ABSTRACT
Introduction
Symptomatic testing and asymptomatic screening for SARS-CoV-2 continue to be essential tools for mitigating virus transmission. Though COVID-19 diagnostics initially defaulted to oropharyngeal or nasopharyngeal sampling, the worldwide urgency to expand testing efforts spurred innovative approaches and increased diversity of detection methods. Strengthening innovation and facilitating widespread testing remains critical for global health, especially as additional variants emerge and other mitigation strategies are recalibrated.
Areas covered
A growing body of evidence reflects the need to expand testing efforts and further investigate the efficiency, sensitivity, and acceptability of saliva samples for SARS-CoV-2 detection. Countries have made pandemic response decisions based on resources, costs, procedures, and regional acceptability – the adoption and integration of saliva-based testing among them. Saliva has demonstrated high sensitivity and specificity while being less invasive relative to nasopharyngeal swabs, securing saliva’s position as a more acceptable sample type.
Expert opinion
Despite the accessibility and utility of saliva sampling, global implementation remains low compared to swab-based approaches. In some cases, countries have validated saliva-based methods but face challenges with testing implementation or expansion. Here, we review the localities that have demonstrated success with saliva-based SARS-CoV-2 testing approaches and can serve as models for transforming concepts into globally-implemented best practices.
1. Introduction
The ‘gold standard’ sample types for SARS-CoV-2 detection defaulted early in the pandemic to nasopharyngeal or oropharyngeal swabs, as these are established diagnostic practices used in identifying other respiratory viruses [Citation1,Citation2]. However, swab-based sample collection can be costly, invasive, and put health personnel at risk by necessitating close contact during sample collection. Furthermore, the rapid evolution of the pandemic placed pressure on supply chains, increasing the need for alternative diagnostic tools to enable testing expansion (an overview of common approaches are shown in ). This led to the consideration of additional specimen types with saliva emerging as a quality alternative [Citation3], a strategy supported by the continued growing body of evidence demonstrating optimization of saliva-based methods.
Figure 1. Overview of the most common sample types and testing methods for SARS-CoV-2 detection. The table in blue summarizes characteristics for the different sample types depicted, beneath. The table in grey summarizes typical characteristics of the testing methods depicted above.‘Nasal swabs’ include both mid-turbinate and anterior nasal swabs. Rapid antigen tests may also be known as lateral flow assays. POC = point of care. H = hour. Created with BioRender.com.
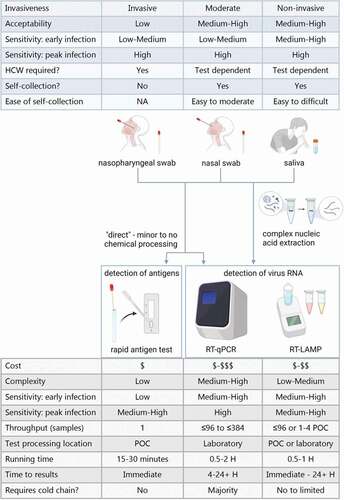
With appropriate treatment, saliva can be more sensitive than swabs for SARS-CoV-2 detection [Citation4], particularly in early infections where saliva can be twelve times more sensitive compared to mid-turbinate swabs [Citation5–7]. Additionally, less-invasive forms of testing are often preferred by patients over other specimen-collection options [Citation8]. Public preference and accessibility of desired options directly influence testing participation rates [Citation9]. Adding options like saliva-based testing that have clear diagnostic benefits and broad public acceptance serve to both remove barriers and strengthen public health response strategies. As a result, saliva-based testing continues to increase worldwide health security.
Many countries have demonstrated the successful introduction of saliva-based diagnostic testing as part of their public health responses. At the same time, misinformation and skepticism generated by the debate surrounding saliva as a reliable sample type have impeded the implementation and expansion of saliva testing programs. To demonstrate the current state of saliva-based SARS-CoV-2 testing internationally, this review highlights the best practices employed by successful testing operations, including effective methodologies and the use of low-cost materials, while addressing barriers and challenges that should be considered when developing or implementing salivary diagnostic programs. With this information, national leaders, health agencies, and research institutes hesitant about implementation can better consider saliva-based testing as a viable addition to the robust testing programs seeking to reduce preventable loss of life. As local context and populations requiring testing (e.g. asymptomatic adults vs. symptomatic children) vary, optimal methods for successful program implementation may differ. A protocol that is most suitable for one country’s population may not be the most appropriate for another. With a sound knowledge base, diverse populations can tailor methodologies and practices from the plethora of successful validation studies that have emerged globally throughout this pandemic and build a program that reflects their needs.
