ABSTRACT
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and fourth-leading cause of cancer death. While drug discovery to improve disease survival was historically poor, there is now evidence of significant potential for immune checkpoint inhibitors (ICPIs) in treatment of the disease, and indeed such drug approvals are beginning to emerge.
Areas covered
HCC typically arises in the context of cirrhosis and chronic liver disease (CLD), and HCC exhibits significant biological heterogeneity, in part reflecting the broad range of etiologies of CLD. Different classes and combinations of ICPI-based therapy exist, but not all patients will respond and predictive biomarkers are not yet available to guide clinician decision-making, unlike some other cancer types. In this review, we discuss the emerging biomarkers for ICPI sensitivity in HCC, including tumor genomic features, perturbation of the gut microbiome, and systemic inflammatory markers.
Expert opinion
Additional profiling studies are required to appreciate existing trends with clinical outcome and to further drive clinical studies in disease stratification by response. This will only be possible within collaborative and international efforts, especially regarding biopsy collection. A close collaboration between basic scientists and clinicians will be the key to shape the next future of HCC biomarker research.
1. Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and the fourth highest cause of cancer-related deaths worldwide [Citation1]. Due to its low proportion of early-stage diagnoses, overall survival (OS) is still poor, <15% at 5 years [Citation2,Citation3]. Even after curative resection five-year disease-free survival rates remain as low as 24% [Citation4]. Varying etiologies and biological heterogeneity make it challenging to develop broadly effective treatments and identify who will respond to a given therapy. Over the last decade, immunotherapy has transformed the treatment of HCC, in particular, following the introduction of immune checkpoint inhibitors (ICPIs) [Citation5].
Clinically available ICPIs target co-inhibitory pathways such as the programmed cell death protein-1/programme cell death ligand-1 (PD-1/PD-L1) axis and CTLA-4/CD80/CD86 axis. Although these therapies have led to transformative changes in the systemic management of various malignancies, therapeutic targeting of these pathways as a monotherapy has not resulted in significant improvements in survival of HCC patients [Citation6].
The anti-CTLA-4 monoclonal antibody (mAb) tremelimumab has in fact demonstrated modest antitumor activity in advanced disease with concurrent hepatitis C virus (HCV) infection, with an overall response rate (ORR) of 17% and a time to progression (TTP) of approximately 6 months [Citation7]. While capable of inducing antitumor responses in <20% of patients [Citation8,Citation9], the forerunner PD-1 inhibitors nivolumab and pembrolizumab have unfortunately fallen short in demonstrating a significant improvement of survival compared to sorafenib in the first-line Checkmate-459 study [Citation10] and compared to placebo in the second-line Keynote-240 clinical trial [Citation11].
More recently, the combination of atezolizumab, an anti-PD-L1 mAb, and bevacizumab, an antivascular endothelial growth factor (VEGF) mAb, has become the standard of care for first-line treatment of patients with unresectable or metastatic HCC [Citation12], following the publication of the results of the IMbrave150 trial [Citation13]. This multicenter, randomized, phase III trial met both its coprimary end points, showing the superiority of the combination over sorafenib in terms of OS and progression-free survival (PFS). According to the most recently presented update [Citation14], after a median follow-up of 15.6 months, the combination achieved a median OS of 19.2 months (95% confidence interval [CI], 17.0–23.7) vs 13.4 months (95% CI, 11.4–16.9) with sorafenib (hazard ratio [HR], 0.66 [95% CI, 0.52–0.85]; p = 0.0009). Similarly, the combination of sintilimab, an anti-PD-1 mAb, and a bevacizumab biosimilar has demonstrated evidence of efficacy as a first-line therapy for Chinese patients with advanced HCC in the phase II/III ORIENT-32 trial [Citation15].
While it is now recognized that ICPIs benefit a proportion of patients with HCC, the development of immunotherapy has been largely empirical in this oncological indication. This is in contrast to other indications such as non-small-cell lung cancer (NSCLC), where clinically available biomarkers such as PD-L1 expression, albeit imperfect, have become a useful companion diagnostic tests that identifies patient subgroups likely to benefit from PD-1 monotherapy [Citation16]. According to landmark studies of ICPIs in HCC such as Checkmate-040 [Citation8] and Keynote-224 [Citation9], 33–49% of patients with HCC are refractory to PD-1/PD-L1 inhibition, with only 14–18% of patients responding radiologically to ICPIs. Even in patients receiving combination immunotherapy for HCC, approximately 20% are refractory to atezolizumab/bevacizumab, illustrating that immunotherapeutic approaches do not benefit all patients [Citation13].
HCC pathogenesis is multifaceted, and such a level of complexity has traditionally represented a major hurdle in the development of precision medicine and predictive biomarkers of response [Citation17]. As systemic treatment options for HCC increase significantly, predictive correlates of response and survival benefit from immunotherapy have become an area of high unmet need in HCC. Predictive biomarkers would facilitate a priori identification of responders, therefore optimizing clinical decision-making in routine practice.
Recognizing the potential for molecularly based prediction of clinical outcomes from immunotherapy, this review highlights opportunities and challenges in the identification of predictive biomarkers of response to immune-based therapies in HCC, focusing on a restricted series of traits ranging from tumor genomic features to host characteristics such as perturbation of the gut microbiome, which have shown potential for disease stratification and are actively being explored in HCC .
2. Genomic characteristics
There have been numerous attempts to interrogate the genomic heterogeneity of HCC in pursuit of biomarkers for treatment stratification. The most notable example has been the targeting of MET with tivantinib, a tyrosine-kinase inhibitor (TKI). MET is the tyrosine kinase receptor for the hepatocyte growth factor (HGF), and its overexpression (MET-high) was linked to worse prognosis in HCC patients [Citation18]. Tivantinib seemed to be capable of reverting the prognosis of these patients, with the drug being particularly active in MET-high sorafenib-pretreated patients. However, the phase III METIV-HCC trial failed to meet its primary end point, with tivantinib not being superior to placebo in terms of survival for the second-line treatment of patients with MET-high advanced HCC [Citation19]. Another example involves the targeting of the pathway fibroblast growth factor 19 (FGF19) and its receptor FGFR4, which has been extensively studied as HCC growth driver [Citation20]. Promising early-phase results were obtained by a selective FGFR4 inhibitor, fisogatinib [Citation21]. However, these findings were not validated in larger, randomized clinical trials, thus interrupting the potential development of the agent.
Comprehensive genomic studies have been conducted in an attempt to identify consistent patterns within the heterogeneity of HCC and predictive biomarkers of response to treatment. These profiling studies have classified HCCs into two main molecular classes: proliferative, which are considered more aggressive, and nonproliferative [Citation22]. Proliferative HCCs are characterized as such due to the overactivation of cell proliferation pathways such as PI3K/Akt/mTOR, transforming growth factor β (TGF-β), insulin-like growth factor, and RAS-MAPK [Citation23]. This class is widely heterogeneous with enhanced chromosomal instability and epigenetic variations such as increased DNA methylation characteristic of progenitor cells [Citation23,Citation24]. Conversely, nonproliferative HCCs are characterized as less aggressive, with an increased level of histological differentiation [Citation25]. These two subgroups can also be correlated with certain immunological features. For example, the proliferative class presents an immune-active subclass that responds positively to ICPIs, characterized by high numbers of helper (CD4+) and cytotoxic (CD8+) T cell infiltrates, as well as an immune-exhausted subclass associated with TGFβ-mediated immunosuppression and a T-cell exhaustion signature [Citation26]. Despite these intriguing observations, these profiling studies are descriptive and should be considered only as hypothesis-generating: there is currently no clinical evidence that these different classes have a prognostic or predictive role, especially in immunotherapy.
