1. Introduction
Routinely, patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) are screened for mutations and fusions to direct therapy decision. The majority of molecular laboratories perform a multitude of different tests for the work up of all biomarkers. Especially with the fast emerging development of new drugs, testing for specific fusion genes has gained much attention within the last years.
RNA-Seq can be achieved by either comprehensive whole transcriptome- or targeted approaches [Citation1]. Both allow the expression analysis of a defined gene set. Targeted RNA-sequencing, as a method for enrichment-sequencing of specific transcripts, is of growing importance in molecular diagnostics. Depending on the technology used, enrichment-based approaches further enable the detection of known and novel gene fusion events. In comparison with whole-transcriptome RNA-Seq, targeted RNA-Seq, focuses only on selected transcripts, thereby allowing increased sensitivity, reduced costs as well as increased throughput opportunities [Citation2].
According to the statement of the ESMO Precision Medicine Working Group, multigene sequencing on DNA or RNA level is highly recommended to increase patient´s access to approved or innovative drugs and speeding up clinical research [Citation3].
2. Current status of fusion detection in NSCLC
Since the first discovery of chromosomal rearrangements as oncogenic drivers, a large number of fusions in various tumor types could be detected through technological advancements [Citation4]. The method, which is currently most widely used, for formalin-fixed, paraffin-embedded (FFPE) tissue is in situ hybridization (ISH). Due to its high sensitivity, specificity and short turnaround time, this assay is still the gold standard in most laboratories. However, ISH analysis suffers from resolution limits and complex rearrangements are not easily detectable and can lead to false-negative results [Citation5].
Regarding fusions leading to an overexpression of the target gene, immunohistochemistry (IHC) can be a time saving and inexpensive tool to perform screening for rearrangements by protein localization. However, there are no antibodies for all clinically relevant fusions. Additionally, this approach has a high rate of false-positive events and a second method is needed to verify fusions found by IHC [Citation1].
Both approaches, ISH and immunohistochemistry, have the major disadvantage to lack multiplexing opportunities. Another drawback of ISH and IHC is that at least one fusion partner has to be already known or assumed. Nonetheless, the identification of unknown fusion partners is becoming increasingly important because the partners can influence treatment choices and can be of prognostic value [Citation6].
Next-Generation Sequencing (NGS) can identify known and unknown fusions on DNA and RNA level including both fusion partners in a single multiplex approach and therefore seems to be more promising.
3. Comprehensive technologies: from WGS to WTS
Among NGS strategies, whole-genome sequencing (WGS) can be used to find a greater number of rearrangements and discover novel fusions. It is possible to characterize exact breakpoints, including those in non-coding regions. However, WGS is expensive and time-consuming due to its enormous data output and intensive analysis [Citation7]. An alternative to WGS can be whole-exome sequencing (WES). Nevertheless, only rearrangements with breakpoints near or in exons can be detected. Since that is true for only a few events, WES has no meaning in fusion detection [Citation8].
As with WGS, whole-transcriptome sequencing (WTS) is sufficient to discover several gene fusions but is at the same time more efficient and sensitive [Citation9]. Even with these advantages, WTS is still too expensive and data analyses and interpretation requires specialized personnel.
4. DNA vs RNA- based targeted sequencing
Besides approaches analyzing large areas of the genome, targeted sequencing can be used to isolate and sequence a subset of genes and detect fusions in a more distinct manner. By enrichment, targeted sequencing results in a higher coverage of the included genomic areas and the sensitivity for detecting fusions increases simultaneously. Further, data output is moderate compared to WGS or WTS and therefore more suitable for daily routine testing [Citation10].
