ABSTRACT
Introduction: The availability of simple, tolerable, therapies for hepatitis C virus (HCV) infection with responses >95% is one of the greatest medical advances in decades, offering an opportunity to reverse the rising burden due to HCV and strive towards HCV elimination. A key challenge moving forward will be to ensure that those who are undiagnosed are made aware of their infection, receive HCV therapy and achieve viral cure. The availability of point-of-care tests for HCV infection has the potential to simplify testing algorithms, increase diagnoses, and facilitate linkage to treatment.
Areas covered: This commentary explores why point-of-care tests for HCV are needed, what markers of HCV can be measured, methods for sample collection, where HCV testing can occur, and the remaining challenges for HCV point-of-care testing.
Expert commentary: Currently, we have reached an era where there are now several commercial assays to detect HCV RNA (active HCV infection) in 60–90 min, and have reached a single visit HCV diagnosis. In the future, it is hoped that further technological advances will enable access to low-cost, rapid, and accurate assays for HCV RNA detection, improving the number of people diagnosed with HCV infection and contributing to global elimination efforts.
1. Introduction
Globally, 71 million people are living with hepatitis C virus (HCV) infection [Citation1] and HCV-related morbidity and mortality is rising [Citation2]. The availability of simple, tolerable, pan-genotypic direct-acting antiviral (DAA) therapies with responses of >95% is one of the greatest medical advances in recent decades and offers an opportunity to reverse the rising burden due to HCV [Citation3]. WHO has set an ambitious goal of eliminating HCV as a major global public health threat by 2030 [Citation4]. WHO targets include reducing new HCV infections by 80%, and the number of HCV deaths by 65% between 2015 and 2030, and increasing HCV diagnoses from <20% to 90% and the proportion of eligible persons receiving HCV treatment from <10% to 80% [Citation4]. Achieving the WHO targets will require increased HCV testing to ensure that undiagnosed people are made aware of their infection, receive HCV therapy, and achieve viral cure.
The availability of point-of-care tests for HCV infection has the potential to simplify testing algorithms, increase diagnoses, and facilitate linkage to treatment. Experts on HIV and tuberculosis diagnostic testing have defined a point-of-care test as ‘a diagnostic test that is performed near the patient or treatment facility, has a fast turnaround time, and may lead to a change in patient management’ [Citation5]. This is different from a rapid diagnostic test (RDT) which is often defined as a medical diagnostic that is quick and easy to perform. Simplified point-of-care diagnostic testing across a number of different platforms has provided significant advances in other infectious diseases, including HIV [Citation6–Citation8], tuberculosis [Citation7,Citation9], chlamydia [Citation10–Citation12], syphilis [Citation8,Citation13], and gonorrhea [Citation10,Citation11,Citation14]. This commentary explores testing for HCV infection and how point-of-care diagnostics are contributing to the pursuit of a single visit HCV diagnosis.
2. Why are point-of-care tests for hepatitis C needed?
The current HCV testing paradigm requires the detection of antibodies (anti-HCV) against HCV to confirm previous exposure. Among people who are anti-HCV antibody positive, active infection is then confirmed by HCV RNA testing. This two-step algorithm is driven by the fact that HCV RNA testing has historically been considerably more expensive and requires more laboratory facilities than anti-HCV testing. All studies of the continuum of care for HCV show that the consequence of requiring two separate tests for diagnosis is that a significant fraction of people who are anti-HCV antibody positive never receive confirmatory HCV RNA testing. In studies from Australia, Canada, and the United States, among people testing anti-HCV antibody positive, only 46–73% of people received confirmatory HCV RNA testing [Citation15–Citation20].
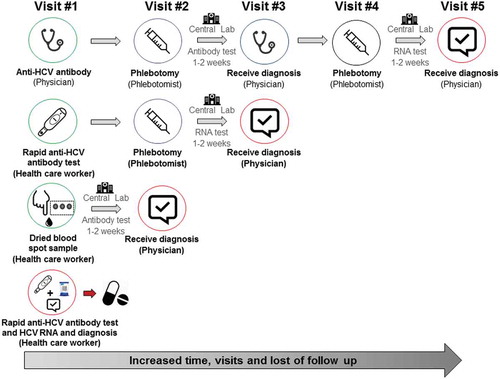
Several factors contribute to the drop-off in confirmatory HCV RNA testing following a positive anti-HCV antibody test result. First, a number of separate visits are required (up to five visits) from initial anti-HCV antibody testing, to HCV RNA testing and to receipt of an HCV diagnosis (). At the initial visit, the practitioner discusses and then orders an anti-HCV test. The individual must then go to a phlebotomist to have this test done and then return to the practitioner to get that result. Assuming it is positive, he/she then must again go to the phlebotomist for a second blood test and finally return, sometimes weeks later, to the practitioner for the final result. If any of these steps is missed or delayed, a confirmed diagnosis of active HCV infection may not be made. This is particularly problematic in remote/rural areas and among marginalized populations with less regular contact with the health-care system, such as people who inject drugs (PWID).
