ABSTRACT
Background: Here we compare the performance of the high-throughput BD COR System (COR) to the Viper LT System (Viper) using the BD Onclarity HPV assay.
Research Design and Methods: Remnant clinical specimens, contrived specimens in SurePath (BD) and PreservCyt (Hologic) media, and prospective clinical specimens in BD Cervical Brush Diluent (CBD) were tested. Outcomes included intra-laboratory agreement of Onclarity results on COR and inter-system agreement between COR and Viper.
Results: Onclarity reproducibility on COR resulted in standard deviation and correlation of variation of Ct values ranging from 0.14 to 1.98 and 0.49% to 2.15%, respectively, for contrived specimens, and 0.9–3.08 and 2.89–9.21%, respectively, for clinical specimens. In the COR and Viper clinical agreement study, OPA for Onclarity ranged from 97.1%-98.9%, depending on the collection media type. PPA values for pooled, HPV(+) specimens at low positive (C95), and moderate positive (3XC95) target concentrations were ≥95.0% and 100%, respectively; PPA values associated with HPV 16, 18, 31, 45, 33/58, 52, 35/39/68, 51, and 56/59/66, individually, ranged from 93.8%-100%.
Conclusions: Onclarity performance on COR is equivalent to Viper, and is accurate and reproducible for detection of all high-risk HPV genotypes, with a throughput of 330 results from a single 8-hour shift.
1. Introduction
The global molecular diagnostic business is expected to grow to an estimated USD 13.8 billion by 2025 with a compound annual growth rate of 7.1% [Citation1]. In the US that demand is driven, in part, by increased spending for the detection of infectious agents and the consolidation of testing into larger, more efficient testing facilities concurrent with the emergence of larger hospital networks (Integrated Delivery Networks). Thus, there is an urgent need for large, centralized core facilities that can quickly receive and process specimens, obtain test results, and upload the results into the laboratory information system (LIS) for health care provider review in an efficient and effective manner [Citation1,Citation2]. A number of large systems have been introduced into the marketplace that have the ability to produce several hundred tests in a single shift [Citation2–5]. However, these systems do not integrate pre-analytical processing (integration of sorting, aliquoting, vortexing, pre-warming/cooling, and specimen storage) and thus a bottleneck occurs upstream of the automated analytic system(s) that requires multiple pre-analytic systems and manual intervention steps to ‘feed’ these higher throughput systems and leverage their assay capacity [Citation2]. This reduces efficiency, promotes an attendant risk from manual intervention to the chain of custody of the specimens, and permits the very real potential for cross-contamination.
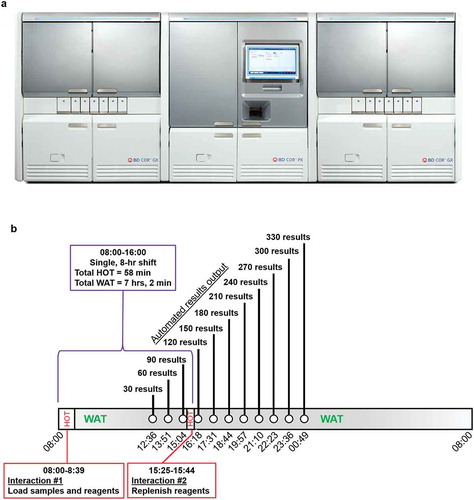
To address these problems, The BD COR™ System (‘COR’; Becton, Dickinson and Company, BD Life Sciences – Integrated Diagnostic Solutions, Sparks, MD, USA) is designed to automate and integrate the entire workflow from specimen processing to result reporting in a single, self-contained system. The system provides a single, flexible, random access portal that enables multiple specimen types for processing and analysis, simultaneously, with minimal user intervention subsequent to primary specimen loading. It accepts multiple specimen types stored in diverse transport media, including cervical brush diluent (CBD; Becton, Dickinson and Company, BD Life Sciences – Integrated Diagnostic Solutions, Burlington, NC, USA) tubes, primary liquid based cytology (LBC) vials (BD SurePath [SurePath; Becton, Dickinson and Company, BD Life Sciences – Integrated Diagnostic Solutions, Burlington, NC, USA] and Hologic PreservCyt® [PreservCyt; Hologic, Inc. Marlborough, MA, USA]), as well as manually aliquoted LBC specimens. The pre-analytical module performs all primary specimen conversions including uncapping/capping, vortexing, aliquoting, heating (as needed), specimen holding (as needed), and control rehydration. The processed specimens and controls are then sent to the analytic module equipped with full assay automation (without user intervention). The system consists of a central pre-analytical module (PX) that can be configured with each of two different analytical modules (GX: the HPV analytical module and MX: the molecular diagnostics analytical module). Depending on the particular needs of the laboratory, the pre-analytical PX module can be connected with up to two independent GX or MX modules, thus offering flexible design options. User intervention is minimal and the system has high throughput with an individual GX capable of producing 540 results in 24 hours. The system can also access and process samples automatically if the test orders originate in the LIS.