We note that this review is not systematic in nature, nor is this review a meta-analysis. Rather, the purpose of this review of the available literature is to highlight the regional acceptability, and the successes of various saliva-based methodologies for the detection of SARS-CoV-2 across the globe. It is intended to showcase examples as well as generate leads that researchers, public health decision-makers, and others can take from the trials experienced during the COVID-19 pandemic and apply to ongoing or future response efforts. Both systematic review and meta-analysis methodologies would have been too narrow to gather the insights this review has generated, as our primary concern was not just published research, but how research on saliva-based testing has been received by governments and regulatory bodies as well. Furthermore, meta-analyses conducted on this topic to date, often mention the differences in sample collection and processing that exist, but do not take these factors into consideration in the analysis, leading to confounded results and reporting. Our search strategy thus included reviewing each major geographic region, using a variety of search engines, to find published research, government documents, press releases, and other materials to understand how saliva as a sample type has been explored or implemented for the detection of SARS-CoV-2. The examples reported on were studies and/or technologies that showed the high performance of saliva as compared to swab-based approaches. We hope that this review encourages additional international collaboration to share and employ best practices for COVID-19 diagnostics, screening, and surveillance, and guide future research efforts.
2. Geographic regions
2.1. North America
In Canada, saliva was first suggested to be a viable diagnostic sample type in February 2021 [Citation10]. As of March 2022 [Citation11], the Canadian Government acknowledges saliva as a sample type for general COVID-19 diagnostics, but has yet to approve any of the saliva tests that have been submitted for consideration to their health agency (Health Canada). Despite this, the British Columbia Center for Disease Control has approved a gargle-and-spit testing method, involving the gargling of a saline solution then expelling saliva into a tube [Citation12].
Saliva testing in the United States of America (USA) has been expansive: the Food and Drug Administration (FDA) has awarded Emergency Use Authorization (EUA) to 33 saliva-based molecular assays for SARS-CoV-2 detection [Citation13]. Saliva as a sensitive sample type was first formally recognized when the FDA awarded an EUA to a PCR test developed by Rutgers University [Citation14] in early April 2020 [Citation15]. Other universities across the USA have also been integral to the innovation of saliva methods, working to address ease, affordability, and the ability to circumvent collapsing swab supply chains, including the Yale School of Public Health’s open-source SalivaDirect™ protocol [Citation16], and the University of Illinois Urbana-Champaign’s covidSHIELD program for asymptomatic screening [Citation17]. Many other universities in the USA have implemented high-volume, saliva-based testing programs specifically for their campuses and communities [Citation18–24]. Saliva-based assays have also permitted safer returns to community centers, refugee resettlement projects, workplaces, and summer camps across the nation [Citation25,Citation26]. With lower vaccine coverage among children relative to adults [Citation27], the urgency of testing vulnerable school populations was accommodated by Mirimus Labs (Brooklyn, NY), who were early innovators that offered pooled saliva testing affordably to schools so they could retain in-person learning for hundreds of thousands of students [Citation28].
Commercialized saliva-based testing is also prevalent across the USA, servicing multiple private sector industries. Biotechnology companies have developed convenient saliva-based tests that can offer safer at-home sample collection by reducing viral exposure compared to testing centers [Citation29], avert the more costly RNA extraction step [Citation30,Citation31], process results with portable PCR machines [Citation32,Citation33] or point-of-care instruments [Citation34] to increase accessibility, streamline test processing through automated laboratory equipment [Citation32,Citation33] and enable sample pooling [Citation35], or the detection of other respiratory illnesses via multiplexing [Citation36,Citation37].
Biotechnology companies have also partnered with state health departments to deliver saliva testing services to the public sector [Citation38–42]. For example, the Howard County Health Department in Maryland contracted a company to provide free PCR saliva testing to the general public for both on-site or at-home testing [Citation38], while the Minnesota Department of Health has offered on-site saliva testing for all Minnesota residents at no cost [Citation40]. Biotechnology companies have also made partnerships with the aviation sector to provide saliva-based testing for inbound and outbound airport travelers [Citation43–45].