There is also a poor knowledge and competence among primary care providers about the natural history and testing for HCV infection [Citation21–Citation23]. Some providers may not order confirmatory HCV RNA testing following a detectable anti-HCV antibody test [Citation23]. As such, patients testing detectable for anti-HCV antibodies may be inappropriately diagnosed as having active infection, leading to situations where people who have spontaneously cleared infection are unaware that they do not have active infection. There is also poor knowledge among patients about HCV testing and the difference between anti-HCV antibody and RNA testing [Citation24,Citation25]. Further work is needed to educate both patients and providers on HCV testing or to investigate innovative laboratory testing algorithms which may reflexively test HCV RNA when indicated.
3. What markers of HCV infection can be measured?
Initial infection with HCV is characterized by the appearance of the virus in the blood within 2–14 days of exposure and the gradual appearance of anti-HCV antibodies within 32–150 days of exposure [Citation26–Citation29]. Commercial assays are available to detect both anti-HCV antibodies [Citation30,Citation31] and HCV RNA [Citation30,Citation32–Citation36]. Among people exposed to HCV infection, 25% will spontaneously clear infection within the first 6–12 months after exposure [Citation37,Citation38] and will be undetectable for HCV RNA, but most will have detectable anti-HCV antibodies that persist life long. As a result, confirmatory HCV RNA testing is required to detect active infection. Spontaneous clearance very rarely occurs after the onset of chronic infection (~6–12 months postexposure) and thus a positive HCV RNA result years after exposure is adequate to confirm chronic infection [Citation37]. There has been a renewed interest in HCV core antigen (HCV cAg), a viral protein that is part of the HCV virion and thus is detectable in the serum, as a potentially stable, affordable alternative to HCV RNA testing for diagnosis of active infection, particularly in low- and middle-income settings [Citation39–Citation44]. Also, core antigen testing does not require dedicated specimens required for HCV RNA testing. Core antigen testing is somewhat less sensitive than HCV RNA; however, field studies have shown that it performs with adequate sensitivity for detecting chronic infection and likely for post-treatment confirmation of viral cure. Currently, HCV genotyping is an additional test that is still required, which can be done using a combination of methods including line-probe assays and sequencing [Citation45–Citation47]. However, pangenotypic treatment regimens may obviate the need for genotyping in the future, allowing for further simplification of diagnostic algorithms. On-treatment HCV RNA testing and monitoring are also no longer needed with current DAA therapies, further simplifying HCV diagnostic requirements.
4. How can samples for HCV testing be collected?
Standard-of-care testing for anti-HCV antibodies and RNA requires the collection and processing of serum and plasma via venepuncture (). The collection of samples for HCV testing is now also possible through oral fluids [Citation48–Citation52] and capillary blood from finger-stick [Citation49–Citation55]. With respect to finger-stick whole-blood collection, possible modes of collection include capillary whole-blood rapid diagnostic testing [Citation49–Citation54], dried blood spots [Citation55–Citation58], and minivette collection tubes [Citation59].
5. Where can HCV sample testing occur?
5.1. Centralized laboratory testing
Following plasma sample collection and processing, samples are often shipped off-site to centralized laboratories for anti-HCV antibody or RNA testing on commercial assay platforms. The advantages of centralized testing include well-established and approved gold standard assays to test different analytes, with high standards and established quality assurance programs.
There are a number of limitations to centralized laboratory testing. On-site processing of serum and sterile plasma and shipment requirements can complicate logistics. Central laboratory testing requires an established infrastructure, including laboratory networks and sample transport pathways. This is particularly problematic in remote/rural settings and resource-constrained countries with limited laboratory capacity. In sites without on-site phlebotomy, patients need to be referred to off-site phlebotomy services and may not present for testing. Difficult venous access is a major barrier to obtaining blood samples by venepuncture among some populations, such as PWID [Citation60], which is often a reason why some people may not present for testing at all. Also, during the two-step process of anti-HCV testing, patients are lost to follow-up, given the need to attend up to five visits to get a final diagnosis (). Although some laboratories have reflex testing in place (RNA testing on a previous stored sample), this is uncommon in most settings, given different sample collection and preparation processes for the testing of RNA as compared to HCV antibodies, as well as the cost involved in keeping samples in ultracold (−70°C) storage. Results from central laboratories are also often not available for 1–2 weeks or longer. However, many laboratories are introducing automated testing and systems which enable results to be electronically downloaded directly to clinics.