To date, HCC profiling studies remain largely descriptive, providing limited evidence of their use for disease stratification, particularly in response to ICPIs. However, Wnt–β-catenin signaling, a widely studied pathway in HCC, is an emerging example of a trait with potential implications for HCC stratification. This pathway appears to be overactivated in approximately 50% of HCC cases [Citation25], alongside telomerase amplification [Citation27,Citation28]. β-catenin is a subunit of the cadherin protein complex and plays an important role in cell adhesion and cell cycle regulation. Its interaction with Wnt leads to the transcriptional upregulation of several oncogenes, and it also promotes immune resistance, as demonstrated in other tumor types [Citation29]. The hyperactivation of the pathway in HCC is caused by activating mutations in CTNNB1 (encoding β-catenin) or by inactivating mutations of AXIN1 or APC (inhibitors of Wnt pathway) [Citation25]. In HCC mouse models, the deregulation of the Wnt–β-catenin pathway suppresses immune response through the impaired recruitment of CD103+ dendritic cells (DC), and the consequent reduction of CD8 + T cell activity [Citation30]. The same mechanism of resistance was found in human HCC samples, where β-catenin-mutant cells significantly lacked DCs and T cell-related transcript [Citation31]. Importantly, the overactivity of β-catenin has been associated with a reduction in the T-cell-inflamed tumor phenotype across cancers [Citation31], inferring a potential role as a negative predictive biomarker for ICI-based therapy. A recent study conducted on HCC samples from 34 patients treated with anti-PD-1 mAb confirmed that Wnt/β-catenin activation was negatively associated with disease control and also with PFS [Citation32].
Since other studies have not found any association between PFS and Wnt-related mutations, it is essential to further explore these in larger cohorts [Citation33,Citation34]. Interestingly, the nonproliferative HCC subclass exhibits a T-cell-inflamed tumor microenvironment and high activity of the interleukin 6 (IL6) – Janus kinase (JAK) – signal transducer and activator of transcription (STAT) pathway [Citation26].
Similar to melanoma, β-catenin activation in HCC leads to T-cell exclusion and resistance to ICPIs through mechanisms employing the downregulation of certain chemokines that facilitate dendritic cell recruitment [Citation29,Citation30]. Fittingly, loss-of-function mutations in the JAK-STAT pathway can also lead to resistance to PD-1 blockade in melanoma patients [Citation35]. In both HCC and melanoma, inflammatory gene signature scores based on PD-L1, CD8A, and STAT1 were associated with better improved OS [Citation36]. The IL-6/STAT3 signaling pathway can also influence hepatocarcinogenesis by promoting the protumorigenic M2 phenotype over the antitumoral M1 phenotype in macrophages [Citation37]. As a key inflammatory pathway promoting HCC, the IL6-JAK-STAT axis and its various components are interesting candidates for further response biomarker studies.
3. Programmed cell death ligand-1 (PD-L1)
PD-1 is a master regulator of immune cell tolerance and is expressed on the cell surfaces of T lymphocytes where it promotes repression of effector function when bound to programmed death-ligand 1 (PD-L1) [Citation38]. PD-L1 expression can be found in different cell lineages within the liver including hepatocytes [Citation39], hepatic stellate cells [Citation40], and Kupffer cells [Citation41]. The PD-1/PD-L1 complex is essential in ensuring T lymphocytes are self-tolerant and are not responsible for autoimmune organ destruction [Citation9]. PD-1/PD-L1 interaction causes colocalization with the T-cell receptor (TCR) and dephosphorylation of the CD3ζ TCR subunit and other kinases by SHP2 (Src homology 2 domain-containing tyrosine phosphatase 2), suppressing TCR signaling via the PI3K/Akt or MAPK pathways and reducing cell growth and survival [Citation42]. Increased expression of PD-1 on the surface of tumor-infiltrated T lymphocytes can lead to lymphocytes entering an exhausted state as a result of chronic inflammation. This is a phenomenon that occurs when the proinflammatory immune response is defective, leading to reduced cytokine production [Citation43] and impaired cytotoxic activity of CD8+ cells.
In cancer, the PD-1/PD-L1 pathway is subverted as a mechanism of immune escape; one of the ten hallmarks of cancer [Citation44]. PD-L1 expression can be driven by genetic events such as EGFR mutations [Citation45] Overexpression of the PD-1/PD-L1 axis inhibits T lymphocyte activation, proliferation, and survival and reduces proinflammatory cytokine production [Citation46]. For example, PD-1 high cytotoxic T cells associated with HCC present a genetic exhaustion signature including LAYN overexpression leading to reduced IFN-γ production and overexpression of immune exhaustion markers (TIM3, LAG3) [Citation47].
PD-L1 expression can be measured with different immunohistochemistry assays, either on tumor cells as the tumor proportional score (TPS) or on tumor and surrounding immune cells as the combined positive score (CPS) [Citation48]. PD-L1 expression by immunohistochemistry (IHC) was initially recognized as a potential predictive biomarker to PD-1/PD-L1 pathway inhibitors in preliminary phase I trials [Citation49] and then validated in larger clinical trials [Citation50], thus paving the way for IHC evaluation of PD-L1 in clinical practice for several cancer types including NCSLC [Citation15,Citation51,Citation52], breast [Citation53], and advanced esophageal cancer [Citation54]. The utility of PD-L1 as a response biomarker for PD-1/PD-L1 pathway inhibitors in HCC is less definitive.
Early studies investigating the prognostic role of PD-L1 in HCC have found its expression to be an independent predictive marker of survival. Gao et al. [Citation55] performed IHC staining on 246 HCC specimens and reported that patients with PD-L1-positive tumors had a significantly worse OS than those with PD-L1-negative tumors (29.6 months vs. 59.4 months). PD-L1-positive cancers were also associated with vascular invasion, with 57% of patients with PD-L1-positive tumors displaying vascular invasion compared to 42% of PD-L1-negative specimens (p = 0.043) [Citation56].
Despite this, early associations of poor survival were not corroborated by later studies. A meta-analysis exploring the clinical significance of PD-L1 expression in HCC found no significant associations with improved survival or response rates. This study, however, did report PD-L1 expression to be associated with crucial clinicopathological features, such as alpha fetoprotein (AFP) secretion (HR 1.45, 95% CI 1.02–2.00, p = 0.04) and CD8+ tumor-infiltrating lymphocytes (HR 3.76, 95% CI 1.42–9.93, p = 0.04) [Citation57]. This is substantiated by Calderaro et al. [Citation58] who investigated the relationship between PD-L1 expression in HCC and clinical features. They reported serum AFP > 20 ng/ml (69% vs 47%, p = 0.038), the presence of microvascular (83% vs 45%, p < 0.001), and macrovascular invasion (56% vs 11%, p < 0.001) to be associated with raised PD-L1 in neoplastic cells. As such, this study was potentially underpowered for a survival association, as these clinicopathological features are associated with poorer survival.
PD-L1 expression therefore lacks convincing evidence of utility as a biomarker of survival in HCC. Its utility as a biomarker for ICPI response has also been interrogated, but once again is not highly convincing. In the phase I Checkmate-040 trial [Citation8], which aimed to assess efficacy and safety of nivolumab alone or in combination with ipilimumab in patients with advanced HCC, PD-L1 did not discriminate between responders, with both PD-L1-positive and -negative patients demonstrating comparable response rates. Additionally, a phase 1b study assessing the efficacy and safety of atezolizumab in combination with bevacizumab [Citation59] retrospectively analyzed PD-L1 expression by IHC staining in 86 tumor samples and found clinical response regardless of PD-L1 status.
The Keynote-224 study [Citation9] analyzed responses in 52 patients assessed for PD-L1 expression in both immune and tumor cells by IHC. No correlations were observed between the response rate and PD-L1 expression restricted to neoplastic cells; however, an association between response and total PD-L1 expression of ≥1 in immune and tumor cells (combined positive score, CPS) was observed: 32% patients with CPS ≥1 responded to pembrolizumab compared to 20% with CPS < 1 (p = 0.021).