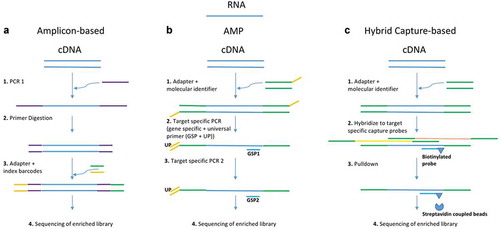
It is possible to use either DNA or RNA as input material for targeted sequencing. DNA-based fusion detection is limited due to the possibility that fusion breakpoints may occur in intronic areas. Thus, large regions have to be covered. RNA-based approaches can overcome this problem by using transcripts for the detection of fusions. Nevertheless, their sensitivity is dependent on fusion gene expression levels [Citation11]. Currently, there are different approaches available to detect fusions in a targeted setup: hybrid capture-based methods, amplicon-based methods and anchored multiplex PCR (see ). Various off-the-shelf panels are available for all approaches as summarized by De Luca et al. and compared by Heydt et al. [Citation7,Citation12]. Hybrid capture-based approaches have the advantage to cover larger areas including flanking regions of the target area. Thereby the possibility to miss a fusion is reduced. However, the input of nucleic acids needed for hybrid capture is relatively high. In contrast, amplicon-based approaches using fusion-specific primers need only a low input, making it applicable for routine diagnostics especially with FFPE material. New fusions or unknown fusion partners cannot be detected though. A more recent way to find fusions is the anchored multiplex PCR (AMP-PCR). Since this approach uses gene-specific primers anchored to exon-intron boundaries and universal reverse primers, it can be used to enrich unknown genomic regions of interest. Even unknown fusion partners can be detected by this approach. A comparative analysis for targetable fusions revealed that assays using AMP-PCR or hybrid capture enrichment detect a broader spectrum of fusions than other RNA-based assays [Citation5].
5. Major challenges and optimization opportunities
The major challenge for many laboratories is to find a way to optimally navigate diagnostic assays without exhausting the limited tumor tissue of small biopsies or even cytological specimen. To meet this, the diversity of diagnostic procedures has to be reduced. A method to definitely save material is to combine DNA and RNA extraction for further NGS on both [Citation12–14]. This can be achieved by using RNA specified extraction kits without the DNA digestion step and adjusting the extraction volume to an optimum. This allows simultaneous analysis of DNA and RNA for NGS instead of sequential analysis. Extraction kits for total nucleic acid extraction (tNA) from FFPE material with following AMP-based RNA-seq have been described and compared extensively by Kresse et al., 2020 (AllPrep DNA/RNA FFPE Kit(Qiagen Inc.); truXTRAC FFPE RNA Kit (Covaris Inc.)) and Heydt et al., 2020 (Maxwell RSC RNA FFPE Purification Kit (Promega). For DNA-free RNA, the genomic DNA was removed from the tNA extracts using the TURBO DNA-free Kit (Thermo Fisher Scientific)) [Citation12,Citation14]. Once established, it can be a powerful tool to save tissue and shorten turnaround time.
n addition, sequencing of RNA from FFPE tissue seems to be a challenge itself, since it is often highly degraded and fixation processes often lead to chemical modifications of the nucleic acid. Nevertheless, various studies have successfully proven the utility of RNA derived from FFPE for different RNA-seq approaches [Citation15–17].
Another important point is the rapidly growing knowledge on fusion events in certain tumor entities. Due to new insights on the significance of fusion partners and the development of new therapeutics, the number of diagnostically relevant genes is growing constantly. Thus, NGS panels that allow a flexible design with the possibility to boost areas of interest should be preferred. Their content can be easily adapted to new requirements. An alternative to find unknown fusions are large comprehensive panels or WTS. Their advantage is diminished by the fact that these panels are to date too expensive for use in routine diagnostics, need high sequencing capacities and complex bioinformatic algorithms for evaluation.