5.2. Point-of-care and dried blood spot testing
Point-of-care HCV testing can include oral fluid rapid diagnostic testing [Citation48–Citation52], finger-stick whole-blood rapid diagnostic testing [Citation49–Citation54], on-site venepuncture-based testing [Citation35,Citation61], and finger-stick capillary whole-blood testing [Citation59]. Although dried blood spot testing is not strictly point of care, the ability to collect a finger-stick sample at the point of care simplifies sample collection, transportation to the laboratory, and diagnosis [Citation55–Citation58]. Point-of-care and DBS testing have been shown to increase uptake of HCV testing [Citation57,Citation62–Citation66] and linkage to HCV care [Citation64,Citation65,Citation67]. Further, point-of-care and DBS testing has the potential to reduce non-attendance to off-site phlebotomy and provides more immediate results to facilitate enhanced counseling, education, and linkage to care. This is particularly useful for remote/rural and outreach settings.
Finger-stick [Citation49–Citation54] and oral fluid [Citation48–Citation52] RDTs for HCV infection are available and have been shown to increase testing [Citation63,Citation66] and linkage to care [Citation64–Citation66]. However, many of these tests are limited in that they only measure HCV antibodies (previous exposure), not HCV RNA (active infection) [Citation41]. As such, a two-step approach with rapid diagnostic testing and confirmatory HCV RNA testing is required. However, rapid diagnostic tests for HCV are quick and inexpensive, so offer an important tool to enhance HCV antibody testing, particularly in populations with low HCV prevalence and/or where resources are limited.
Dried blood spot testing has been shown to enhance HCV testing [Citation57,Citation62,Citation63] and linkage to HCV care [Citation67]. The benefits of finger-stick capillary dried blood spot testing are that (1) capillary blood sampling avoids the need for phlebotomy, a major advantage where venous access is difficult (e.g. PWID) or where phlebotomy services are unavailable; (2) serological testing can be linked to reflex virological testing for HCV RNA confirmation using additional spots from the same filter paper, thus enabling a definitive diagnosis without the need for the person to return for resampling; (3) dried blood spots are stable once dried and easy to transport and store, without the need for a stable cold chain, thus providing a convenient sampling solution in resource-limited settings with long transport times and high temperatures; (4) dried blood spot samples can be used for other purposes, such as HCV sequencing, and testing for other viruses (such as HIV and HBV); (5) collection can be performed by peers or other community workers with limited training. One disadvantage of dried blood spot testing is that it still requires testing to be performed at centralized laboratories and people have to come back for a second visit to get their result, which is a potential barrier in remote areas and among marginalized populations (e.g. PWID). Further, the smaller sample volume requires that multiple pricks need to be collected and in some instances this may yield a lower HCV RNA titer, which may impact the sensitivity of assays.
Point-of-care assays are now available that facilitate HCV RNA confirmation and diagnosis in a single visit [Citation68]. Point-of-care assays available or under late-stage development include the Xpert HCV Viral Load (manufactured by Cepheid), HCV ID Kit (manufactured by Genedrive), and Truenat HCV (manufactured by Genedrive).
The Xpert HCV Viral Load is the first of these assays to receive CE marking and WHO prequalification. In the first iteration of this system, EDTA venous blood samples are collected via venepunture, centrifuged on-site, plasma placed into an Xpert HCV Viral Load cartridge, and loaded into a GeneXpert instrument with a quantitative HCV RNA result in 108 min. Several studies have demonstrated good sensitivity (94–98%) and specificity (98–100%) for quantification of HCV RNA in samples collected via venepuncture using the Xpert HCV Viral Load assay [Citation35,Citation61]. Advantages of this system include the fact that it is modular (enables testing of other infections), semi-portable, random access (does not require batch testing), and HCV RNA results can be provided on the same day. Disadvantages include the need for venepuncture, the time to result, and the initial up-front costs in purchasing the GeneXpert instrument (although several different models are available with varying number of modules). In addition, the cost per assay is still likely to be more expensive than anti-HCV antibody testing, making it less relevant in low prevalence settings. However, this approach may be particularly attractive in higher prevalence populations such as PWID.
A finger-stick capillary whole-blood version of this assay is also under development. In a study of people attending drug health and homelessness services in Australia, samples were collected via a finger stick and tested using the Xpert HCV Viral Load, with a good sensitivity (98%) and specificity (99%) observed [Citation59]. Although the time to result was not ideal (2 h including collection), a version with a time to result of 1 h is in development and being evaluated in clinical trials. This offers a potential new tool to enhance HCV testing, attaining HCV confirmation and diagnosis in a single visit.
Other point-of-care HCV RNA assays are also under late-stage development [Citation68]. The HCV ID Kit (Genedrive) is a point-of-care HCV RNA test that has also received CE marking, enabling HCV RNA testing without requiring the purchase of additional equipment to enable testing. The disadvantage of the HCV ID Kit system is that it only accepts plasma (so venepuncture is required) and requires a number of manual steps to perform the test [Citation68]. At this time, the HCV ID Kit (Genedrive) has not obtained WHO prequalification. The Truenat HCV assay (Molbio Diagnostics) is another point-of-care HCV RNA test under late-stage development, which is cartridge-based and fully automated, but does not have CE marking and has not received WHO prequalification [Citation68]. Further studies are needed to evaluate the performance of novel assays in different settings and populations. A broader number of different HCV RNA detection assays are urgently needed that are rapid (<10 min), cheaper, accurate, and do not require significant equipment requirements (e.g. purchasing of machines).