Defining the threshold for PD-L1 positivity and the choice of different antibodies to detect PD-L1 protein expression in tissue specimens are challenges to its utility and consistency as a biomarker. The five antibody clones that are predominantly used for PD-L1 IHC staining are E1LN3, 28–8, 22c3, SP263, SP142 [Citation45]. Studies have explored the analytical heterogeneity of these PD-L1 antibodies, with the Blueprint-HCC study showing the most variation between SP263 and 28–8 (R2 = 0.080) and significant differences in the quantity and intensity of PD-L1 expression in immune cells [Citation60]. Due to the characteristic immune-cell-rich HCC microenvironment, it is crucial to recognize the difference in the antibodies ability to recognize PD-L1 expression in both the tumor and its peripheries.
Moreover, there is evidence to suggest preanalytical heterogeneity in PD-L1 expression between primary HCC tumors and paired metastasis [Citation61], with one study reporting primary tumors to be less responsive to ICPI therapy than extrahepatic metastases (ORR 22.4% in primary tumors, 41.2% lung metastases, and 38.9% other intraabdominal metastases) [Citation62]. Studies have also shown PD-L1 levels to fluctuate as disease progresses, highlighting the need for standardization in both the methodology and interpretation of PD-L1 expression [Citation63,Citation64]. Establishing the utility of PD-L1 expression as a biomarker of response to ICPI would have prognostic utility, however this may not directly affect decision to treat PD-L1-negative patients with ICPIs as responses are still seen in these cases. As such, the role of PD-L1 as a biomarker for response should be assessed distinctly for ICPI monotherapy and combination therapy.
4. Tumor mutational burden
Tumor mutation burden (TMB) is defined as the number of DNA mutations per megabase (muts/Mb) in the coding genome of tumors [Citation65]. Tumors with a high TMB are associated with a greater expression of neoantigens [Citation66], which require identification by T lymphocytes to trigger an immune response. Consequently, the TMB-high (TMB-H) status, defined as the presence of more than 10 muts/Mb, has been explored as predictive biomarker of response to PD-1/PD-L1 inhibitors. The phase 2 Keynote-158 study showed a significantly improved response to pembrolizumab in multiple advanced TMB-H solid tumors, with an ORR of 29% in TMB-H patients compared to 6% in the TMB-low subgroup [Citation67]. These findings led the United States (US) Food and Drug Administration (FDA) to grant pembrolizumab a site-agnostic approval for the treatment of unresectable or metastatic TMB-H cancer regardless of its primary site [Citation68]. However, the trial did not enroll any patients with HCC, with TMB-H status being an infrequent finding in this tumor type [Citation69].
High TMB tends to be secondary to specific mutagenic processes (particularly UV light and smoking exposure), befittingly rendering TMB-high tumors enriched in cancer types intrinsically sensitive to immunotherapies, such as melanoma and NSCLC [Citation70]. A study of 1000 HCC specimens revealed a median of 5 coding somatic mut/Mb [Citation71] lower than melanoma (15 mut/Mb) and non-small-cell lung cancer (NSCLC) (9 mut/Mb). High TMB is therefore less likely to be a relevant biomarker for ICPI response but could be considered for retrospective analysis.
Another challenge relies on the methodological discrepancies utilized to obtain TMB data. Wong et al. [Citation72] found the median TMB to be higher in paraffin-embedded HCC samples compared to fresh-frozen samples (958.39 vs 2.5 Mut/Mb, respectively, p < 0.001) when optimizing the use of next-generation sequencing to determine TMB. Moreover, discrepancies in TMB between patients of different geographical origin (and diverse risk factors for chronic liver disease) have been documented, with a recent study by Tang et al. reporting 9.3% of Chinese patients with HCC having a high TMB compared to 1% of western ethnicity [Citation73].
Evidence thus far suggests TMB-high to correspond to a minority of HCC patients, thereby restricting the clinical applicability of TMB as a predictive biomarker in HCC.
5. Microsatellite instability
Carcinogenesis is characterized by the acquisition of a cancer mutator phenotype, where somatic mutations happen at sharply increased rate compared to normal cells [Citation74,Citation75]. In the context of cancer progression, somatic changes to the DNA sequence led to functional changes in so-called oncogenic drivers (i.e. activating mutations of oncogenes and suppressing mutations of gatekeepers), which are often pathogenic in cancer progression. The probability of mutations to arise during the process of DNA synthesis is limited by instant proofreading of replication errors and postreplicative mismatch repair (MMR) mechanisms. Somatic hypermutation is the result of several mechanisms, encompassing the deficit of the DNA repair system.
Many pathways work simultaneously to repair and maintain DNA integrity, including the MMR pathway that rectifies DNA base substitution and frameshift mutations [Citation76]. Microsatellites are short regions of DNA with tandem repeats and are inherently more vulnerable to impaired DNA repair. Deficit in the MMR pathway (dMMR) leads to a status of microsatellite instability (MSI). Tumors can be differentiated into MSI-high or MSI-low by measuring the size of a panel of microsatellite regions in tumor tissue using PCR and capillary electrophoresis. Additionally, IHC staining of four key genes of the MMR (MLH1, MSH2, MSH6, and PMS2) can be used to differentiate between MMR-deficient and MMR-proficient tumors and is in common clinical practice [Citation77].
MSI status has been considered a potential biomarker for response to ICPI therapy. In 2015, the FDA granted pembrolizumab its first site-agnostic accelerated approval for any MSI-h or dMMR unresectable tumor type, based on the response rates observed in the phase I KEYNOTE-016 study [Citation78]. However, MSI-high is a rare trait in patients with HCC, leading to a limited utility as a predictor of response to ICPIs, with <3% of HCCs harboring an MSI status [Citation76].
Goumard et al. [Citation79] performed polymerized chain reaction (PCR) on 122 HCC samples and found none of these to resemble an MSI-high phenotype. While limited studies have explored the relationship between MSI-high and response rate in HCC, Kawaoka et al. [Citation80] recently used PCR to determine the incidence on MSI-high in 82 Japanese patients with advanced HCC and correlated this with response to pembrolizumab. This study demonstrated only 2/82 (2.4%) of the patients enrolled had an MSI-high phenotype, with one of these demonstrating a complete response to pembrolizumab therapy which was maintained for 10 months post treatment.
Similarly, a case report details a 64-year-old patient with unresectable HCC who was treated with pembrolizumab after biopsy results reported MSI-high [Citation81]. Despite previously not responding to sorafenib, this patient demonstrated a complete response to ICPI therapy. However, this remarkable response cannot solely be attributed to MSI-high, as the tumor immunophenotype (PD-L1+ and high CD8+ expression) was likely to predispose to a successful response irrespective of MSI-high tumor. Such case reports provide limited prognostic evidence and warrant further profiling studies on a diverse spectrum of HCC phenotypes before MSI can play a role in response stratification.
Despite this anecdotal evidence, the evaluation of MSI status as an ICPI response biomarker would be of low impact in HCC due to its low prevalence. Further studies are required to highlight the clinical value of MMR evaluation, within the perspective of a wider series of biomarkers.
6. Tumor microenvironment characteristics
HCCs have a complex tumor microenvironment (TME), composed of both cellular and noncellular components. The TME is pivotal in facilitating the synergy of immune suppression mechanisms, allowing resistance to immunotherapy to develop [Citation5]. The TME includes tumor-infiltrating lymphocytes such as cytotoxic CD8+ cells, which directly induce cell death upon stimulation of T-cell receptors. Proliferation of these lymphocytes is stimulated by the release of proinflammatory markers (e.g. IL-2, IL-1α, IL-1β, and IFN-γ) [Citation82], produced by regulatory CD4+ cells that are crucial in stimulating an antitumor response. Thus, the over-expression of proinflammatory cytokines has been considered a harbinger of improved survival and reduced metastases [Citation83].