6. Detection of rare structural variants by RNA-Seq: examples from clinical practice
Employing RNA-Seq in routine diagnostics revealed that it is also a helpful tool in detecting activating aberrations, like the MET exon 14 skipping event or duplications of the EGFR kinase domain. Especially for the characterization whether mutations and deletions in MET within or in close proximity to splice regions lead to skipping of exon 14, RNA-Seq has proven reliability. A large variety of so-called activating MET exon 14 skipping mutations has been described by Awad et al and Frampton et al. [Citation18–20]. However, for some deletions and mutations within the splice region the effect on splicing is only assumed or even unknown. Indeed, for such cases showing unknown deletions and/or mutations within the splice region of MET exon 14 skipping can be proven by subsequent targeted RNA sequencing. Noteworthy also silent mutations within the splice site could lead to exon 14 skipping. Carrying out NGS on DNA level only might miss the correct interpretation of such mutations as they could easily be overlooked. Silent mutations within the splice site do not alter the amino acid encoded, but the alteration on nucleic acid level could lead to the inactivation of splice sites resulting in exon skipping [Citation21].
Additionally, interesting and putative targetable rare alterations of the EGFR gene can be detected. Such include VSTM2A-EGFR fusions, EGFR kinase domain duplications and the EGFR variant III. They all affect the activity of the EGF receptor, but therapy options vary strongly. The duplication of the EGFR kinase domain region results from the so-called EGFR ITD (internal tandem duplication), which is a fusion of EGFR exon 18 and exon 25 [Citation22]. This fusion consequently leads to constitutively activated signaling of the receptor. It has been shown to be sensitive to second-generation EGFR TKI offering a strong treatment opportunity [Citation23]. Additionally, the fusion of EGFR exon 1 with EGFR exon 8 is known to result in a structural variant of EGFR (known as EGFR vIII). The kinase domain itself is not affected but a constitutive downstream signaling of the EGF receptor has been shown [Citation24]. Unfortunately, EGFR TKI are not effective against this variant, but promising efficacy of EGFR antibodies has been shown in glioblastoma patients carrying this aberration [Citation25]. Finally, the VSTM2A–EGFR fusion has not been characterized so far and it remains to be ambiguous whether it has a driving effect via the EGF receptor and which therapeutics might present a treatment opportunity.
As already mentioned, RNA-Seq allows to determine the exact fusion partner and the fused exons. By now it is known that the fusion partner of certain drivers can influence therapy response and patient outcome. For the EML4–ALK fusion different variants are described. The most frequent ALK variants in NSCLC patients are ALK-EML4 variant 1 (EML4 (exon 13)-ALK (exon 20)) and 3 (EML4 (exon 6)-ALK (exon 20)). Although the fusion partner gene is the same, the fused regions vary and lead to obvious differences in resistance acquisition under therapy. Especially the well-described resistance mutation under ALK inhibitor therapy p.G1202R is only acquired in variant 3 patients [Citation26].
Another example for the importance to determine the fusion partner has been described by Li and coworkers. They analyzed the survival data of ROS1 translocated patients. Patient cohorts were separated into CD74-ROS1 and non-CD74–ROS1 patients. Progression-free survival (PFS) as well as overall survival (OS) was significantly higher within the non-CD74–ROS1 group [Citation27].
7. Conclusion and expert opinion
With regard to our experience, we recommend targeted RNA sequencing as a reliable method to evaluate known and novel gene-fusions. Nevertheless, the question still remains, whether RNA-seq will replace currently used molecular methods such as FISH for the detection of fusion events in NSCLC. To date, molecular diagnostic labs that apply RNA-seq still use IHC and FISH. The current clinical setting relies on the time- and cost-effective screening of targetable fusions via IHC (e.g ALK, ROS rearrangements) and FISH (e.g. RET rearrangements). Further validation of the screened fusion is then performed with NGS-based approaches, which also allows the determination of the fusion partner. In addition, patients without any targetable driver mutation are often subjected to RNA-seq fusion analysis.
Therefore, the applied NGS panels should be flexible to be easily adapted to clinically emerging requirements. In order to adequately save tissue material multigene testing as well as simultaneous RNA- and DNA extraction can be implemented into diagnostic procedures. Examples from clinical practice strongly underline the importance to correctly identify the exact fusion partner. Moreover, the detection of even new but probably targetable fusions and structural variants opens treatment options and drives clinical research toward innovative drugs.