An alternative saliva testing methodology, loop-mediated isothermal amplified testing (RT-LAMP), has been adapted in the USA as a simplified COVID-19 diagnostic option relative to PCR. The performance of some RT-LAMP assays is comparable to RT-PCR [Citation46], demonstrating concordance as high as 98.8% [Citation47], with options for extraction-free approaches [Citation48], multiplexing for SARS-CoV-2 and influenza [Citation49], or optimized for point-of-care devices [Citation50,Citation51].
More recently, American researchers have investigated the use of less common alternative diagnostic technologies, developing a silver nanotriangle array-based localized surface plasmon resonance (LSPR) sensor for use on saliva samples (February 2022) [Citation52] and chip technology for detection of SARS-CoV-2 in saline gargle samples via smartphone (March 2022) [Citation53]; both with the goal of providing quicker, easier testing. Overall, the abundance of robust saliva methods that have emerged in the USA validate saliva as a sustainable, reliable sample type to facilitate mass-testing programs and allow communities to more safely re-open.
In Mexico, saliva has reliably detected SARS-CoV-2 in healthcare workers [Citation54], patients [Citation55,Citation56], and children [Citation55,Citation56]. The inaccessibility of RNA extraction kits forced health authorities to validate RNA-extraction-free protocols at a local level [Citation57]. Saliva was successfully validated and implemented for primary school surveillance testing [Citation57] as well as sample pooling for high-throughput testing [Citation58]. As a result of nasopharyngeal swab shortages, saliva was also identified as a suitable sample type for testing asymptomatic healthcare workers (April 2021) [Citation59]. Thus, saliva permitted more affordable and simpler diagnostic procedures appropriate for scalable SARS-CoV-2 detection [Citation58].
2.2. Central America and the Caribbean
Countries throughout Central America and the Caribbean have been operating against intense resource and staffing constraints, challenging the region’s ability to effectively respond to the pandemic [Citation60]. Hospitals and community health centers have had to prioritize the acquisition of other essential healthcare resources, such as ventilators and personal protective equipment, limiting the efficiency and availability of testing [Citation61]. The lack of PCR testing options has imposed further stress on many of the region’s fragile healthcare systems by impeding the identification and isolation of infected healthcare professionals and other populations vulnerable to SARS-CoV-2 infection. Most of the region follows testing recommendations set forth by the Pan American Health Organization, which has called for an expansion of testing networks with a focus on rapid antigen tests (January 2022) [Citation62,Citation63].
Much of the Central America and Caribbean region abides by the current global standards of testing for incoming and departing international travelers, making the availability of PCR tests especially crucial in a time of high regional tourism. To meet testing demands related to international travel, some countries, including Costa Rica, have utilized saliva-based assays [Citation64] or will accept saliva-based tests for border entry [Citation65]. Island nations with high tourism rates, such as Aruba, Bonaire, and the Dominican Republic, boasted convenient saliva PCR testing locations and concierge services for outbound travelers in collaboration with Delta Airlines [Citation66–68].
Earlier in the pandemic, a Nicaragua-based trial among healthcare workers (July 2020) found saliva-based LAMP to be an attractive testing option in the face of scarcity and inefficient government testing [Citation69]. For testing of the general public, the SalivaDirect™ protocol was implemented in the U.S. Virgin Islands [Citation70] and The Bahamas (November 2020) to expand testing for asymptomatic individuals [Citation71], and a saliva-based PCR test developed in Puerto Rico (March 2021) is now used in major laboratories across the territory [Citation72,Citation73]. Although saliva-based PCR testing has been considered, most Caribbean nations solely rely on nasopharyngeal or oropharyngeal swab PCR testing for incoming travelers [Citation74,Citation75]. Thus, the region still faces a slow uptake of saliva-based PCR testing for COVID-19, despite demonstrations of its feasibility in implementation [Citation76].
2.3. South America
The rapid and dramatic spread of COVID-19 throughout many South American health care systems drove the need for expansive testing efforts. Throughout the region, saliva collection has been praised as a non-invasive approach [Citation77] that poses lower risk to healthcare providers [Citation77,Citation78] and is well-suited for low-resource settings [Citation79]. Studies conducted in Argentina (September 2020)[Citation80], French Guiana (October 2020) [Citation81], Chile (February 2021) [Citation77], and Colombia (October 2021) [Citation79] all found saliva to have high sensitivity (91–100%) and to serve as an effective alternative sample type to nasopharyngeal swabs. In Peru (March 2021), a study found high household incidence of COVID-19 through testing saliva specimens [Citation82]. In Ecuador, National Reference Laboratories have been recommended to use saliva samples alongside nasal and nasopharyngeal swabs when validating diagnostics for SARS-CoV-2 [Citation83]. In Brazil, RT-PCR screening of saliva samples was found to be a comparable method for the detection of certain SARS-CoV-2 variants, as a time-, cost-, and contact-saving alternative to genomic surveillance [Citation84].