Unlike cytotoxic T lymphocytes, natural killer (NK) cells secrete proinflammatory cytokines to induce apoptosis without requiring antigen presentation [Citation5]. These cells are often dysfunctional in tissues of chronic inflammation [Citation84] which is characteristic of HCC. Studies have also found patients with intratumoral NK cells that overexpress CD96 to be at higher risk of a poor overall survival [Citation85].
In the case of nonproliferative HCCs, a specific subclass exhibiting frequent CTNNB1 gain-of-function mutations (or other associated mutations that lead to Wnt–β-catenin signaling pathway activation) has been associated with a paucity of immune cells in the tumor microenvironment [Citation86]. This immune-excluded (‘cold’ tumor) phenotype with an increase of regulatory T cells might predict primary resistance to ICPIs, as seen in a small number of patients (n = 27) with shorter median progression-free survival (PFS; 2.0 vs. 7.4 months // HR = 9.2, 95% CI: 2.9–28.8, p < 0.0001), as well as in preclinical/mouse HCC models [Citation30,Citation87]. This raises important questions about the potential of the Wnt/β-catenin mutational status as a predictive biomarker for immunotherapy outcome.
In HCC, a greater T lymphocyte infiltrate has traditionally been associated with prolonged PFS and OS [Citation88], with recent studies by Chew et al. [Citation89] emphasizing that high-density CD8 + T lymphocyte and CD56+ NK cell infiltrate are correlated with improved survival (HR = 7.9, p < 0.0001 and HR = 3.7, p = 0.016 respectively). Additionally, in a post-hoc analysis of the CheckMate-040 trial, an increased CD8+ expression was found to be associated with improved OS in patients treated with nivolumab, albeit not significantly [Citation36]. Also, this study explored the predictive role of the ‘immune class’ gene signatures described by Sia et al. [Citation26], identifying a significant correlation of a 4-gene signature (CD274 [PD-L1], CD8A, LAG3, STAT1) with ORR and OS in nivolumab-treated patients.
A pivotal study showed a possible association between HCC etiology and ICPI response. In mouse models of HCC secondary to nonalcoholic fatty liver disease (NAFLD), researchers found an accumulation of exhausted CD8+/PD-1+ CD8 + T cell population, leading to a lack of response to ICPI [Citation90]. Surprisingly, for NAFLD-related HCC, the administration of prophylactic PD-1 inhibitor was found to even promote the development of HCC in CD8+ proficient mice, while this detrimental effect was not observed in CD8+ depleted mice. The finding of exhausted immune phenotype in mouse models of NAFLD-related HCC is corroborated by a meta-analysis of three large phase III trials, investigating ICPI-based therapy for advanced HCC. Patients with nonviral etiology did not seem to benefit from immunotherapy; however, because of the lack of prospective evidence, ICPI-based therapy should still be considered for all HCC patients, regardless of the underlying etiology [Citation91].
A more recent study performed by Hectors et al. [Citation92] compared MRI radiomic features with the immune and genomic profile of resectable HCCs. They report radiomic features that correlate with CD3 + T lymphocytes, CD68+ macrophages, and protein-level expression of PD-L1, indicating a potentially noninvasive immunoprofiling technique allowing for disease stratification.
7. Gut microbiome
The gut microbiome consists of over one trillion microorganisms that live in a symbiotic relationship with the host. With diversity in proportions and richness of species, the gut microbiome plays an essential role in regulating immunity [Citation93,Citation94] and developing tolerance to commensal bacteria. Similar to a localized immune response, the microbiota plays a multifaceted role in molding systemic immune responses through influencing the innate and adaptive immunity of the host [Citation95]. The gut microbiome has also been implicated in carcinogenesis and even variations in response to cancer therapies.
Initial preclinical studies in mice with metastatic melanoma revealed gut Bifidobacterium to be associated with antitumor effect: administering oral Bifidobacterium in combination with PD-L1 inhibitors significantly decreased the tumor volume [Citation96]. This effect was mediated by an amplification in primed CD8 + T cells accumulating in the TME. Further studies in patients have shown changes in the gut microbiome to improve sensitivity to ICPIs, with Chaput et al. [Citation97] reporting patients with an increase in gut Faecalibacterium as opposed to Bacteroides, which demonstrate a prolonged OS and PFS when treated with ipilimumab.
While the liver does not bear a microbiome of its own, the gut microbiome plays an essential role in HCC pathogenesis through the gut–liver axis [Citation98]. This axis has implications over liver inflammation, chronic fibrosis, and liver cirrhosis [Citation98], as well as implications in HCC progression, antitumor immunity, and resistance to ICPIs [Citation99]. Chronic liver disease is associated with increased gut permeability, thus favoring the migration of a number of microbial components from the intestinal mucosa to the portal blood and ultimately to the liver, including bacterial nucleic acid, lipopolysaccharides (LPS), and toxins, collectively referred to as microbial-associated molecular patterns (MAMPs) or pathogen-associated molecular patterns (PAMPs) [Citation100]. Pre-clinical studies in mice have revealed the importance of gut microbial metabolite deoxycholic acid in promoting HCC, with higher levels in obesity [Citation101]. These changes are hypothesized to have extra-intestinal effects by encouraging hepatic stellate cells to secrete proinflammatory and oncogenic factors such as IL-6 and TGF-β.
There is limited evidence of the effect of the gut microbiota on ICPI response when used for HCC treatment. Zheng et al. [Citation98] investigated the variety of gut microbiome in HCC patients treated with camrelizumab, a PD-1 inhibitor. Eight patients were enrolled on this study – three responders to camrelizumab and five nonresponders. Metagenomic sequencing from fecal samples revealed that responders have a consistently higher microbiota richness (100 vs 85 different species, p = 0.046), with a stark rise in Proteobacteria in nonresponders, making up 25% of the gut microbiome as early as 3 weeks after treatment with camrelizumab and 75% after 12 weeks of therapy. This rise in Proteobacteria in nonresponders was largely due to Escherichia coli compared to Klebsiella pneumonia in the responders. These results suggest that, more than a single bacterial strain, the overall richness of species in the gut microbiome can be associated with the response to ICPI. The dynamic changes of the microbiota during the course of the treatment could be an intriguing predictor of response, even if the causative rather than purely associative nature of these findings still needs to be addressed by larger, translational studies.
Despite these intriguing initial results, larger-scale studies tracking changes in the gut flora throughout therapy with PD-1/PD-L1 inhibitors and follow-up are required. Additionally, studies have found discrepancies in oral and fecal microbiota in cancer patients [Citation102], suggesting that both populations should be considered in further investigations. A key limitation when considering the gut microbiome as a clinical prognostic factor is susceptibility to environmental factors, including the use of antibiotics and changes in diet. David et al. report rapid changes in the composition of gut microbiota consequent to short-term shifts between purely animal or plant-based diets [Citation103], while multiple studies corroborate an impaired response to therapy in conjunction with antibiotics, pointing to the disruption of eubiosis as a possible explanation [Citation104,Citation105].
8. Inflammatory markers
Inflammation plays a crucial role in most, if not all, cases of HCC regardless of etiology, particularly with the progression of liver fibrosis to carcinoma being dependent on the activation of a number of proinflammatory pathways within the liver [Citation83]. Key examples of raised inflammatory cytokines in chronic hepatitis include IL-6, TNF-α, and TGF-β, which engender an oncogenic phenotype in liver progenitor cells. Inflammation can also determine the prognosis of HCC: studies have shown that cytokine shift within the tumor microenvironment from a TH1 to a TH2 response is associated with the occurrence of metastasis, thereby increasing recurrence and mortality following resection [Citation106].