In conclusion, the exploration of fusions plays an important role in the understanding of cancer. Thus, the development and improvement of NGS-based molecular approaches such as RNA-sequencing are growing and will lead perspectively to a better treatment of NSCLC patients.
Declaration of interest
S Wagener-Ryczek has received honoraria from Invitae. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Siemanowski J, Heydt C, Merkelbach-Bruse S. Predictive molecular pathology of lung cancer in Germany with focus on gene fusion testing: methods and quality assurance. Cancer Cytopathol. 2020 Sep;128(9):611–621. PubMed PMID: 32885916; eng.
- Hong M, Tao S, Zhang L, et al. RNA sequencing: new technologies and applications in cancer research. J Hematol Oncol. 2020 Dec 4;13(1):166. PubMed PMID: 33276803; PubMed Central PMCID: PMCPMC7716291. eng.
- Mosele F, Remon J, Mateo J, et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO precision medicine working group. Ann Oncol. 2020 Nov;31(11):1491–1505. PubMed PMID: 32853681; eng.
- Barr FG. Fusion genes in solid tumors: the possibilities and the pitfalls. Expert Rev Mol Diagn. 2016 Sep;16(9):921–923. PubMed PMID: 27550633; PubMed Central PMCID: PMCPMC6699740.
- Heydt C, Ruesseler V, Pappesch R, et al. Comparison of in situ and extraction-based methods for the detection of ROS1 rearrangements in solid tumors. J Mol Diagn. 2019 Nov;21(6):971–984. PubMed PMID: 31382035.
- Childress MA, Himmelberg SM, Chen H, et al. ALK fusion partners impact response to ALK inhibition: differential effects on sensitivity, cellular phenotypes, and biochemical properties. Mol Cancer Res. 2018 Nov;16(11):1724–1736. PubMed PMID: 30002191; PubMed Central PMCID: PMCPMC6214753.
- De Luca A, Esposito Abate R, Rachiglio AM, et al. FGFR fusions in cancer: from diagnostic approaches to therapeutic intervention. Int J Mol Sci. 2020 Sep 18;21(18):6856. PubMed PMID: 32962091; PubMed Central PMCID: PMCPMC7555921. eng.
- Schroder J, Kumar A, Wong SQ. Overview of fusion detection strategies using next-generation sequencing. Methods Mol Biol. 2019;1908:125–138. PubMed PMID: 30649725.
- Stengel A, Shahswar R, Haferlach T, et al. Whole transcriptome sequencing detects a large number of novel fusion transcripts in patients with AML and MDS. Blood Adv. 2020 Nov 10;4(21):5393–5401. PubMed PMID: 33147338; PubMed Central PMCID: PMCPMC7656918.
- Heyer EE, Deveson IW, Wooi D, et al. Author correction: diagnosis of fusion genes using targeted RNA sequencing. Nat Commun. 2020 Apr 8;11(1):1810. PubMed PMID: 32269228; PubMed Central PMCID: PMCPMC7142116.
- Davies KD, Lomboy A, Lawrence CA, et al. DNA-based versus RNA-based detection of MET Exon 14 skipping events in lung cancer. J Thorac Oncol. 2019 Apr;14(4):737–741. PubMed PMID: 30639620.
- Heydt C, Wölwer CB, Velazquez Camacho O, et al. Detection of gene fusions using targeted next-generation sequencing: a comparative evaluation. BMC Med Genomics. 2021 Feb 27;14(1):62. PubMed PMID: 33639937; PubMed Central PMCID: PMCPMC7912891. eng.
- Wei H, Smith Z, Doh J, et al. Simultaneously DNA and RNA extraction from formalin-fixed paraffin embedded (FFPE) tissue. J Biomol Tech. 2019;30(Suppl):S37–S37. PubMed PMID: PMC6938039; eng.