Here we illustrate the utility of the COR System using the BD Onclarity™ HPV Assay (‘Onclarity;’ Becton, Dickinson and Company, BD Life Sciences – Integrated Diagnostic Solutions, Sparks, MD, USA), a 15-target (14 HPV genotypes and 1 internal control), multiplex, real-time PCR assay for cervical cancer screening. The Centers for Disease Control and Prevention estimate that approximately 70% of US women get a Pap smear [Citation6] and surveys from the College of American Pathologists estimate over 60% of labs offer the option of HPV genotyping [Citation7]. Onclarity is compatible with multiple form factors, including SurePath and PreservCyt LBC media, and CBD. From a lab workflow perspective, cervical cancer screening requires both an absolute chain of custody (result reporting directly tied to diagnosis) and strict cross-contamination countermeasures to avoid misdiagnosis. The COR GX instrument is designed to run Onclarity with additional assays running on the MX instrument. Therefore, Onclarity is a useful test case for the COR system since it is a high complexity, multi-target assay that requires high throughput. To our knowledge, the COR system is the first fully integrated ‘specimen processing to results output’ system that can process cervical specimens. For Onclarity, a single operator can achieve 330 results from an 8-hour shift with just two instrument interventions (once in the morning to load the LBC vials, consumables, and reagents; and once in the late afternoon to replenish supplies), with a total walk-away time of 7 hours.
Onclarity received FDA approval in 2018 with claims for HPV primary screening, co-testing with cytology, and triage for patients with ASC-US cytology [Citation8–13]. It was initially approved in the US to detect HPV 16, HPV 18, and HPV 45 with the 11 other types as a group, and received additional approval to report all genotypes in 2020 – the 9-valent vaccine types (HPV 31, HPV 52, or HPV 33/58 [pooled]), and the remaining seven high-risk types, which are less likely to cause cervical disease (HPV 51, HPV 35/39/68 [pooled], and HPV 56/59/66 [pooled]) [Citation14,Citation15]. Thus, it is well positioned to respond to changes in the US cervical cancer management guidelines announced by the ASCCP in April 2020 [Citation16,Citation17] and the corresponding screening guidelines published by the American Cancer Society in July 2020 [Citation18] with their focus on HPV testing and quantitative risk estimates to guide patient management. In addition, Onclarity was also clinically validated against international criteria for HPV testing within the framework of cervical cancer screening [Citation19].
Here we describe the performance of the assay (limit of detection [LOD] confirmation, precision and reproducibility, percent agreement for Onclarity results tested on COR and compared to Viper LT in a clinical agreement study, endogenous/exogenous interfering substances) as well as provide an overview of the system capabilities (including cross-contamination) and assay throughput.
2. Patients and methods
2.1. COR instrument throughput
The BD COR system ()) is capable of working completely unattended with a daily walk-away time for HPV testing of approximately 7 hours. The system is capable of producing 330 results with just two interventions during the day, the first to load primary (e.g. liquid-based cytology) vials and reagents and the second in the afternoon to replenish consumables ()). System throughput was validated at two different clinical sites as part of this study.
Figure 1. (a) PX, GX, and MX modules that constitute the BD COR system. The PX module automates pre-analytical sample processing and associated steps for each sample type. The PX module is the operational core of the system, upon which the GX module depends. This instrument is specifically designed to automatically perform a number of manual steps that are similar to Viper LT sample processing. All specimen loading and unloading occurs at the PX instrument. Pre-analytical steps performed via the PX include sample vortexing, aliquoting clinical specimens into a molecular tube with the correct diluent, sorting/grouping of samples, sample pre-warming and cooling (where required), and sample transport to the GX module. Automated extraction and amplification/detection occurs within the GX module. GX is designed to perform the Onclarity Assay using core technology that exists in the Viper LT system; however, GX is designed for a larger capacity. The MX module can also be utilized with PX to perform various molecular assays for diagnostic purposes that are not specifically addressed in this report. The COR system allows for multiple configurations that are centered on the PX module including GX:PX:MX, GX:PX:GX, MX:PX:MX, GX:PX, and MX:PX. (1b). Throughput of specimen processing and analysis via PX-GX modules within the BD COR System. GX throughput was validated as part of this study and is capable of delivering 330 HPV results in an 8-hour shift, with just two contact points – one in the morning to load samples and reagents, and a second brief interaction in the afternoon to replenish supplies to enable the system to complete this number of specimens
2.2. COR analytical sensitivity confirmation (LOD), precision, and reproducibility studies
The analytical sensitivity of Onclarity on COR was evaluated by confirming previously established LODs for Onclarity on Viper LT. Clinical specimens in SurePath LBC, PreservCyt LBC, or CBD media were screened for all 14 high-risk HPV targets using Onclarity on COR or Viper LT systems and found to be negative for HPV 16, 18, and 45 genotypes. LODs for HPV 16, 18, and 45 were determined as described previously on Viper LT [Citation20]. Serial dilution of HPV positive cell lines, SiHa (HPV 16 positive), HeLa (HPV 18 positive), and MS751 (HPV 45 positive) spiked into SurePath and PreservCyt clinical specimens, and Onclarity specimen diluent for CBD specimens.