The presence of a local proinflammatory response within the tumor microenvironment is often associated with the systemic release of cytokines from the tumor and host cells [Citation107]. Systemic inflammation is often associated with tumor progression and poor survival outcomes [Citation108], and while this can be asymptomatic in most patients with cancer, a proportion of patients often present with weight loss, fever, night sweats, and nutritional decline. These systemic changes are reflected in changes to blood parameters, which can be used as biomarkers of systemic inflammation.
One such marker for systemic inflammation is the neutrophil–lymphocyte ratio (NLR), which increases as patients with HCC experience relative neutrophilia and lymphopenia [Citation109]. As neutrophils are an eminent source of VEGF, a relative rise has been hypothesized to promote tumor angiogenesis and metastasis [Citation110]. A raised NLR has also been associated with the proinflammatory tumor microenvironment: Motomura et al. [Citation111] demonstrated both serum and peritumoral IL-17 to be raised in patients with NLR > 4 (33.6 vs 1.3 pg/ml, p = 0.02), as well as an increased density of peritumoral CD163+ macrophages (60 in NLR > 4 vs 45 CD163+ cells in NLR < 4, p = 0.002). The correlation between a raised NLR and poor prognosis in patients with HCC has been reproducibly linked with poorer survival across multiple HCC therapeutic modalities, including resection, transplantation, and TACE [Citation112].
Dharmapuri et al. [Citation113] demonstrated the predictive and prognostic potential of NLR in patients with advanced HCC, treated with nivolumab. Both pretreatment NLR < 5 (23 vs 10 months, p = 0.004) and post-treatment NLR < 5 (35 vs 9 months, p < 0.0001) were associated with significantly improved OS relative to patients with NLR ≥ 5. Another recent study confirmed the association between NLR ≥ 5 and worse OS, PFS, and ORR in a large, global cohort of patients treated with immunotherapy for advanced HCC [Citation114]. In this study, NLR was confirmed to have a negative prognostic role in patients receiving a number of different immunotherapy regimens. Additionally, previous studies have shown that sorafenib-treated HCC patients with NLR < 4 have a significantly greater OS than those with an NLR > 4 (12.5 vs 6.5, p = 0.01) [Citation115].
Another score with a potential prognostic value is the platelet–lymphocyte ratio (PLR). A raised PLR is indicative of relative thrombocytosis and lymphopenia. While thrombocytopenia is more common in patients with HCC and is a sign of portal hypertension [Citation116], Carr et al. identified patients with thrombocytosis to be at a higher risk of poor outcomes [Citation117]. For patients with advanced HCC treated with ICPIs, high PLR was found to be a negative prognostic factor for survival [Citation113,Citation114].
Despite these encouraging early findings, inflammation status inevitably fluctuates during disease development and progression, and thus, both NLR and PLR will vary depending on the time of sampling. The utility of dynamic changes in NLR, PLR, and other inflammatory markers during treatment of HCC with ICPIs is a subject of further interest. Overall, the validity of these associations requires further investigation in patients with HCC before they can be considered for clinical use.
9. Conclusion
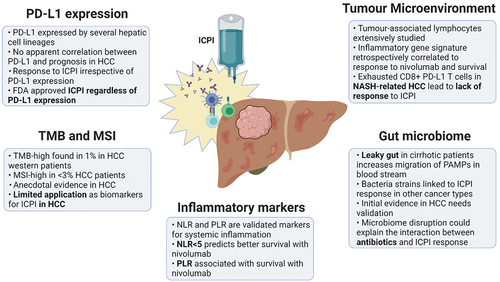
In conclusion, no single biomarker has consistently demonstrated accuracy as a predictive factor for response to ICPI in HCC (). Efforts have been made to stratify HCC into subclasses according to genomic characteristics, however profiling studies have not translated to clinical benefit for HCC patients. Despite holding a primary role in orienting the treatment algorithm of other cancer types, PD-L1 has not shown substantial predictive value for HCC, and all approved ICPI-based therapies are administered regardless of PD-L1 status. Other biomarkers, such as TMB or MSI status have revealed to be of little value in HCC, due to their low prevalence. Promising data could come from further studies on the interaction between the tumor microenvironment, the gut microbiome, and the ICPI efficacy, but evidence for utility is still in the early stage for HCC.
Table 1. Clinical evidence on predictive and prognostic biomarkers of response in patients with advanced hepatocellular carcinoma treated with immune checkpoint inhibitors (ICPI)
10. Expert opinion
The introduction of ICPI in clinical practice has represented a true paradigm shift in the management of HCC after more than a decade of universal TKI use. The results of the IMbrave150 trial [Citation13] and the consequent approval of atezolizumab plus bevacizumab as first-line treatment have corresponded to a significant benefit for patients with HCC, in light of convincing evidence for survival benefit. PD-1 monotherapy with pembrolizumab remains an FDA-approved therapy in sorafenib experienced patients, although significant OS benefit was never demonstrated. While further improving the outlook of patients diagnosed with HCC, these radical changes in the treatment landscape of liver cancer bring new challenges. Indeed, only a minority of patients seem to respond to ICPI, while primary disease progression occurs in approximately 20–30% of the patients [Citation14]. The lack of predictive biomarkers of therapeutic benefit to ICPI is one of the areas of highest unmet needs in hepato-oncology, where the a priori identification of responders would allow to individualize ICPI administration, sparing nonresponders from the added risk of immune-related toxicities. Efforts have been made to guide scientific research in this domain [Citation118], however – conclusive and practice-changing evidence is still lacking.
Many researchers have focused their work on validating, in HCC, the role that PD-L1 has in NSCLC, where it is a well-known predictive factor for ICPI [Citation48]. However, when considered as an isolated marker, PD-L1 expression has a limited and unverified role in prognosticating response to ICPI in HCC. While studies assessing the role of PD-L1 expression in combination therapy remain limited, current ICPI therapies are administered irrespective of PD-L1 status. It is conceivable that, due to its high intratumor and intrapatient variability and the lack of test standardization [Citation60], a number of biomarkers, rather than PD-L1 alone, may form part of the assessment of intrinsic tumor immunogenicity [Citation36]. Furthermore, the profound HCC heterogeneity, considering both tumor genomic and the tumor microenvironment, calls into question the value of analyses performed on a single archival tissue sample, especially if collected within a significant time interval from initiation of immunotherapy. For the purpose of biomarker qualification, the importance of fresh biopsies and repeat biomarker assessment at the point of disease progression remains invaluable. The use of radiological criteria for HCC diagnosis, while reducing the need of an expensive and potentially dangerous procedure, may decrease the availability of tissue samples for retrospective research. For this reason, international societies advocate the importance of multi-institutional, global consortia, in an effort to promote shared human biological material collection in the setting of prospective studies [Citation118].
While a number of studies have documented coherent HCC molecular subclassifications [Citation26], prospective validation and adoption in clinical studies is the only pathway to ensure clinical application, obviating from the risk of remaining purely descriptive pathologic traits. In this context, conduction of pre-registrative drug development programs of immune-based therapies should be complemented by a rationale biomarker development plan involving prospective collection of biological samples in order to complement the important clinical advances with precious translational insights.