Government authorization and commercial implementation currently varies considerably by country in South America. In Brazil, self-collected saliva specimens for PCR testing [Citation85], rapid antigen testing [Citation86,Citation87], and electrochemical RNA detection [Citation86,Citation88] are available for at-home testing and at drive-through community testing sites. Chile’s Health Minister has endorsed saliva testing in primary care settings [Citation89] and saliva testing results are accepted by Chilean airports [Citation90]. A novel, saliva CRISPR-Cas-based COVID-19 test was developed in Peru [Citation91] and two new isothermal amplification-based tests (iAMP and LAMP) using saliva have been validated in El Salvador and Paraguay [Citation92]. Although strong uptake by the private sector has yet to occur, South American research demonstrates continental investment in saliva for the detection of SARS-CoV-2.
2.4. Oceania
In Oceania, the COVID-19 response has varied among nations and their internal geographies. Some island nations have reported limited COVID-19 case data, such as Micronesia which reported no cases within their borders through 2021 [Citation93]. However other countries, such as Fiji and Papua New Guinea, reported zero or very few COVID-19 cases in 2020, but saw increases in case numbers during 2021, prompting a need for heightened testing and prevention measures [Citation94].
Saliva test findings in this region have mainly emerged from Australia and New Zealand. Although saliva was recognized early in the pandemic (April 2020) in Australia as a viable diagnostic sample type [Citation95], uptake was slow due to initial reports of reduced sensitivity relative to nasopharyngeal swabs. While this earlier data was potentially skewed by historic cases or methods not yet optimized for sensitive detection in this sample type, the authors later reported saliva to be effective for the detection of SARS-CoV-2 (December 2020) [Citation96] and an observational study (October 2020) found higher positive predictive agreement (PPA) for saliva (72%) than for combined oropharyngeal-nasal swabs (63%) [Citation97]. Furthermore, the study ran an optional preferences survey for participants finding that the saliva collection method was preferred by 79% of the respondents, including 92% of children under 10 years old [Citation97].
In early 2021, the Australian government called for heightened use of saliva testing for vulnerable populations and essential workers that require regular testing [Citation98]. Since November 2021, saliva testing has been utilized for essential workers within the New South Wales Ministry of Health’s COVID-19 Saliva Surveillance Program [Citation98]. There are currently three COVID-19 saliva tests approved by the Australian government, meaning that most Australian laboratories are required to perform their own validation of assays for saliva testing [Citation99]. A saliva rapid antigen test for at-home use has also been approved [Citation100]. A proof-of-concept screening method using infrared light technology is in development that measures the SARS-CoV-2 RNA signature (sensitivity = 93%) in the infrared spectra of saliva in symptomatic hospital patients [Citation101]. The portable test displays results in ~5 minutes greatly supporting reduced response times, a significant factor for interrupting transmission chains. The Australian Department of Health acknowledges the literature on saliva testing includes numerous methods that have demonstrated high sensitivity, but states that further studies are required to evaluate the best collection and processing methods for saliva [Citation98]. The current Australian Testing Framework (updated 7 March 2022) includes reference to many Australian laboratories that have validated saliva for RT-PCR testing to facilitate expanded surveillance. It also posits the use of saliva as an alternative specimen to nasopharyngeal swabs as a way to increase testing uptake and frequency as well as sample pooling and extraction-free PCR methods [Citation98].