Moving forward, strategies to enhance HCV testing, linkage to care, and treatment will require broader access to point-of-care HCV testing across a variety of settings. This includes community health centers and drug treatment clinics with no access to on-site phlebotomy, needle, and syringe program services (which lack phlebotomy services), prisons (particularly at the time of reception), remote/rural regions, homelessness settings, supervised drug consumption rooms, and residential rehabilitation/detoxification facilities.
6. What are the remaining challenges ahead for point-of-care testing?
6.1. Improving the number of people diagnosed for HCV infection
Globally, the biggest challenge for HCV testing is that the majority of people remain undiagnosed [Citation69–Citation71]. Strategies are needed to enhance testing (both HCV antibody and RNA) in many countries, particularly those with a high HCV prevalence. Targeted testing campaigns are needed to raise awareness about testing and identify infected individuals. Specific strategies for targeted testing to improve diagnosis will vary by country and will require specific insight into the epidemiology of HCV within that setting to inform potential testing strategies to improve diagnosis.
6.2. Achieving more rapid and accurate HCV RNA testing
Although there are a number of HCV RDTs through salivary [Citation48–Citation52] and finger-stick [Citation49–Citation54] blood collection, the majority of these tests only test for HCV antibody (exposure), not HCV RNA (active infection). The only point-of-care HCV RNA assay which is CE marked and WHO prequalified is the Xpert HCV Viral Load test. Although the currently approved Xpert HCV Viral Load requires sample collection via venepunture and has a time to result of 120 min, a second-generation cartridge is under development enabling blood collection via finger-stick and a time to result of 1 h which has a high sensitivity and specificity [Citation59]. There is considerable potential to improve point-of-care HCV testing with this technology, but it is not yet clinically approved. Also, the time to result is still not ideal and further efforts are needed to develop a truly rapid HCV RNA test with results in 5–10 min. Also, if massive screening efforts in low prevalence populations are to be achieved, there needs to be a significant decrease in the price of point-of-care nucleic acid-based assays. Further efforts are needed to ensure that other novel point-of-care HCV RNA assays are CE marked and WHO prequalified.
6.3. Accelerated product development for HCV diagnostics
Accelerated clinical evaluation and approval of diagnostic tests are needed to have more rapid translation of novel diagnostic discoveries, thereby improving translation into routine clinical practice leading to better provision of HCV services, and improved overall patient health. This is often challenging for diagnostic companies, given often limited resources and multiple competing priorities. However, companies must find ways to accelerate clinical development and support in-country applications for diagnostic approval. This could be achieved through more streamlined collaborations with other stakeholders (researchers, practitioners, government, policy makers, the affected community), particularly with respect to supporting implementation research to translate research outcomes and drive changes in health policy and practice.
One concern that has been raised is that whether the size of the HCV market is big enough to ‘justify’ large-scale investment in HCV diagnostics. However, diagnostic companies need to reevaluate how they assess the market potential for HCV diagnostic tests. Instead of basing projections on the number of people with undiagnosed HCV infection that require HCV testing, companies need to more closely consider the total numbers of people that need to be tested in order to identify those who are positive. The proportion of undiagnosed individuals in countries globally ranges from 20% to 80% and broad-scale test and treat strategies across a broad range of populations will be required to identify the undiagnosed fraction. Further health economic research is needed to better inform decisions about the potential role of HCV point-of-care testing globally.
6.4. Reducing the price of diagnostics for low-income countries
For low-income and even some middle-income countries to achieve the WHO elimination target to improve diagnosis, the prices of diagnostics must come down. Low-price DAA generic therapies are now available in many countries. In some countries, the costs for diagnostic testing actually exceed the cost of HCV therapies. Also, the cost and access to platforms is almost as big a barrier as the cost of a test itself. Some centers or countries actually restrict the use of platforms for a certain disease (e.g. HIV or TB) so it is not able to be used for HCV testing. While many diagnostic companies offer high disease burden country discounts for tests, strategies for lowering the costs for low-income countries, while maintaining incentives for companies to invest in diagnostics for sale in mid- and high-income countries, are urgently required.
6.5. Quality assurance and linkage to central laboratories
The roll out of point-of-care testing must be accompanied by programs to ensure quality assurance. Ongoing maintenance of platforms and ensuring the accuracy of testing performed by health-care workers in the field is critical. In many instances, point-of-care testing occurs in the clinic or a community-based setting where trained laboratory staff are not involved. It is vital to ensure health-care workers involved in testing are trained in the key elements of laboratory quality management including pre- and post-analytical processes to ensure testing is provided to an optimal standard. Where possible, linkage to local central laboratories with existing expertise and a history of long-term history of maintaining quality assurance programs using commercial HCV assays may provide one solution to ensure quality assurance.