Alongside more traditional human biological material collection (i.e. tumor tissue and blood), stool collection has become essential for the study of the intestinal microbiome. Evidence for a significant interaction between gut bacterial richness and diversity and outcomes from immunotherapy has become undeniable [Citation71]. Even though the pathogenesis and progression of HCC has been linked to the unopposed release of intestinal MAMPs and PAMPs [Citation93], a large chapter needs still be written regarding the role of gut microbiota and immunotherapy efficacy in HCC. Further studies will enlighten this aspect, and maybe they will provide a key to interpreting the mounting clinical evidence surrounding the interaction between antibiotics and response to ICPI in HCC, with a possible underlying role of microbiota disruption [Citation119].
n the next future, clinical studies investigating ICPI in HCC will have to take into account the differential responsiveness to immunotherapy shown in patients with NASH [Citation90]. While, at the moment, the evidence is not robust enough to exclude these patients from receiving immunotherapy, future trials will need to consider etiology of chronic liver disease as a stratifying factor, taking into account different immunobiologic mechanisms of progression that might differentially impact ICPI efficacy across etiologies of HCC [Citation91]. On the other hand, further mechanistic studies are also warranted to improve our understanding of ICPI combination therapy in HCC. While multi-technology assessment of human biological materials has become commonplace in biomarker development, the use of simple prognostic tools, readily available from routine blood tests such as NLR or PLR [Citation113,Citation114], should not be disregarded, as they can be a more easily accessible alternative in clinical practice or in resource-deprived areas. Moreover, while ICPIs are increasingly being used in the management of patients with advanced HCC, predictive biomarkers of response are needed to disentangle the complex therapeutic landscape. Along with atezolizumab plus bevacizumab [Citation13], other ICPI combinations are likely to add new treatment options for patients with advanced HCC in the next future [Citation120,Citation121]. For this reason, further research into the prognostic and predictive value of the aforementioned biomarkers in the presence of combination therapy is warranted, including double ICPIs or anti-VEGF agents, to facilitate meaningful application in clinical practice. In the context of a highly heterogeneous oncological indication with multiple disease processes contributing as potential source of clinically available biomarkers; a closer collaboration between clinicians and translational researchers is of paramount importance to shape the ongoing quest for biomarkers in HCC.
Declaration of Interest
A D’Alessio received congress support from Roche. A Cortellini received consulting fees from MSD, BMS, AstraZeneca, Roche; speakers’ fee from AstraZeneca, MSD, Novartis and Astellas. DJ Pinato received lecture fees from ViiV Healthcare, EISAI, IPSEN and Bayer Healthcare and travel expenses from BMS and Bayer Healthcare; consulting fees for Mina Therapeutics, EISAI, Roche, IPSEN, DaVolterra, Mursla and Astra Zeneca; received research funding (to institution) from MSD and BMS. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Article highlights
Only a minority of patients respond to immune checkpoint-inhibitors (ICPI), and the search of predictive biomarkers of response is crucial to optimize the treatment outcomes
While PD-L1 expression has been associated with improved response, it lacks a predictive role in hepatocellular carcinoma treatment
Tumour mutational burden and microsatellite instability are a rare finding in HCC, and they are of little value as predictors of response
Inflammatory cells in the tumour microenvironment modulate the response to immunotherapy, as seen for exhausted CD8+ T cell in patients with NASH not responding to ICPI
Gut microbiome plays a role in HCC pathogenesis and its interaction with the response to ICPI will be probably clarified in the next future
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
A D’Alessio is supported by the NIHR Imperial BRC and by grant funding from the European Association for the Study of the Liver (Andrew Burroughs Fellowship). A Cortellini is supported by the NIHR Imperial BRC. DJ Pinato is supported by grant funding from the Wellcome Trust Strategic Fund (PS3416) and acknowledges grant support from the Cancer Treatment and Research Trust (CTRT) and infrastructural support by the Cancer Research UK Imperial Centre and the NIHR Imperial Biomedical Research Centre. The authors are fully responsible for all content and editorial decisions. The image was realized with biorender.com.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. Available from.
- Capocaccia R, Sant M, Berrino F, et al. Hepatocellular carcinoma: trends of incidence and survival in Europe and the United States at the end of the 20th century. Am J Gastroenterol. 2007;102(8):1661–1670. Available from.
- Sarveazad A, Agah S, Babahajian A, et al. Predictors of 5 year survival rate in hepatocellular carcinoma patients. J Res Med Sci. 2019;24:86. Available from.
- Chen XP, Qiu FZ, Wu ZD, et al. Long-term outcome of resection of large hepatocellular carcinoma. Br J Surg. 2006;93(5):600–606. Available from.
- Pinato DJ, Guerra N, Fessas P, et al. Immune-based therapies for hepatocellular carcinoma. Oncogene. 2020;39:3620–3637. Available from.
- Rizzo A, Ricci A, Brandi G. Immune-based combinations for advanced hepatocellular carcinoma: shaping the direction of first-line therapy. Future Oncol. 2021;17(7):755–757. Available from.
- Sangro B, Gomez-Martin C, de La Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59(1):81–88. Available from.
- Yau T, Kang YK, Kim TY, et al. Efficacy and Safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the checkmate 040 randomized clinical trial. JAMA Oncol. 2020;6(11):e204564–e204564. Available from.
- Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940–952. Available from.
- Yau T, Park JW, Finn RS, et al. CheckMate 459: a randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2019;30:v874–v875. Available from.
- Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38(3):193–202. Available from.
- Rizzo A, Ricci A, Brandi G. Atezolizumab in advanced hepatocellular carcinoma: good things come to those who wait. Immunotherapy. 2021;13(8):637–644. Availale from.
- Finn RS, Qin S, Ikeda M, et al.Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. Available from
- Finn RS, Qin S, Ikeda M, et al. IMbrave150: updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC). J Clin Oncol. 2021;39(3_suppl):267. Available from.
- Ren Z, Fan J, Xu J, et al. LBA2 Sintilimab plus bevacizumab biosimilar vs sorafenib as first-line treatment for advanced hepatocellular carcinoma (ORIENT-32)2. Ann Oncol. 2020;31:S1287. Available from.
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. Available from.
- Faivre S, Rimassa L, Finn RS. Molecular therapies for HCC: looking outside the box. J Hepatol. 2020;72(2):342–352. Available from.
- Santoro A, Rimassa L, Borbath I, et al. Tivantinib for second-line treatment of advanced hepatocellular carcinoma: a randomised, placebo-controlled phase 2 study. Lancet Oncol. 2013;14(1):55–63. Available from.
- Rimassa L, Assenat E, Peck‑Radosavljevic M, et al. Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): a final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol. 2018;19(5):682–693. Available from.
- Kanzaki H, Chiba T, Ao J, et al. The impact of FGF19/FGFR4 signaling inhibition in antitumor activity of multi-kinase inhibitors in hepatocellular carcinoma. Sci Rep. 2021;11(1):1–12. Available from.
- Kim RD, Sarker D, Meyer T, et al. First-in-human phase i study of fisogatinib (BLU-554) validates aberrant FGF19 signaling as a driver event in hepatocellular carcinoma. Cancer Discov. 2019;9(12):1696–1707. Available from.
- Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2(1):1–23. Available from.
- Zucman-Rossi J, Villanueva A, Nault JC, et al. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology. 2015;149(5):1226–1239.e4. Available from.
- Villanueva A, Portela A, Sayols S, et al. DNA methylation-based prognosis and epidrivers in hepatocellular carcinoma. Hepatology. 2015;61(6):1945–1956. Available from.
- Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(6):1–28. Available from.
- Sia D, Jiao Y, Martinez-Quetglas I, et al.Identification of an immune-specific class of hepatocellular carcinoma, based on molecular features. Gastroenterology. 2017;153(3):812–826. Available from
- Nault JC, Mallet M, Pilati C, et al. High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat Commun. 2013;4(1):1–7. Available from.
- Schulze K, Imbeaud S, Letouzé E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Gen. 2015;47(5):505–511. Available from.
- Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature. 2015;523(7559):231–235. Available from.
- de Galarreta M R, Bresnahan E, Molina-Sánchez P, et al.β-catenin activation promotes immune escape and resistance to anti-PD-1 therapy in hepatocellular carcinoma. Cancer Discov. 2019;9(8):1124–1141. Available from
- Luke JJ, Bao R, Sweis RF, et al. WNT/β-catenin pathway activation correlates with immune exclusion across human cancers. Clin Cancer Res. 2019;25(10):3074–3083. Available from.