Probit analysis was performed on COR to calculate high-negative specimens (C5; specimens called positive approximately 5% and negative 95% of the time), low-positive specimens (C95; specimens called positive approximately 95% and negative 5% of the time), and moderate-positive specimens (3x C95; specimens approximately 3 times above the C95 level and expected to be positive 100% of the time), relative to the clinical cutoff of Onclarity. HPV negative C33A cells were used to generate negative panels. Target genotypes were co-spiked into a single tube with mixed HPV target levels. For example, HPV 16 high negative, HPV 18 low positive, and HPV 45 moderate positive might be present in a single tube. In addition to contrived specimen utilization, positive and negative clinical SurePath and PreservCyt specimens were used to prepare panels representing HPV 16, 18, 45, 31, 33/58, and 52 genotypes at a moderate positive concentration. CBD specimens were only utilized as contrived specimens and without any clinical positive specimens.
For LOD confirmation, each panel member was tested by two analysts across a total of 15 days with 3 lots of reagents, yielding a minimum of 90 replicates for each panel member per specimen. For each specimen type and positivity level, paired specimens were analyzed on Viper LT to assess the equivalence to the previous (Viper LT) LOD. Comparison of cycle threshold (Ct) scores was made between the test system (COR) and reference system (Viper LT) using a two-sample, t-test (one-sided) for equivalence. A predefined clinically appropriate equivalence margin of 0.75 Ct score relative to the mean was established. Equivalence is established when the 95% confidence interval for the mean difference in Ct scores between the test and reference system was contained within the equivalence margin.
Precision and reproducibility experiments were conducted for SurePath and PreservCyt LBC media types at two external locations and one internal (BD Life Sciences – Integrated Diagnostic Solutions, Sparks, MD, USA) location; precision experiments were conducted at the internal location. Precision was evaluated by testing two runs per day over a period of 12 days across two operators. Reproducibility studies were conducted over a period of 5 days with two runs per day. Variance component analysis of Ct score was performed to evaluate within run (repeatability), between run, between day, and total (reproducibility) variability for each HPV target and viral load using SAS 9.4 PROC MIXED statistical software.
2.3. Clinical comparison studies
Remnants from prospectively collected SurePath and PreservCyt clinical specimens from the BD Onclarity Clinical Trial (NCT01944722) were utilized to create panels as either individual specimens, pooled positive clinical specimens, or negative clinical specimens spiked with positive clinical specimen or HPV+ cell line(s). Prospective collection occurred for all CBD specimens used in the agreement study. HPV 16, 18, and 45 panel members for SurePath and PreservCyt specimens were prepared such that 60–80% of the specimens were near the clinical cutoff (HPV 16: ≥32.3 and ≤38.3 Ct; non-HPV 16: ≥28.2 and ≤34.2 Ct). The remaining positives were prepared as high positives (HPV 16: ≥10 and <32.3 Ct; non-HPV 16: ≥10 and <28.2 Ct). Negative panels were prepared so that 30–40% were high negatives and the remaining were true negatives. The 11 other genotypes (those tested in the G2 PCR tube [HPV 31, 33/58, and 56/59/66] and G3 PCR tube [HPV 51, 52, and 35/39/68]) were grouped and distributed such that 60–80% of the positives were near the cutoff, with the remaining positives covering the analytical range of the assay. Although the testing primarily involved clinical specimens, in some cases, contrived specimens were used to increase certain genotype group numbers. Each specimen was tested once on COR and once on Viper LT in a randomized order. Each specimen was pre-warmed once, which occurred on the first system used for testing of that specimen. The panels were tested on COR at three different sites (two external sites and one internal site). All Viper LT testing occurred at BD Life Sciences – Integrated Diagnostic Solutions (Sparks, MD, USA) and Viper LT results served as reference. CBD testing on COR and Viper LT was performed internally at BD.
To determine system equivalence, results from the COR were compared to those from Viper LT. Clinical testing was conducted across three studies (one per specimen type). Only specimens with a valid result on both systems were included in the analysis. Positive percent agreement (PPA), negative percent agreement (NPA), and overall percent agreement (OPA) values between COR and Viper LT were calculated along with 95% confidence intervals (CI). In addition, performance was stratified by instrument and genotype information (G1: HPV 16, 18, and 45, G2: HPV 31, 33/58, and 56/59/66 and G3: HPV 51, 52, and 35/39/68). For the SurePath and PreservCyt testing, PPA was stratified by specimen category (true negative, high negative, low positive, moderate positive, high positive). Scatterplots by Ct score were generated for COR versus Viper LT with descriptive statistics for all instruments combined. Deming regression [Citation21] was performed to estimate the systematic difference between the Ct score generated by COR and Viper LT for all instruments combined. Paired t-tests with 95% confidence intervals were performed to estimate the systematic difference between the Ct score generated by COR and Viper LT across all genotypes combined.