7. Expert commentary
We have reached an era where there are now several commercial assays to detect HCV RNA (active HCV infection) in 60–90 min, offering a single visit HCV diagnosis. However, there are still limitations with respect to the time to result, cost, and the need for additional equipment to perform point-of-care HCV RNA testing. Technology is moving at such a rapid pace that in the next 4–5 years, the ultimate goal for the field of HCV diagnostics would be to have a quick (<10 min), user-friendly, and inexpensive diagnostic test for HCV RNA detection. Achieving this goal will require further investment from companies in the diagnostic space, researchers, and nonprofit organizations like the Foundation for Innovative Diagnostics, who are working to unlock the HCV diagnostics market. Hopefully, further investment will enable more rapid evaluation of new technologies, more rapid CE marking and device approvals in other countries, and a greater number of point-of-care HCV RNA assays with WHO prequalification. As we move forward, researchers, practitioners, governments, the affected community, and the diagnostic industry must work together to ensure that novel diagnostic discoveries are translated into clinical practice, thereby improving the health of people living with HCV infection globally and achieving the WHO goal of eliminating HCV infection as a major global public health threat by 2030.
8. Five-year view
In the next 5 years, there will be continued standardized collection of venipuncture samples with shipment and testing within centralized laboratories. There will also be the emergence of decentralized testing models in settings such as drug and alcohol clinics, community health centers, sexual health clinics, prisons, needle and syringe programs, supervised consumption rooms, and other community-based mobile outreach services. Dried-blood spot sample collection with HCV RNA testing will be more common in various settings (particularly those lacking the resources for on-site phlebotomy, particularly rural/remote areas and low- and middle-income countries). Also, there will be a variety of different commercial HCV RNA assays from samples collected from dried blood spots and finger-stick point-of-care HCV RNA assays that will receive regulatory approval (including CE-marking) and obtain WHO prequalification. Technological advances will lead to the development of a quick (<10 min), user-friendly, and inexpensive finger-stick point-of-care assay (perhaps which is battery powered and without the requirement for additional equipment). A single-visit diagnosis will be the norm in most settings. These advances will lead to expanded HCV testing and diagnosis globally, improve linkage to HCV care and treatment, and bring us closer to achieving the WHO HCV elimination targets.
Key issues
Improved HCV diagnostics and point-of-care technologies are urgently needed to improve testing/diagnosis and achieve HCV elimination globally
There are a number of point-of-care assays for HCV antibody detection, but further assays are needed for HCV RNA detection (active infection)
Several commercial assays can detect HCV RNA (active HCV infection) in 60–90 min, offering a single-visit diagnosis
Limitations include the time to result, cost, and the need for additional equipment to perform point-of-care HCV RNA testing
Remaining challenges include improving the number of people diagnosed, achieving more rapid and accurate HCV RNA testing, accelerated product development, price reductions, and quality assurance and linkage to central laboratories
Technology is moving at such a rapid pace that in the next 4–5 years, the ultimate goal for the field of HCV diagnostics would be to have a quick (<10 min), user-friendly, and inexpensive diagnostic test for HCV RNA detection
Declaration of interest
J Grebely is a consultant/advisor and has received research grants from AbbVie, Bristol–Myers Squibb, Cepheid, Gilead Sciences and Merck/MSD. J Grebely is supported by a National Health and Medical Research Council Career Development Fellowship. T Applegate is a consultant/advisor for Cepheid and has received research grants from Abbott Diagnostics and Cepheid. P Cunningham is consultant/advisor and/or has received research support from Abbott, Cepheid, Hologic, and Roche Molecular. JJ Field is consultant/advisor and/or has received research support from AbbVie, Achillion, Boehringer-Ingelheim, Gilead Sciences, Janssen, Merck, Roche and Vertex. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. A reviewer on this manuscript has disclosed participation as an investigator in Cepheid test development in US and is director of SC Liver Research Consortium a clinical site network that helped Cepheid enroll their trial.
Contributors
JG, JJF, PC, and TLA contributed to the commentary concept. JG wrote the first draft of the commentary. All authors contributed to the writing and review of the commentary.
Additional information
Funding
References
- The Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastro Hepatol. 2017;2:161–176.
- Stanaway JD, Flaxman AD, Naghavi M, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the global burden of disease study 2013. Lancet. 2016;388:1081–1088.
- Dore GJ, Feld JJ. Hepatitis C virus therapeutic development: in pursuit of “perfectovir”. Clin Infect Dis. 2015;60:1829–1836.
- WHO. Global health sector strategy on viral hepatitis 2016-2021. Geneva, Switzerland: WHO Press. 2017.
- Schito M, Peter TF, Cavanaugh S, et al. Opportunities and challenges for cost-efficient implementation of new point-of-care diagnostics for HIV and tuberculosis. J Infect Dis. 2012;205 Suppl 2:S169–180.