- Morita M, Nishida N, Sakai K, et al. Immunological microenvironment predicts the survival of the patients with hepatocellular carcinoma treated with anti-PD-1 antibody. Liver Cancer. 2021;10:380–393.
- Haber PK, Torres-Martin M, Dufour J-F, et al. Molecular markers of response to anti-PD1 therapy in advanced hepatocellular carcinoma. J Clin Oncol. 2021;39(15_suppl):4100.
- Von Felden J, Craig AJ, Garcia-Lezana T, et al. Mutations in circulating tumor DNA predict primary resistance to systemic therapies in advanced hepatocellular carcinoma. Oncogene. 2021;40(1):140–151. Available from.
- Chen G, Huang AC, Zhang W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560(7718):382–386. Available from.
- Sangro B, Melero I, Wadhawan S, et al. Association of inflammatory biomarkers with clinical outcomes in nivolumab-treated patients with advanced hepatocellular carcinoma. J Hepatol. 2020;73(6):1460–1469. Available from.
- Yin Z, Ma T, Lin Y, et al. IL-6/STAT3 pathway intermediates M1/M2 macrophage polarization during the development of hepatocellular carcinoma. J Cell Biochem. 2018;119(11):9419–9432. Available from.
- Yu MC, Chen CH, Liang X, et al. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology. 2004;40(6):1312–1321. Available from.
- Kassel R, Cruise MW, Iezzoni JC, et al. Chronically inflamed livers up-regulate expression of inhibitory B7 family members. Hepatology. 2009;50(5):1625–1637. Available from.
- Qin W, Hu L, Zhang X, et al. The diverse function of PD-1/PD-L1 pathway beyond cancer. Front Immunol. 2019;10:2298. Available from.
- Yan Y, Zheng L, Du Q, et al. Interferon regulatory factor 1 (IRF-1) and IRF-2 regulate PD-L1 expression in hepatocellular carcinoma (HCC) cells. Cancer Immunol Immunother. 2020;69(9):1891–1903. Available from.
- Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, et al. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med. 2012;209(6):1201–1217. Available from.
- Naito Y, Saito K, Shiiba K, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58(16):3491–3494.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. Available from.
- Kythreotou A, Siddique A, Mauri FA, et al. PD-L1. J Clin Pathol. 2018;71(3):189–194. Available from.
- Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Can Res. 2020;10(3):727–742. Available from: PMID: 32266087.
- Kim HD, Song GW, Park S, et al. Association between expression level of PD1 by tumor-infiltrating CD8+ T cells and features of hepatocellular carcinoma. Gastroenterology. 2018;155(6):1936–1950.e17. Available from.
- Doroshow DB, Bhalla S, Beasley MB, et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat Rev Clin Oncol. 2021;18(6):345–362. Available from.
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. Available from.
- Daud AI, Wolchok JD, Robert C, et al. Programmed death-ligand 1 expression and response to the anti-programmed death 1 antibody pembrolizumab in melanoma. J Clin Oncol. 2016;34(34):4102–4109. Available from.
- Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915–1928. Available from.
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. Available from.
- Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–2121. Available from.
- Shah MA, Adenis A, Enzinger PC, et al. Pembrolizumab versus chemotherapy as second-line therapy for advanced esophageal cancer: phase 3 KEYNOTE-181 study. J Clin Oncol. 2019;37(15_suppl):4010. Available from.
- Gao Q, Wang XY, Qiu SJ, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15(3):971–979. Available from.
- Feun LG, Li YY, Wu C, et al. Phase 2 study of pembrolizumab and circulating biomarkers to predict anticancer response in advanced, unresectable hepatocellular carcinoma. Cancer. 2019;125(20):3603–3614. Available from.
- Liu GM, Li XG, Zhang YM. Prognostic role of PD-L1 for HCC patients after potentially curative resection: a meta-analysis. Cancer Cell Int. 2019;19(1):22. Available from.
- Calderaro J, Rousseau B, Amaddeo G, et al. Programmed death ligand 1 expression in hepatocellular carcinoma: relationship with clinical and pathological features. Hepatology. 2016;64(6):2038–2046. Available from.
- Lee MS, Ryoo BY, Hsu CH, et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol. 2020;21(6):808–820. Available from.
- Pinato DJ, Mauri FA, Spina P, et al.Clinical implications of heterogeneity in PD-L1 immunohistochemical detection in hepatocellular carcinoma: the blueprint-HCC study. Br J Cancer. 2019;120(11):1033–1036. Available from
- L-C L, Lee Y-H, Chang C-J, et al. Increased expression of programmed death-ligand 1 in infiltrating immune cells in hepatocellular carcinoma tissues after sorafenib treatment. Liver Cancer. 2019;8(2):110–120. Available from.
- L-C L, Hsu C, Shao -Y-Y, et al. Differential organ-specific tumor response to immune checkpoint inhibitors in hepatocellular carcinoma. Liver Cancer. 2019;8(6):480–490. Available from.
- Vilain RE, Menzies AM, Wilmott JS, et al. Dynamic changes in PD-L1 expression and immune infiltrates early during treatment predict response to PD-1 blockade in melanoma. Clin Cancer Res. 2017;23(17):5024–5033. Available from.
- Fessas P, Spina P, Boldorini RL, et al. Phenotypic characteristics of the tumour microenvironment in primary and secondary hepatocellular carcinoma. Cancers (Basel). 2021;13(9):2137. Available from.
- Xu J, Zhang Y, Jia R, et al. Anti-PD-1 antibody SHR-1210 combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: an open-label, dose escalation and expansion study. Clin Cancer Res. 2019;25(2):515–523. Available from.
- Efremova M, Finotello F, Rieder D, et al. Neoantigens generated by individual mutations and their role in cancer immunity and immunotherapy. Front Immunol. 2017;8:1679. Available from.
- Marabelle A, Fakih M, Lopez J, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21(10):1353–1365. Available from.
- Boyiadzis MM, Kirkwood JM, Marshall JL, et al. Significance and implications of FDA approval of pembrolizumab for biomarker-defined disease. Journal for ImmunoTherapy of Cancer. 2018;6(1):1–7. Available from.
- Ang C, Klempner SJ, Ali SM, et al. Prevalence of established and emerging biomarkers of immune checkpoint inhibitor response in advanced hepatocellular carcinoma. Oncotarget. 2019;10(40):4018–4025. Available from.
- Yarchoan M, Albacker LA, Hopkins AC, et al. PD-L1 expression and tumor mutational burden are independent biomarkers in most cancers. JCI Insight. 2019;4(6). Available from. DOI:https://doi.org/10.1172/jci.insight.126908.
- Vétizou M, Pitt JM, Daillère R, et al.Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–1084. Available from
- Wong CN, Fessas P, Dominy K, et al. Qualification of tumour mutational burden by targeted next‐generation sequencing as a biomarker in hepatocellular carcinoma. Liver Int. 2021;41(1):192–203. Available from.
- Tang X, Fan L, Chen G, et al. Higher level of tumor mutational burden and 11q13 amplification in Chinese hepatocellular carcinoma patients. Cancer Res. 2018;78(13_s):4349. Available from.
- Loeb LA, Bielas JH, Beckman RA. Cancers exhibit a mutator phenotype: clinical implications. Cancer Res. 2008;68(10):3551–3557. Available from.
- Roberts SA, Gordenin DA. Hypermutation in human cancer genomes: footprints and mechanisms. Nat Rev Cancer. 2014;14(12):786–800. Available from.
- Eso Y, Shimizu T, Takeda H, et al. Microsatellite instability and immune checkpoint inhibitors: toward precision medicine against gastrointestinal and hepatobiliary cancers. J Gastroenterol. 2020;55(1):15–26. Available from.