2.4. Interfering and contamination studies
To determine the impact of exogenous or endogenous substances on the detection of HPV 16, HPV 18, or HPV 45 by Onclarity on the COR, contrived specimens were prepared at moderate positive concentrations as described above (using SiHa, HeLa, or MS751 cell lines) in SurePath or PreservCyt media. The three cell lines were combined and tested as mixed specimens. C33A cells were used for HPV-negative studies (cellular background only). Six interfering substances were tested on both positive and negative specimens and 24 replicates were tested per media type (two), per substance (six), and per positive or negative HPV matrix (total number of replicates was 576). Studies of well-to-well contamination, cross-over (within the same run), or carry-over (run-to-run) were also performed on the COR in SurePath and PreservCyt LBC media. The study design utilized a ‘checkerboard’ specimen loading pattern where positive and negative specimens were alternated during processing. High positive specimens in both LBC media types were prepared using CaSki cells diluted into the LBC at a concentration of ~8×104 cells/mL. The execution schedule included Onclarity testing covering six consecutive runs (30 reactions per run), on three separate COR systems per day, for 5 days; resulting in a minimum of 1,350 positive and 1,350 negative specimens for both SurePath and PreservCyt LBC media types. The acceptance criteria for COR to pass cross- and carry-over contamination was a ≤ 0.5% false positive rate among the negative specimens in runs comprising a 50% positivity rate.
3. Results
3.1. Limit of detection confirmation and assay reproducibility for Onclarity on COR
Contrived specimens (created using cells expressing HPV 16 [SiHa], HPV 18 [HeLa], and HPV 45 [MS751]) were utilized for LOD analysis with the Onclarity assay on the COR system. Using the mean difference in Ct values for Onclarity on COR and Viper LT as the criteria for LOD confirmation, equivalence was established between the two systems for detection of HPV 16, 18, and 45 (used as representative genotypes) in SurePath (n = 1,620), PreservCyt (n = 1,620), and CBD (n = 1,780) (Table S1).
Contrived (n = 1,473; positive for HPV 16, 18, and 45) and clinical specimens (n = 1,077; positive for HPV 16, 18, 45, 31, 33/58, or 52), prepared in SurePath or PreservCyt LBC media, were utilized for reproducibility testing on the COR system (). The highest level of standard deviation (SD) and percent coefficient of variation (%CV) came from within-run assay testing (aliquot to aliquot repeatability) for both contrived and pooled clinical specimens. For the within-run reproducibility analysis on specimens prepared in SurePath, the SD and %CV values for the contrived specimens ranged from 0.16 to 0.81 and from 0.52% to 2.23%, respectively; for the pooled clinical specimens, the SD and %CV values ranged from 0.70 to 2.16 and 2.32% to 6.65%, respectively. For the within-run reproducibility analysis on specimens prepared in PreservCyt, the SD and %CV values for the contrived specimens ranged from 0.10 to 0.66 and from 0.36% to 1.98%, respectively; for the pooled clinical specimens, the SD and %CV values ranged from 0.80 to 3.08 and 2.58% to 9.21%, respectively. Between site, between day, and between run reproducibility analyses showed lower variance values for both contrived and pooled clinical specimens ().
Table 1. Variance component analysis – site to site reproducibility for the Onclarity assay results on the COR system
3.2. COR analytical and clinical agreement with Viper LT using Onclarity assay
For studies to determine Onclarity assay agreement in SurePath (n = 940) and PreservCyt (n = 930) media on COR versus Viper LT, specimen panels were created that consisted of a combination of clinical and contrived specimens (Table S2). The CBD agreement study leveraged prospectively collected individual samples. As shown in (), PPA values for contrived and pooled clinical specimens, combined, were 98.3% (95% CI: 96.9, 99.1; n = 48 and n = 533, respectively), 98.6% (95% CI: 97.3, 99.3; n = 13 and n = 574, respectively), and 97.5% (95% CI: 94.2, 98.9; n = 198; clinical specimens only) for SurePath, PreservCyt, and CBD media, respectively. NPA values for contrived and pooled clinical specimens, combined, were 95.3% (95% CI: 92.5, 97.0; n = 12 and n = 347, respectively), 95.9% (95% CI: 93.3, 97.6; n = 13 and n = 341, respectively), and 100% (95% CI: 98.6, 100; n = 277; clinical specimens only) for SurePath, PreservCyt, and CBD media, respectively. The OPA values were similar for SurePath (97.1% [95% CI: 95.9, 98.0]), PreservCyt (97.6% [96.4, 98.4]), and CBD (98.9% [97.6, 99.5]). The PPA and NPA were lower around the clinical cutoff (low positive and high negative) as expected due to higher variability at those levels (Table S3). Table S4 represents the PPA and NPA values for SurePath, PreservCyt, and CBD media stratified by different COR instruments (both internal and external study sites). PPA (low, moderate, and high positive) values ranged from 95.0% to 100% across instruments and media types. NPA (low and high negative) values ranged from 94.9% to 100% across instruments and media types.