- Pant Pai N, Balram B, Shivkumar S, et al. Head-to-head comparison of accuracy of a rapid point-of-care HIV test with oral versus whole-blood specimens: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:373–380.
- Drain PK, Hyle EP, Noubary F, et al. Diagnostic point-of-care tests in resource-limited settings. Lancet Infect Dis. 2014;14:239–249.
- Gliddon HD, Peeling RW, Kamb ML, et al. A systematic review and meta-analysis of studies evaluating the performance and operational characteristics of dual point-of-care tests for HIV and syphilis. Sex Transm Infect. 2017 Jul 26. pii: sextrans-2016-053069. doi: 10.1136/sextrans-2016-053069. [Epub ahead of print].
- Drobniewski F, Cooke M, Jordan J, et al. Systematic review, meta-analysis and economic modelling of molecular diagnostic tests for antibiotic resistance in tuberculosis. Health Technol Assess. 2015;19:1-188, vii-viii.
- Causer LM, Hengel B, Natoli L, et al. A field evaluation of a new molecular-based point-of-care test for chlamydia and gonorrhoea in remote Aboriginal health services in Australia. Sex Health. 2015;12:27–33.
- Natoli L, Maher L, Shephard M, et al. Point-of-care testing for chlamydia and gonorrhoea: implications for clinical practice. PloS One. 2014;9:e100518.
- Hislop J, Quayyum Z, Flett G, et al. Systematic review of the clinical effectiveness and cost-effectiveness of rapid point-of-care tests for the detection of genital chlamydia infection in women and men. Health Technol Assess. 2010;14:1-97, iii-iv.
- Causer LM, Kaldor JM, Conway DP, et al. An evaluation of a novel dual treponemal/nontreponemal point-of-care test for syphilis as a tool to distinguish active from past treated infection. Clin Infect Dis. 2015;61:184–191.
- Watchirs Smith LA, Hillman R, Ward J, et al. Point-of-care tests for the diagnosis of Neisseria gonorrhoeae infection: a systematic review of operational and performance characteristics. Sex Transm Infect. 2013;89:320–326.
- Yehia BR, Schranz AJ, Umscheid CA, et al. The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PloS One. 2014;9:e101554.
- Patel RC, Vellozzi C, Smith BD. Results of hepatitis C birth-cohort testing and linkage to care in selected U.S. Sites, 2012-2014. Public Health Rep. 2016;131 Suppl 2:12–19.
- Mera J, Vellozzi C, Hariri S, et al. Identification and clinical management of persons with chronic hepatitis C virus infection - cherokee nation, 2012-2015. MMWR Morb Mortal Wkly Rep. 2016;65:461–466.
- Snow K, Scott N, Clothier HJ, et al. Limited provision of diagnostic services to Victorians living with hepatitis C antibodies, 2001-2012: a multi-level modelling analysis. Aust N Z J Public Health. 2016 Aug 14. doi: 10.1111/1753-6405.12560. [Epub ahead of print].
- Janjua NZ, Kuo M, Yu A, et al. The population level cascade of care for hepatitis C in British Columbia, Canada: the BC Hepatitis Testers Cohort (BC-HTC). EBioMedicine. 2016;12:189–195.
- Iversen J, Grebely J, Catlett B, et al. Estimating the cascade of hepatitis C testing, care and treatment among people who inject drugs in Australia. Int J Drug Policy. 2017 Sep;47:77-85.
- Cox J, Graves L, Marks E, et al. Knowledge, attitudes and behaviours associated with the provision of hepatitis C care by Canadian family physicians. J Viral Hepat. 2011;18:e332–340.
- Gupta L, Shah S, Ward JE. Educational and health service needs of Australian general practitioners in managing hepatitis C. J Gastroenterol Hepatol. 2006;21:694–699.
- Shehab TM, Sonnad SS, Lok AS. Management of hepatitis C patients by primary care physicians in the USA: results of a national survey. J Viral Hepat. 2001;8:377–383.
- Marshall AD, Micallef M, Erratt A, et al. Liver disease knowledge and acceptability of non-invasive liver fibrosis assessment among people who inject drugs in the drug and alcohol setting: the LiveRLife Study. Int J Drug Policy. 2015;26:984–991.
- Treloar C, Hull P, Dore GJ, et al. Knowledge and barriers associated with assessment and treatment for hepatitis C virus infection among people who inject drugs. Drug Alcohol Rev. 2012;31:918–924.
- Busch MP. Insights into the epidemiology, natural history and pathogenesis of hepatitis C virus infection from studies of infected donors and blood product recipients. Transfus Clin Biol. 2001;8:200–206.
- Cox AL, Netski DM, Mosbruger T, et al. Prospective evaluation of community-acquired acute-phase hepatitis C virus infection. Clin Infect Dis. 2005;40:951–958.