In New Zealand, USA-developed RNA extraction-free, saliva-based PCR tests have been validated locally and offered commercially [Citation102,Citation103]. In May 2021, routine saliva testing was mandated for individuals at high risk of exposure [Citation104]. The initial legislative act on saliva testing required confirmatory nasopharyngeal swab testing for positive saliva results. However, this order was amended in December 2021 as New Zealand’s Ministry of Health updated their position on the use of saliva for diagnostic COVID-19 testing, reducing the required frequency of testing and eliminating the need for confirmatory testing of nasopharyngeal swabs. The update also endorsed the use of saliva as a sample for diagnostic testing in the aviation, maritime, and education sector [Citation105]; there are now designated locations for employees of these sectors to self-sample at home and then drop-off their samples in collection boxes [Citation106]. In the private sector, saliva-based testing is used for elder care settings [Citation103], certain workplaces, sports teams, and pre-departure travel certificates [Citation107]. In January 2022, a New Zealand-based, inexpensive, and portable RT-qPCR device was added onto the SalivaDirect protocol’s FDA EUA [Citation108] after demonstrating performance comparable to larger, immobile instruments [Citation109]. In addition to its implementation in the maritime sector, this has also been implemented in an endoscopy clinic for saliva-based screening of patients and staff [Citation110].
2.5. East and Central Asia
East Asian nations have spearheaded saliva-based COVID-19 testing, especially as some East Asian governments have been the first in the world to normalize it as the predominant sample type through legislation.
Hong Kong has been at the forefront of much of the saliva-based SARS-CoV-2 diagnostic research, innovation, and implementation. As early as February 2020, researchers in Hong Kong had identified that saliva samples contained SARS-CoV-2 in infected patients and therefore concluded that saliva showed promise as a noninvasive specimen for diagnosis, monitoring, and infection control in COVID-19 patients [Citation2]. Importantly, differences in viral shedding dynamics were observed between sample types, with tests utilizing nasopharyngeal swabs taking longer to turn negative than those that utilized saliva [Citation111]. This finding was particularly significant with respect to quarantine measures, as many countries have required a negative test to end isolation. A saliva-based RT-LAMP test was developed for mass on-site screening (July 2020) [Citation112], and self-collected mouth gargle samples demonstrated similar sensitivity for COVID-19 detection compared to deepthroat saliva samples (December 2020) [Citation113]. The Hong Kong government has a mixed-specimen approach to SARS-CoV-2 diagnostics, and recommends that oropharyngeal saliva specimens be used for asymptomatic individuals while symptomatic individuals receive both a nasal and a throat swab from a community testing center [Citation114].
In Taiwan (October 2020), researchers argued that self-collected saliva should be included in the arsenal of COVID-19 diagnostics, particularly as it protects healthcare workers and the broader community from unnecessary exposure and is minimally invasive [Citation115]. In South Korea (March 2020), the early identification of saliva as a useful specimen for diagnostic purposes led to the adoption of saliva-based methods by testing centers [Citation116,Citation117]. Since then, South Korean laboratory and health agencies have released guidelines on acceptable clinical diagnostics of SARS-CoV-2 (February 2022), recognizing saliva as a sample type that may benefit patients who require repetitive or less invasive sample collection [Citation118]. In Japan, after a direct SARS-CoV-2 salivary detection kit (one that does not require RNA extraction or purification) was developed (June 2020) [Citation119] and self-collected saliva demonstrated comparable sensitivity to nasopharyngeal swabs (June-July 2020) [Citation120,Citation121], saliva became the preferred sample-type for certain mass screening programs [Citation122,Citation123]. These findings drove innovation and enabled the implementation of saliva-based COVID-19 testing. As early as July 2020, Taiwan utilized PCR testing of oropharyngeal saliva samples for entry into the country [Citation124], and had developed rapid detection approaches that use biosensors (electrical double layer (EDL)-gated field-effect transistor-based biosensor [BioFET]) for use in screening programs [Citation125,Citation126]. At least two different saliva-based chemiluminescent enzyme immunoassays have been approved by the Japanese Ministry of Health, Labor, and Welfare, and are currently used in airports [Citation127]. For the purpose of contact tracing, most health care centers in Japan collect saliva samples, demonstrating its commonplace use as a screening tool for potentially-infected individuals. A self-collected PCR test developed in South Korea gained approval both domestically and internationally early in 2021 [Citation128]. In November 2021, a saliva-based RT-PCR kit was authorized by the Taiwan Food and Drug Administration [Citation129].
With early work from China demonstrating the utility of saliva for monitoring SARS-CoV-2 infection dynamics [Citation130], China has embarked on many new saliva-based diagnostic endeavors in 2022. Among these are the development and evaluation of a MALDI-TOF MS (Matrix Assisted Laser Desorption Ionization-time of Flight Mass Spectrometry) assay coupled with isothermal gene amplification from saliva (February 2022) [Citation131], the establishment of an enhanced substrate to be used saliva samples (February 2022) [Citation132], the amplification of nanoparticles for rapid detection of SARS-CoV-2 in saliva (March 2022)[Citation133], and the proposition of an electrochemical biosensor that can identify viral targets in samples containing only 10% saliva (April 2022) [Citation134].