Table 2. Performance agreement with specimen type for Onclarity results between COR and Viper LT systems – PPA, NPA, and OPA
PPA was calculated for all nine HPV genotype results reported by Onclarity (representing six individual genotype results and three pooled results) in specimens prepared in SurePath and PreservCyt (). For specimens in SurePath, the PPA ranged from 93.8% to 100%; for PreservCyt, the values ranged from 94.1% to 100%. No trend was observed in which PPA values were higher or lower based on a given genotype result in any of the media types.
Table 3. Positive percent agreement with HPV genotype using the Onclarity assay on the COR system versus the Viper LT system
Analyses were performed for all specimens (contrived and pooled clinical) combined within each clinical matrix media type to determine the mean cycle threshold for an HPV positive result by the Onclarity assay when performed on the COR or Viper LT (). The average mean Ct scores were close between SurePath and PreservCyt specimens on both the COR (31.21 and 30.87, respectively) and Viper LT (31.37 and 30.97, respectively). For CBD, the mean Ct scores on the COR and Viper LT were 25.97 and 25.70, respectively. Deming regression was performed to determine any systematic differences between the COR and Viper LT platforms regarding Onclarity assay performance (). The regression analysis resulted in Pearson’s r values close to +1 for runs conducted in all three media types.
Figure 2. Deming regression plots which represent linear regression, incorporates values for errors in observations generated by both Viper LT (x-axis) and COR (y-axis) for (a) SurePath, (b) PreservCyt, and (c) CBD media types are shown. All specimen results with a Ct value <40 were included in the analysis shown
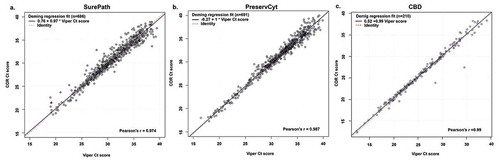
Table 4. Ct score comparisons for Onclarity assay results between the COR system and the Viper LT system
3.3. Interfering substances and contamination
Onclarity on COR was challenged with endogenous and exogenous substances that may be present in clinical specimens (Table S5). For each substance, serial titrations were prepared and evaluated in the presence and absence of representative HPV targets for the possibility of false negative and positive results, respectively. At the concentrations of substances shown, no false negatives in HPV-positive specimens were observed and no false positives were detected in HPV-negative specimens (Figure S1).
The COR system is designed to automate the nucleic acid extraction and PCR steps in a high-throughput manner, which are traditionally performed manually in a low throughput setting. Using HPV 16 high-positive specimens, Onclarity on COR produced zero false positive results in both SurePath or PreservCyt LBC, and one false positive in CBD; resulting in a contamination rate of 0.0%, 0.0%, and 0.22%, respectively (Figure S2).
4. Discussion
This was a migration study to test both the reproducibility and the agreement of Onclarity HPV results, in three different specimen types (SurePath, PreservCyt, and CBD), when performed on a new high-throughput system, COR, compared to results on Viper LT. The reproducibility results observed here for the Onclarity assay regarding contrived and clinical specimens are consistent with previous work. For example, Ejegod and colleagues showed high reproducibility with 98.6% intra-laboratory agreement (kappa value of 0.967) and 98.4% inter-laboratory agreement (kappa value of 0.962) for Onclarity assay results from specimens collected in PreservCyt media in a subpopulation of an English screening population [Citation22]. Ejegod and colleagues also published good intra- and inter-laboratory reproducibility for Onclarity in SurePath specimens in a study from a Danish population [Citation23]. As previously observed for the Onclarity assay on the Viper LT [Citation20], the greatest variation on COR for both contrived and clinical specimens was seen within assay runs. LBC specimens are inherently non-homogenous as they are comprised of exfoliated sheaths of fixed cells. In addition, HPV infections are focal in nature (HPV is a non-lytic virus). These conditions act to increase the Ct score spread between aliquots, even though the mean Ct score is largely unaffected. The other factors, including between site, between day, and between runs, showed relatively low variation in results compared to that observed for the within-run results. Therefore, as with Onclarity results on Viper LT, Onclarity results on COR should be consistent regardless of external factors (site location, operator, time of day) when utilized according to the manufacturer’s instructions.
Onclarity results for HPV 16, 18, and 45 on Viper LT were previously shown to have at least a 98% PPA with observed results for contrived specimens using SurePath LBC. The PPA values in this study with expected results for HPV 16, 18, or 45 on contrived specimens prepared in SurePath, PreservCyt, and CBD were 100%, ≥98.9%, and ≥94.0%, respectively, for COR and Viper LT (Table S1), and the equivalence criteria for Onclarity performance between the two systems were met. Moreover, OPA values for Onclarity on COR, versus that on Viper LT, ranged from 97.1%-98.9%, depending on the collection media used (). In addition, differences in mean Ct scores between Viper LT and COR for HPV (+) specimens across all three media types ranged from −0.169 to 0.2749 (), which were within the predetermined criteria for establishing equivalence.
