olympus Europa, Baxter; he participated in a company sponsored speaker’s bureau: Healthcare, Intuitive Surgical Inc., GlaxoSmithKline. C. Marchetti received grants/research support from AstraZeneca, MSD, GSK, Pharmamar Menarini; she received honoraria/consultation fees from AstraZeneca, Pharmamar. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Moore KN, Angelergues A, Konecny GE, et al. Mirvetuximab Soravtansine in FRα-positive, platinum-resistant ovarian cancer. N Engl J Med. 2023;389(23):2162–2174. doi: 10.1056/NEJMoa2309169
- Ghose A, Gullapalli SVN, Chohan N, et al. Applications of proteomics in ovarian cancer: Dawn of a new era. Proteomes. 2022;10(2):16. doi: 10.3390/proteomes10020016
- Ortiz M, Wabel E, Mitchell K, et al. Mechanisms of chemotherapy resistance in ovarian cancer. Cancer Drug Resist. 2022;5:304–316. doi: 10.20517/cdr.2021.147
- Skor MN, Wonder EL, Kocherginsky M, et al. Glucocorticoid receptor antagonism as a novel therapy for triple-negative breast cancer. Clin Cancer Res. 2013;19(22):6163–6172. doi: 10.1158/1078-0432.CCR-12-3826
- Veneris JT, Darcy KM, Mhawech-Fauceglia P, et al. High glucocorticoid receptor expression predicts short progression-free survival in ovarian cancer. Gynecol Oncol. 2017;146(1):153–160. doi: 10.1016/j.ygyno.2017.04.012
- Isikbay M, Otto K, Kregel S, et al. Glucocorticoid receptor activity contributes to resistance to androgen-targeted therapy in prostate cancer. Horm Cancer. 2014;5(2):72–89. doi: 10.1007/s12672-014-0173-2
- Greenstein AE, Hunt HJ. Glucocorticoid receptor antagonism promotes apoptosis in solid tumor cells. Oncotarget. 2021;12(13):1243–1255. doi: 10.18632/oncotarget.27989
- Gruver-Yates AL, Cidlowski JA. Tissue-specific actions of glucocorticoids on apoptosis: a double-edged sword. Cells. 2013;2(2):202–223. doi: 10.3390/cells2020202
- Longui CA, Santos MC, Formiga CB, et al. Antiproliferative and apoptotic potencies of glucocorticoids: nonconcordance with their antiinflammatory and immunosuppressive properties. Arq Bras Endocrinol Metabol. 2005;49(3):378–383. doi: 10.1590/S0004-27302005000300008
- Goyeneche AA, Carón RW, Telleria CM. Mifepristone inhibits ovarian cancer cell growthIn vitro and in vivo mifepristone inhibits ovarian cancer cell growth in vitro and in vivo. Clin Cancer Res. 2007;13(11):3370–3379. doi: 10.1158/1078-0432.CCR-07-0164
- Stringer-Reasor EM, Baker GM, Skor MN, et al. Glucocorticoid receptor activation inhibits chemotherapy-induced cell death in high-grade serous ovarian carcinoma. Gynecol Oncol. 2015;138(3):656–662. doi: 10.1016/j.ygyno.2015.06.033
- Melhem A, Yamada SD, Fleming GF, et al. Administration of glucocorticoids to ovarian cancer patients is associated with expression of the anti-apoptotic genes SGK1 and MKP1/DUSP1 in ovarian tissues. Clin Cancer Res. 2009;15(9):3196–3204. doi: 10.1158/1078-0432.CCR-08-2131
- Buonaiuto R, Neola G, Cecere SC, et al. Glucocorticoid receptor and ovarian cancer: from biology to therapeutic intervention. Biomolecules. 2023;13(4):653. doi: 10.3390/biom13040653
- Rocereto TF, Saul HM, Aikins JA, et al. Phase II study of mifepristone (RU486) in refractory ovarian cancer. Gynecol Oncol. 2000;77(3):429–432. doi: 10.1006/gyno.2000.5789
- Rocereto TF, Brady WE, Shahin MS, et al. A phase II evaluation of mifepristone in the treatment of recurrent or persistent epithelial ovarian, fallopian or primary peritoneal cancer: a gynecologic oncology group study. Gynecol Oncol. 2010;116(3):332–334. doi: 10.1016/j.ygyno.2009.10.071