- Kresse SH, Namløs HM, Lorenz S, et al. Evaluation of commercial DNA and RNA extraction methods for high-throughput sequencing of FFPE samples. PloS One. 2018;13(5):e0197456.
- Pennock ND, Jindal S, Horton W, et al. RNA-seq from archival FFPE breast cancer samples: molecular pathway fidelity and novel discovery. BMC Med Genomics. 2019 Dec 19;12(1):195.
- Newton Y, Sedgewick AJ, Cisneros L, et al. Large scale, robust, and accurate whole transcriptome profiling from clinical formalin-fixed paraffin-embedded samples. Sci Rep. 2020 Oct 19;10(1):17597.
- Esteve-Codina A, Arpi O, Martinez-García M, et al. A comparison of RNA-seq results from paired formalin-fixed paraffin-embedded and fresh-frozen glioblastoma tissue samples. PloS One. 2017;12(1):e0170632. PubMed PMID: 28122052; PubMed Central PMCID: PMCPMC5266269. eng.
- Awad MM, Oxnard GR, Jackman DM, et al. MET Exon 14 mutations in non-small-cell lung cancer are associated with advanced age and stage-dependent MET genomic amplification and c-met overexpression. J Clin Oncol. 2016 Mar 1;34(7):721–730. PubMed PMID: 26729443; eng.
- Frampton GM, Ali SM, Rosenzweig M, et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov. 2015 Aug 5;(8):850–859. DOI:10.1158/2159-8290.cd-15-0285. PubMed PMID: 25971938; eng.
- Wolf J, Seto T, Han JY, et al. Capmatinib in MET Exon 14-mutated or MET-amplified non-small-cell lung cancer. N Engl J Med. 2020 Sep 3;383(10):944–957. PubMed PMID: 32877583; eng.
- Montera M, Piaggio F, Marchese C, et al. A silent mutation in exon 14 of the APC gene is associated with exon skipping in a FAP family. J Med Genet. 2001 Dec;38(12):863–867. PubMed PMID: 11768390; PubMed Central PMCID: PMCPMC1734788. eng.
- Wegert J, Vokuhl C, Collord G, et al. Recurrent intragenic rearrangements of EGFR and BRAF in soft tissue tumors of infants. Nat Commun. 2018 June 18;9(1):2378.
- Gallant JN, Sheehan JH, Shaver TM, et al. EGFR Kinase Domain Duplication (EGFR-KDD) is a novel oncogenic driver in lung cancer that is clinically responsive to afatinib. Cancer Discov. 2015 Nov 5;(11):1155–1163. DOI:10.1158/2159-8290.cd-15-0654. PubMed PMID: 26286086; PubMed Central PMCID: PMCPMC4631701. eng.
- Rutkowska A, Stoczyńska-Fidelus E, Janik K, et al. EGFR(vIII): an oncogene with ambiguous role. J Oncol. 2019;2019:1092587. PubMed PMID: 32089685; PubMed Central PMCID: PMCPMC7024087 publication of this paper. eng
- An Z, Aksoy O, Zheng T, et al. Epidermal growth factor receptor and EGFRvIII in glioblastoma: signaling pathways and targeted therapies. Oncogene. 2018 Mar;37(12):1561–1575. PubMed PMID: 29321659; PubMed Central PMCID: PMCPMC5860944. eng.
- Lin JJ, Zhu VW, Yoda S, et al. Impact of EML4-ALK variant on resistance mechanisms and clinical outcomes in ALK-positive lung cancer. J Clin Oncol. 2018 Apr 20;36(12):1199–1206. PubMed PMID: 29373100; PubMed Central PMCID: PMCPMC5903999. eng.
- Li Z, Shen L, Ding D, et al. Efficacy of crizotinib among different types of ROS1 fusion partners in patients with ros1-rearranged non-small cell lung cancer. J Thorac Oncol. 2018 Jul;13(7):987–995. PubMed PMID: 29704675; eng.