Onclarity was originally developed and validated for the specificity of the 14 high-risk target genotypes present in the assay for use with the Viper LT [Citation24]. Validation of Onclarity with microorganisms that may be present in cervical specimens resulted in no detection of any potential cross-reactants [Citation25]. Since the assay chemistry has not been changed for use in COR, no cross-reactivity is expected for viruses, bacteria, fungi and low-risk HPV types. Interference observed was limited to a few compounds and blood, which after testing did not result in assay inhibition or false positives.
When challenged with high viral load positive samples run in a checkerboard pattern with negative samples, COR did not generate false positives for LBC and less than 0.5% for CBD specimens. Therefore, the likelihood of a result being a false positive is very low, which is especially critical in HPV primary screening populations, given the high-throughput sample processing of BD COR.
Results from the submitted work (personal communication from Ejegod et al. 2021) show that the COR System has been clinically validated using the Meijer criteria comparator assay GP5+/6+ enzyme immune assay. On the Viper LT system, Onclarity is internationally validated [Citation19,Citation26] and is FDA-approved and CE marked for detection of HPV during routine screening. It has also been validated against the WHO LabNet panel, validated against Meijer criteria, and has been compared to and validated against other established HPV assays in clinical comparison studies [Citation22,Citation23,Citation27,Citation28], studies that include referral populations [Citation12,Citation13,Citation15,Citation27,Citation29–32], retrospective studies [Citation33,Citation34], and large screening population studies [Citation9,Citation10,Citation14,Citation22,Citation23,Citation35,Citation36]. In addition, Onclarity is clinically validated for HPV detection from both SurePath and PreservCyt LBC media types [Citation37,Citation38]. Recently, FDA approval was granted for Onclarity on the Viper LT to provide extended genotyping information during routine screening to facilitate risk-based information for individuals based on their individual genotype results. Thus, Onclarity provides screening information that aligns with the current ASCCP guidelines for risk-based screening in order to help optimize the balance between sensitivity and specificity for individuals undergoing cervical cancer screening. The results here show good agreement between Onclarity performance on COR for the detection of all nine genotyping results with Onclarity results on Viper LT in both SurePath (ranging from 93.8% to 100%) and PreservCyt (ranging from 94.1% to 100%) media (). This is in agreement with previous work in which acceptable clinical and analytical performance have been established for Onclarity for extended genotyping [Citation14,Citation15,Citation27,Citation30,Citation32,Citation33,Citation39], and has been shown for specimens with both mixed and single infections.
The BD COR System is also designed with reflex testing of primary samples in mind such as is often the case for primary HPV testing, where a positive result may trigger a request for cytology triage. Processed samples are stored in barcoded racks that can easily be retrieved from storage, where positive vials can then be selected for additional testing. In addition, the BD Totalys™ System is a cytology-based automation system that automates slide preparation but can also aliquot a sample for molecular testing either before or after the cytology slide is made. These molecular tubes can then be placed directly on the BD COR System for automated HPV screening. Moving forward, results from Onclarity on COR should be effective in identifying women with NILM cytology who are at high risk for cervical disease, based on HPV results, and require a colposcopy referral. Likewise, Onclarity results on COR should also be able to identify women with equivocal and low-grade cytology that is recommended for follow-up after 1 year, in lieu of an immediate colposcopy, based on an infection with genotypes that confer low risk of cervical disease.
COR is currently the only high throughput fully integrated pre-analytic and analytic system on the market, enabling seamless chain of custody and eliminating the need to transfer primary and secondary vials between systems. This dramatically reduces hands-on time and the number of user interventions, while reducing the risk of contamination or a breach in specimen to result in a chain of custody. The configurability allows each lab to customize the number of GX and MX units needed to meet its testing demands and facilitate test volume expansion without adding additional pre-analytic systems. The ability to test directly from the primary collection vials provides both convenience and flexibility, allowing both LBC media types to be run in a fully automated fashion using a single instrument.
4.1. Limitations
Some of the clinical specimens used in this study were obtained from the Onclarity cervical cancer screening trial, a large study conducted in the US that provided evidence for the pre-market application for Onclarity with the FDA. Therefore, some types of bias associated with the Onclarity trial design or procedures could be applied to these analyses. Some types of partial verification bias when stratifying results based on age or cytology result are possible. Although this was previously addressed in Onclarity reports through statistical methodology to adjust for verification bias, this was not addressed here, as the main goal was to compare results between Onclarity agreement on COR versus Viper LT.