- Page-Shafer K, Pappalardo BL, Tobler LH, et al. Testing strategy to identify cases of acute hepatitis C virus (HCV) infection and to project HCV incidence rates. J Clin Microbiol. 2008;46:499–506.
- Glynn SA, Wright DJ, Kleinman SH, et al. Dynamics of viremia in early hepatitis C virus infection. Transfusion. 2005;45:994–1002.
- Chevaliez S, Pawlotsky JM. How to use virological tools for optimal management of chronic hepatitis C. Liver Int. 2009;29 Suppl 1:9–14.
- Seigneres B, Descamps F, Croise R, et al. Multicenter clinical evaluation of the new 3rd generation assay for detection of antibodies against hepatitis C virus on the VIDAS((R)) system. J Clinical Virology: Official Publication Pan Am Soc Clin Virol. 2016;78:20–26.
- Gourlain K, Soulier A, Pellegrin B, et al. Dynamic range of hepatitis C virus RNA quantification with the Cobas Ampliprep-Cobas Amplicor HCV Monitor v2.0 assay. J Clin Microbiol. 2005;43:1669–1673.
- Chevaliez S, Bouvier-Alias M, Pawlotsky JM. Performance of the Abbott real-time PCR assay using m2000sp and m2000rt for hepatitis C virus RNA quantification. J Clin Microbiol. 2009;47:1726–1732.
- Chevaliez S, Dubernet F, Dauvillier C, et al. The new aptima HCV Quant Dx Real-time TMA Assay Accurately Quantifies Hepatitis C Virus Genotype 1-6 RNA. J Clinical Virology: Official Publication Pan Am Soc Clin Virol. 2017;91:5–11.
- McHugh MP, Wu AHB, Chevaliez S, et al. Multicenter Evaluation of the Cepheid Xpert Hepatitis C Virus Viral Load Assay. J Clin Microbiol. 2017;55:1550–1556.
- Pas S, Molenkamp R, Schinkel J, et al. Performance evaluation of the new Roche cobas AmpliPrep/cobas TaqMan HCV test, version 2.0, for detection and quantification of hepatitis C virus RNA. J Clin Microbiol. 2013;51:238–242.
- Grebely J, Page K, Sacks-Davis R, et al. The effects of female sex, viral genotype, and IL28B genotype on spontaneous clearance of acute hepatitis C virus infection. Hepatology. 2014;59:109–120.
- Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13:34–41.
- Freiman JM, Tran TM, Schumacher SG, et al. Hepatitis C core antigen testing for diagnosis of hepatitis C virus infection: a systematic review and meta-analysis. Ann Intern Med. 2016 Sep 6;165(5):345-355.
- EASL. EASL Recommendations on Treatment of Hepatitis C 2016. J Hepatol. 2017;66:153–194.
- Easterbrook PJ, Group WHOGD. Who to test and how to test for chronic hepatitis C infection - 2016 WHO testing guidance for low- and middle-income countries. J Hepatol. 2016;65:S46–66.
- Chevaliez S, Soulier A, Poiteau L, et al. Clinical utility of hepatitis C virus core antigen quantification in patients with chronic hepatitis C. J Clinical Virology: Official Publication Pan Am Soc Clin Virol. 2014;61:145–148.
- Duchesne L, Njouom R, Lissock F, et al. HCV Ag quantification as a one-step procedure in diagnosing chronic hepatitis C infection in Cameroon: the ANRS 12336 study. J Int AIDS Soc. 2017;20:1–8.
- Lamoury FMJ, Soker A, Martinez D, et al. Hepatitis C virus core antigen: a simplified treatment monitoring tool, including for post-treatment relapse. J Clinical Virology: Official Publication Pan Am Soc Clin Virol. 2017;92:32–38.
- Cunningham EB, Applegate TL, Lloyd AR, et al. Mixed HCV infection and reinfection in people who inject drugs–impact on therapy. Nat Rev Gastroenterol Hepatol. 2015;12:218–230.
- Bouchardeau F, Cantaloube JF, Chevaliez S, et al. Improvement of hepatitis C virus (HCV) genotype determination with the new version of the INNO-LiPA HCV assay. J Clin Microbiol. 2007;45:1140–1145.
- Chevaliez S, Rodriguez C, Pawlotsky JM. New virologic tools for management of chronic hepatitis B and C. Gastroenterology. 2012;142:1303–1313 e1301.
- Drobnik A, Judd C, Banach D, et al. Public health implications of rapid hepatitis C screening with an oral swab for community-based organizations serving high-risk populations. Am J Public Health. 2011;101:2151–2155.
- Jewett A, Smith BD, Garfein RS, et al. Field-based performance of three pre-market rapid hepatitis C virus antibody assays in STAHR (Study to Assess Hepatitis C Risk) among young adults who inject drugs in San Diego, CA. J Clin Virol. 2012;54:213–217.