- Karvonen H, Arjama M, Kaleva L, et al. Glucocorticoids induce differentiation and chemoresistance in ovarian cancer by promoting ROR1-mediated stemness. Cell Death Dis. 2020;11(9):790. doi: 10.1038/s41419-020-03009-4
- Bakour N, Moriarty F, Moore G, et al. Prognostic significance of glucocorticoid receptor expression in cancer: a systematic review and Meta-analysis. Cancers (Basel). 2021;13(7):1649. doi: 10.3390/cancers13071649
- Chen N, Saha P, Rampurwala MM, et al. A randomized phase II trial of nab-paclitaxel with or without mifepristone for advanced triple-negative breast cancer. JCO. 2023;41(16_suppl):e13103–e13103. doi: 10.1200/JCO.2023.41.16_suppl.e13103
- ORIC Pharmaceuticals Reports Fourth Quarter and Full Year. Financial results and operational update | ORIC pharmaceuticals, inc. [Internet]. 2021 [cited 2024 Jan 21]. Available from: https://investors.oricpharma.com/news-releases/news-release-details/oric-pharmaceuticals-reports-fourth-quarter-and-full-year-2021/
- Colombo N, Van Gorp T, Matulonis UA, et al. Relacorilant + nab-paclitaxel in patients with recurrent, platinum-resistant ovarian cancer: a three-arm, randomized, controlled, Open-label phase II study. J Clin Oncol. 2023;41(30):4779–4789. doi: 10.1200/JCO.22.02624
- University of Chicago. A randomized phase II trial of neoadjuvant enzalutamide plus the glucocorticoid receptor antagonist relacorilant versus placebo for patients with high-risk localized prostate cancer [internet]. Clinicaltrials.gov. 2023 [cited 2024 Jan 1]. ( Report No.: NCT05726292). Available from: https://clinicaltrials.gov/study/NCT05726292
- Hunt HJ, Belanoff JK, Walters I, et al. Identification of the Clinical Candidate (R)-(1-(4-Fluorophenyl)-6-((1-methyl-1H-pyrazol-4-yl)sulfonyl)-4,4a,5,6,7,8-hexahydro-1H-pyrazolo[3,4-g]isoquinolin-4a-yl)(4-(trifluoromethyl)pyridin-2-yl)methanone (CORT125134): a selective glucocorticoid receptor (GR) antagonist. J Med Chem. 2017;60(8):3405–3421. doi: 10.1021/acs.jmedchem.7b00162
- Lorusso D, Bagameri A, Bishop E, et al. #205 ROSELLA (GOG-3073, ENGOT-Ov72/MITO): a phase 3 study of relacorilant + nab-paclitaxel vs. nab-paclitaxel in advanced, platinum-resistant ovarian cancer. Int J Gynecologic Cancer [Internet]. 2023 [cited 2024 Jan 21];33. Available from: https://ijgc.bmj.com/content/33/Suppl_3/A411.2
- Hunt H, Donaldson K, Strem M, et al. Assessment of safety, tolerability, pharmacokinetics, and pharmacological effect of orally administered CORT125134: an adaptive, double‐blind, randomized, placebo‐controlled phase 1 clinical study. Clin Pharmacol Drug Dev. 2018;7(4):408–421. doi: 10.1002/cpdd.389
- Munster PN, Greenstein AE, Fleming GF, et al. Overcoming taxane resistance: preclinical and phase 1 studies of relacorilant, a selective glucocorticoid receptor modulator, with Nab-paclitaxel in solid tumors. Clin Cancer Res. 2022;28(15):3214–3224. doi: 10.1158/1078-0432.CCR-21-4363
- Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA Open-label randomized phase III trial. JCO. 2014;32(13):1302–1308. doi: 10.1200/JCO.2013.51.4489
- Van Gorp T, Sabatier R, Konecny GE, et al. Efficacy of mirvetuximab soravtansine in folate receptor alpha high, platinum-resistant ovarian cancer by type and number of prior treatment regimens: an exploratory analysis. In: Presented at the 2023 Annual Global Meeting of the International Gynecologic Cancer Society (IGCS); 2023 Nov 5–7; Seoul, South Korea.