4.2. Conclusions
Overall, the results here characterize the analytical results on contrived and clinical specimens for Onclarity performance on COR versus Viper LT. This migration study shows that Onclarity produces similar results, regardless of the specimen type (i.e. SurePath, PreservCyt, or CBD), on both COR and Viper LT. The clinical data presented here suggest that Onclarity should perform well on COR in a clinical setting and should be an effective assay for risk-based screening through extended genotyping during routine cervical cancer screening, and as a tool for the follow up and management of women with screening or treatment history. Finally, the BD COR System offers the first full end-to-end automation solution that can process both LBC specimen types directly from the vial.
Declaration of interest
K Eckert, M Van Sickler, JA Price, E Gutierrez, AA Rucki, M Lizzi, DM Wolfe, JM Harris, SM Gregory, and L Vaughan are employees of Becton, Dickinson and Company, the study sponsor. All non-BD employees received research funding as part of this study. SN Taylor reports receiving grants to her institution from Becton Dickinson, Hologic, Cepheid, binx, Rheonix, Roche, and Abbott Molecular. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
One peer reviewer has worked on trials which involve independent evaluation of HPV assays on cervical and vaginal self-samples. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Supplemental Material
Download MS Word (762.5 KB)Acknowledgments
The authors would like to thank Devin S. Gary, PhD (Becton, Dickinson and Company, BD Life Sciences – Diagnostic Solutions), for his input on the content of this manuscript and editorial assistance. The authors also thank Aojun Li and Indrias Berhane (Becton, Dickinson and Company, BD Life Sciences – Diagnostic Systems Solutions) for their statistical support. The authors also thank the BD employees who worked to establish the data presented in this report. The individuals acknowledged here have no additional funding or additional compensation to disclose.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
Notes
Abbreviations: PPA, positive percent agreement; NPA, negative percent agreement; OPA, overall percent agreement; CI, confidence interval; CBD, Cervical Brush Diluent
References
- Molecular diagnostics market report, fortune business insights; 2019. https://www.fortunebusinessinsights.com/industry-reports/molecular-diagnostics-market-100086. December 01, 2020
- Public health England guidance – Cervical screening: acceptable HPV tests; 2019. [Cited 2021 Dec 01]. Avaialable from: https://www.gov.uk/government/publications/cervical-screening-acceptable-hpv-tests/cervical-screening-acceptable-hpv-tests
- Aretzweiler G, Leuchter S, Simon CO, et al. Generating timely molecular diagnostic test results: workflow comparison of the cobas® 6800/8800 to panther. Expert Rev Mol Diagn. 2019;19(10):951–957.*
- Oštrbenk Valenčak A, Šterbenc A, Seme K, et al. Alinity m HR HPV assay fulfills criteria for human papillomavirus test requirements in cervical cancer screening settings. J Clin Microbiol. 2019;58(1):e01120–01119.
- Saville M, Sultana F, Malloy MJ, et al. Clinical validation of the cobas HPV test on the cobas 6800 system for the purpose of cervical screening. J Clin Microbiol. 2019;57(2):e01239–01218.
- Centers for Disease Control and Prevention. Use of pap smears among women aged 18 and over, by selected characteristics: United States, selected years 1987–2015; 2015. [Cited 2021 Dec 01]. Available from: https://www.cdc.gov/nchs/hus/contents2018.htm#Table_034
- Zhao C, Crothers BA, Ghofrani M, et al. Human papillomavirus genotyping testing practice in 2014: results of a college of American pathologists national survey. Arch Pathol Lab Med. 2016;140(12):1364–1370.
- Stoler MH, Wright TC, Parvu V, et al. The Onclarity human papillomavirus trial: design, methods, and baseline results. Gynecol Oncol. 2018;149(3):498–505.
- Stoler MH, Wright TC Jr., Parvu V, et al. Stratified risk of high-grade cervical disease using onclarity HPV extended genotyping in women, >/=25years of age, with NILM cytology. Gynecol Oncol. 2019;153(1):26–33.
- Stoler MH, Wright TC, Parvu V, et al. HPV testing with 16, 18, and 45 genotyping stratifies cancer risk for women with normal cytology. Am J Clin Pathol. 2019;151(4):433–442.
- Wright TC Jr., Parvu V, Stoler MH, et al. HPV infections and cytologic abnormalities in vaccinated women 21-34 years of age: results from the baseline phase of the Onclarity trial. Gynecol Oncol. 2019;153(2):259–265.
- Wright TC Jr., Stoler MH, Parvu V, et al. Risk detection for high-grade cervical disease using Onclarity HPV extended genotyping in women, >/=21 years of age, with ASC-US or LSIL cytology. Gynecol Oncol. 2019;154(2):360–367.
- Wright TC Jr., Stoler MH, Parvu V, et al. Detection of cervical neoplasia by human papillomavirus testing in an atypical squamous cells-undetermined significance population: results of the Becton Dickinson Onclarity trial. Am J Clin Pathol. 2019;151(1):53–62.
- Schiffman M, Hyun N, Raine-Bennett TR, et al. A cohort study of cervical screening using partial HPV typing and cytology triage. Int J Cancer. 2016;139(11):2606–2615.