- Smith BD, Teshale E, Jewett A, et al. Performance of premarket rapid hepatitis C virus antibody assays in 4 national human immunodeficiency virus behavioral surveillance system sites. Clin Infect Dis. 2011;53:780–786.
- Smith BD, Drobeniuc J, Jewett A, et al. Evaluation of three rapid screening assays for detection of antibodies to hepatitis C virus. J Infect Dis. 2011;204:825–831.
- Shivkumar S, Peeling R, Jafari Y, et al. Accuracy of rapid and point-of-care screening tests for hepatitis C: a systematic review and meta-analysis. Ann Intern Med. 2012;157:558–566.
- Wong VW, Wong GL, Chim AM, et al. Targeted hepatitis C screening among ex-injection drug users in the community. J Gastroenterol Hepatol. 2014 Jan;29(1):116-120.
- Poiteau L, Soulier A, Rosa I, et al. Performance of rapid diagnostic tests for the detection of antibodies to hepatitis C virus in whole blood collected on dried blood spots. J Viral Hepat. 2016;23:399–401.
- Chevaliez S, Poiteau L, Rosa I, et al. Prospective assessment of rapid diagnostic tests for the detection of antibodies to hepatitis C virus, a tool for improving access to care. Clin Microbiol Infect. 2016;22:459 e451–456.
- Soulier A, Poiteau L, Rosa I, et al. Dried blood spots: a tool to ensure broad access to hepatitis C screening, diagnosis, and treatment monitoring. J Infect Dis. 2016;213:1087–1095.
- Coats JT, Dillon JF. The effect of introducing point-of-care or dried blood spot analysis on the uptake of hepatitis C virus testing in high-risk populations: a systematic review of the literature. Int J Drug Policy. 2015;26:1050–1055.
- Greenman J, Roberts T, Cohn J, et al. Dried blood spot in the genotyping, quantification and storage of HCV RNA: a systematic literature review. J Viral Hepat. 2015;22:353–361.
- Grebely J, Lamoury FMJ, Hajarizadeh B, et al. Evaluation of the Xpert HCV Viral Load point-of-care assay from venepuncture-collected and finger-stick capillary whole-blood samples: a cohort study. Lancet Gastroenterol Hepatol. 2017;2:514–520.
- Day C, White B, Thein H, et al. Experience of hepatitis C testing among injecting drug users in Sydney, Australia. AIDS Care. 2008;20:116–123.
- Gupta E, Agarwala P, Kumar G, et al. Point -of -care testing (POCT) in molecular diagnostics: performance evaluation of GeneXpert HCV RNA test in diagnosing and monitoring of HCV infection. J Clin Virol. 2017;88:46–51.
- Bajis S, Dore GJ, Hajarizadeh B, et al. Interventions to enhance testing, linkage to care and treatment uptake for hepatitis C virus infection among people who inject drugs: a systematic review. Int J Drug Policy. 2017 Sep;47:34-46.
- Meyer JP, Moghimi Y, Marcus R, et al. Evidence-based interventions to enhance assessment, treatment, and adherence in the chronic Hepatitis C care continuum. Int J Drug Policy. 2015;26:922–935.
- Morano JP, Zelenev A, Lombard A, et al. Strategies for hepatitis C testing and linkage to care for vulnerable populations: point-of-care and standard HCV testing in a mobile medical clinic. J Community Health. 2014;39:922–934.
- Bottero J, Boyd A, Gozlan J, et al. Simultaneous human immunodeficiency virus-hepatitis B-hepatitis C point-of-care tests improve outcomes in linkage-to-care: results of a randomized control trial in persons without healthcare coverage. Open Forum Infect Dis. 2015;2:ofv162.
- Beckwith CG, Kurth AE, Bazerman LB, et al. A pilot study of rapid hepatitis C virus testing in the Rhode Island Department of Corrections. J Public Health (Bangkok). 2016;38:130–137.
- McAllister G, Innes H, McLeod A, et al. Uptake of hepatitis C specialist services and treatment following diagnosis by dried blood spot in Scotland. J Clinical Virology: Official Publication Pan Am Soc Clin Virol. 2014;61:359–364.
- MSF. Putting HIV and HCV to the Test: A Product Guide for Point-of-care CD4 Tests and Laboratory-based Point-of-care HIV and HCV Viral Load Tests. Geneva, Switzerland: MSF; 2017.
- Saraswat V, Norris S, De Knegt RJ, et al. Historical epidemiology of hepatitis C virus (HCV) in select countries - volume 2. J Viral Hepat. 2015;22:6–25.
- Liakina V, Hamid S, Tanaka J, et al. Historical epidemiology of hepatitis C virus (HCV) in select countries - volume 3. J Viral Hepat. 2015;22:4–20.
- Bruggmann P, Berg T, Ovrehus AL, et al. Historical epidemiology of hepatitis C virus (HCV) in selected countries. J Viral Hepat. 2014;21:5–33.