- Lee V, Murphy A, Le DT, et al. Mismatch repair deficiency and response to immune checkpoint blockade. Oncologist. 2016;21(10):1200–1211. Available from.
- Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl Med. 2015;372(26):2509–2520. Available from.
- Goumard C, Desbois-Mouthon C, Wendum D, et al. Low levels of microsatellite instability at simple repeated sequences commonly occur in human hepatocellular carcinoma. Cancer Genomics Proteomics. 2017;14:329–339. Available from.
- Kawaoka T, Ando Y, Yamauchi M, et al. Incidence of microsatellite instability-high hepatocellular carcinoma among Japanese patients and response to pembrolizumab. Hepatol Res. 2020;50(7):885–888. Available from.
- Ando Y, Yamauchi M, Suehiro Y, et al. Complete response to pembrolizumab in advanced hepatocellular carcinoma with microsatellite instability. Clin J Gastroenterol. 2020;13(5):867–872. Available from.
- Anguille S, Smits EL, Bryant C, et al. Dendritic cells as pharmacological tools for cancer immunotherapys. Pharmacol Rev. 2015;67(4):731–753. Available from.
- Budhu A, Forgues M, Ye QH, et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10(2):99–111. Available from.
- Sheppard S, Ferry A, Guedes J, et al. The paradoxical role of NKG2D in cancer immunity. Front Immunol. 2018;9:1808. Available from.
- Sun H, Huang Q, Huang M, et al. Human CD96 correlates to natural killer cell exhaustion and predicts the prognosis of human hepatocellular carcinoma. Hepatology. 2019;70(1):168–183. Available from.
- Lachenmayer A, Alsinet C, Savic R, et al. Wnt-pathway activation in two molecular classes of hepatocellular carcinoma and experimental modulation by sorafenib. Clin Cancer Res. 2012;18(18):4997–5007. Available from.
- Harding JJ, Nandakumar S, Armenia J, et al. Prospective genotyping of hepatocellular carcinoma: clinical implications of next-generation sequencing for matching patients to targeted and immune therapies. Clin Cancer Res. 2019;25(7):2116–2126. Available from.
- Ding W, Xu X, Qian Y, et al. Prognostic value of tumor-infiltrating lymphocytes in hepatocellular carcinoma: a meta-analysis. Medicine (Baltimore). 2018;97(50):e13301. Available from.
- Chew V, Chen J, Lee D, et al. Chemokine-driven lymphocyte infiltration: an early intratumoural event determining long-term survival in resectable hepatocellular carcinoma. Gut. 2012;61(3):427–438. Available from.
- Pfister D, Núñez NG, Pinyol R, et al.NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592(7854):450–456. Available from
- Kelley RK, Grete TF. Hepatocellular carcinoma - origins and outcomes. N Engl J Med. 2021;385(3):280–282. Available from.
- Hectors S, Lewis S, Besa C, et al. MRI radiomics features predict immuno-oncological characteristics of hepatocellular carcinoma. Eur Radiol. 2020;30(7):3759–3769. Available from.
- Naqash AR, Kihn-Alarcón AJ, Stavraka C, et al. The role of gut microbiome in modulating response to immune checkpoint inhibitor therapy in cancer. Ann Transl Med. 2021;9(12):1034. Available from.
- Schwabe RF, Greten TF. Gut microbiome in HCC – mechanisms, diagnosis and therapy. J Hepatol. 2020;72(2):230–238. Available from.
- Gopalakrishnan V, Helmink BA, Spencer CN, et al. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell. 2018;33(4):570–580. Available from.
- Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084–1089. Available from.
- Chaput N, Lepage P, Coutzac C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2017;28(6):1368–1379. Available from.
- Zheng Y, Wang T, Tu X, et al. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J Immunother Cancer. 2019;7(1). Available from. DOI:https://doi.org/10.1186/s40425-019-0650-9.
- Cheng WY, Wu CY, Yu J. The role of gut microbiota in cancer treatment: friend or foe? Gut. 2020;69:1867–1876. Available from.
- Tripathi A, Debelius J, Brenner DA, et al. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. 2018;15:397–411. Available from
- Yoshimoto S, Loo TM, Atarashi K, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499(7456):97–101. Available from.
- Galloway-Peña JR, Smith DP, Sahasrabhojane P, et al. Characterization of oral and gut microbiome temporal variability in hospitalized cancer patients. Genome Med. 2017;9(1):21. Available from.
- David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. Available from.
- Iida N, Dzutsev A, Stewart CA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342(6161):967–970. Available from.
- Cortellini A, Tucci M, Adamo V, et al. Integrated analysis of concomitant medications and oncological outcomes from PD-1/PD-L1 checkpoint inhibitors in clinical practice. J Immunother Cancer. 2020;8(2):1361. Available from.
- Sanghera C, Teh JJ, Pinato DJ. The systemic inflammatory response as a source of biomarkers and therapeutic targets in hepatocellular carcinoma. Liver Int. 2019;39:2008–2023. Available from.
- Diakos CI, Charles KA, McMillan DC, et al. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–e503. Available from.
- Roxburgh CSD, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6(1):149–163. Available from.
- Kusumanto YH, Dam WA, Hospers GAP, et al. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis. 2003;6(4):283–287. Available from.
- Mano Y, Shirabe K, Yamashita YI, et al. Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: a retrospective analysis. Ann Surg. 2013;258(2):301–305. Available from.
- Motomura T, Shirabe K, Mano Y, et al. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol. 2013;58(1):58–64. Available from.
- Chan SL, Chan AWH, Chan AKC, et al. Systematic evaluation of circulating inflammatory markers for hepatocellular carcinoma. Liver Int. 2017;37(2):280–289. Available from.
- Dharmapuri S, Özbek U, Lin J, et al. Predictive value of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in advanced hepatocellular carcinoma patients treated with anti–PD‐1 therapy. Cancer Med. 2020;9(14):4962–4970. Available from.
- Muhammed A, Fulgenzi CAM, Dharmapuri S, et al. The systemic inflammatory response identifies patients with adverse clinical outcome from immunotherapy in hepatocellular carcinoma. Cancers (Basel). 2022;14:186. Available from.
- Zheng YB, Zhao W, Liu B, et al. The blood neutrophil-to-lymphocyte ratio predicts survival in patients with advanced hepatocellular carcinoma receiving sorafenib. Asian Pac J Cancer Prev. 2013;14(9):5527–5531. Available from.
- Carr BI, Guerra V, de Giorgio M, et al. Small hepatocellular carcinomas and thrombocytopenia. Oncology. 2012;83(6):331–338. Available from.
- Carr BI, Guerra V. Thrombocytosis and hepatocellular carcinoma. Dig Dis Sci. 2013;58(6):1790–1796. Available from.
- Singal AG, Hoshida Y, Pinato DJ, et al. International Liver Cancer Association (ILCA) white paper on biomarker development for hepatocellular carcinoma. Gastroenterology. 2021;160(7):2572–2584.
- Fessas P, Naeem M, Marron TU, et al. Early antibiotic exposure delays disease progression following immune checkpoint inhibitor therapy for hepatocellular carcinoma: evidence from an observational study. In: Proceedings of the 112th Annual Meeting of the American Association for Cancer Research; 2021 Apr 10-15. Philadelphia (PA): AACR; 2021. Abstract nr 485.
- Kelley RK. Cabozantinib (C) plus atezolizumab (A) versus sorafenib (S) as first-line systemic treatment for advanced hepatocellular carcinoma (aHCC): results from the randomized phase III COSMIC-312 trial. ESMO Virtual Plenary. 2021 November 20.
- Abou-Alfa GK, Chan SL, Kudo M, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA2022. 40(4_suppl): 379–379.