- Schiffman M, Vaughan LM, Raine-Bennett TR, et al. A study of HPV typing for the management of HPV-positive ASC-US cervical cytologic results. Gynecol Oncol. 2015;138(3):573–578.
- Schiffman M, Wentzensen N, Perkins RB, et al. An Introduction to the 2019 ASCCP risk-based management consensus guidelines. J Low Genit Tract Dis. 2020;24(2):87–89.
- Cheung LC, Egemen D, Chen X, et al. 2019 ASCCP risk-based management consensus guidelines. J Low Genit Tract Dis. 2020;24(2):90–101.
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70(5):321-346.
- Arbyn M, Snijders PJ, Meijer CJ, et al. Which high-risk HPV assays fulfil criteria for use in primary cervical cancer screening? Clin Microbiol Infect. 2015;21(9):817–826.
- Young S, Vaughan L, Yanson K, et al. Analytical and clinical sample performance characteristics of the Onclarity assay for the detection of human papillomavirus. J Clin Microbiol. 2020;59(1). DOI:10.1128/JCM.02048-20.
- Trevisan A, Schlecht NF, Ramanakumar AV, et al. Human papillomavirus type 16 viral load measurement as a predictor of infection clearance. J Gen Virol. 2013;94(Pt 8):1850–1857.
- Ejegod DM. Clinical validation of the BD Onclarity? HPV assay using a non-inferiority test. J Med Microbiol Diagn. 2015;s3. DOI:10.4172/2161-0703.S3-003
- Ejegod D, Bottari F, Pedersen H, et al. BD Onclarity HPV assay on samples collected in surepath medium meets the international guidelines for human papillomavirus test requirements for cervical screening. J Clin Microbiol. 2016;54(9):2267–2272.
- BD Onclarity™ HPV assay for the BD viper LT™ system. CE Mark Instructions for use, Ref 442946; 2019.
- BD Onclarity™ HPV assay [package insert]. Becton, Dickinson and Company, Sparks-Glencoe, MD; 2018.
- Meijer CJLM, Berkhof J, Castle PE, et al. Guidelines for human papillomavirus DNA test requirements for primary cervical cancer screening in women 30 years and older. Int J Cancer. 2009;124(3):516–520.
- Ejegod DM, Junge J, Franzmann M, et al. Clinical and analytical performance of the BD Onclarity HPV assay for detection of CIN2+ lesions on SurePath samples. Papillomavirus Res. 2016;2:31–37.
- Wright TC Jr., Stoler MH, Agreda PM, et al. Clinical performance of the BD Onclarity HPV assay using an adjudicated cohort of BD SurePath liquid-based cytology specimens. Am J Clin Pathol. 2014;142(1):43–50.
- Bottari F, Iacobone AD, Boveri S, et al. Onclarity human papillomavirus extended genotyping in the management of cervical intraepithelial neoplasia 2+ lesions. J Low Genit Tract Dis. 2019;23(1):39–42.
- Castle PE, Gutierrez EC, Leitch SV, et al. Evaluation of a new DNA test for detection of carcinogenic human papillomavirus. J Clin Microbiol. 2011;49(8):3029–3032.
- Cuschieri K, Geraets DT, Moore C, et al. Analytical performance of the Onclarity HPV assay using the VALGENT framework. J Clin Microbiol. 2015;53(10):3272–3279.
- Nakamura M, Nakade K, Orisaka S, et al. Comparison study of BD Onclarity HPV with digene HC2 high-risk HPV DNA test and Roche Cobas 4800 HPV for detecting high-risk human papillomavirus in Japan. Am J Clin Pathol. 2019;151(3):263–269.
- Arbyn M, Depuydt C, Benoy I, et al. VALGENT: a protocol for clinical validation of human papillomavirus assays. J Clin Virol. 2016;76(Suppl 1):S14–s21.
- Bonde J, Ejegod DM, Cuschieri K, et al. The Valgent4 protocol: robust analytical and clinical validation of 11 HPV assays with genotyping on cervical samples collected in SurePath medium. J Clin Virol. 2018;108:64–71.
- Cuzick J, Cadman L, Mesher D, et al. Comparing the performance of six human papillomavirus tests in a screening population. Br J Cancer. 2013;108(4):908–913.
- Ogilvie GS, Van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. Jama. 2018;320(1):343–52.
- Bonde JH, Pedersen H, Quint W, et al. Clinical and analytical performance of the BD Onclarity HPV assay with surepath screening samples from the Danish cervical screening program using the VALGENT framework. J Clin Microbiol. 2020;58(2). DOI:10.1128/JCM.01518-19.
- Ejegod DM, Serrano I, Cuschieri KS, et al. Clinical validation of the BD Onclarity™ HPV assay using a non-inferiority test. J Med Microb Diagn. 2013;S3:003.
- Ejegod DM, Pedersen H, Alzua GP, et al. Time and temperature dependent analytical stability of dry-collected Evalyn HPV self-sampling brush for cervical cancer screening. Papillomavirus Res. 2018;5:192–200.