In Central Asia, clinical studies evaluating saliva-based tests are underway in Kazakhstan [Citation135] and Mongolia [Citation136] with the aim to increase testing accessibility, comfort, and turnaround time of SARS-CoV-2 diagnostics in the region.
2.6. Southeast Asia
In January 2021, the Philippine Department of Health approved saliva-based testing by the Philippine Red Cross (PRC)[Citation137]. They validated the SalivaDirect™ protocol [Citation138] to provide a more equitable and affordable option for the region’s testing, which is offered to the public at testing sites and through at-home collection kits [Citation139]. Eventually, the Filipino government officially recognized the PRC’s saliva tests by including positive results in their national case counts [Citation140]. In Thailand (April 2020), a cross-sectional study found saliva to be a suitable alternative to nasopharyngeal swabs especially in low resource areas [Citation141]. As of February 2022, saliva appears to be a mainstream sample type as the Thailand government has authorized 63 at-home saliva-based rapid antigen tests [Citation142], although there is little information available on saliva-based PCR tests. Prior to the emergence of the Omicron variant, quarantine-free entry into Thailand was permitted for specific cities when inbound travelers produced a negative swab or saliva-based RT-PCR test result [Citation143].
In Singapore (August 2020), self-collected saliva specimens outperformed self-collected nasal swabs in sensitivity [Citation144]. Initial studies proposed that nasopharyngeal swabs should be used for confirmatory testing following a positive saliva-based test result [Citation145]. Furthermore, as the validity of saliva as a sample type became more widely accepted in diagnostics, Singapore implemented PCR saliva testing in airports for pre-departure testing [Citation146] the collection kit used in airports is registered as a medical device with the Singaporean Health Sciences Authority (September 2020). Approval of a saliva-based antigen test to be used in airports is still underway; however, one is in development that uses a proprietary on-kit amplification method that reports sensitivity close to laboratory RT-PCR assays [Citation147]. In Vietnam (April 2020), asymptomatic COVID-19 infections have been accurately detected by saliva-based PCR testing, determining that, despite higher yields of viral load being detected in nasal/throat swabs, saliva’s simplified collection process facilitates mass-screening initiatives [Citation148]. Additionally, saliva was validated in Indonesia (August 2021) on nine commercial kits using RT-PCR, including RNA-extraction free methods, to demonstrate its versatility in use across multiple existing workflows [Citation149].
2.7. South Asia
An investigation of the unique impact of the COVID-19 pandemic on low- and middle-income countries (June 2020) focused on Nepal and used saliva alongside other sample types for diagnostic testing [Citation150]. In a large-scale study at the COVID-19 Screening Unit of Dhaka Hospital in Bangladesh (November 2020) [Citation151,Citation152], paired saliva samples and nasopharyngeal swabs were collected from all research participants and the sensitivity of saliva (80%) met the criteria defined by the WHO [Citation151,Citation152].
Studies in India (September 2020 and May 2021) showed the advantages of saliva testing for the detection of SARS-CoV-2 compared to nasopharyngeal swabs, including cost-effectiveness, acceptability, and ease of collection [Citation153,Citation154]. Researchers in India (November 2020) also developed an RNA extraction-free approach (Cas13 Assisted Saliva-based & Smartphone Integrated Testing; CASSPIT), which exhibited a 97% PPA with RNA extraction-based methods [Citation155]. In response to the Delta outbreak in July 2021, the IndiaCOVID SOS volunteer task force developed recommendations for diagnostic approaches to tackle the crisis, including the expansion of rural surveillance using PCR testing of nasal and saliva samples [Citation156]. In August 2021, a Singaporean start-up based in India sought to provide a new method in addition to the traditional nasal samples by utilizing a ‘lollipop-like device’ to collect saliva samples from patients [Citation157]. This new device has enabled frequent testing in public spaces such as schools and airports [Citation158].
2.8. Middle East and North Africa (MENA)
While the region has predominantly used nasopharyngeal swab PCR testing, some MENA nations have demonstrated success with saliva. Notably, the United Arab Emirates (UAE) has achieved one of the highest testing rates worldwide [Citation159]. Studies in the UAE (July 2020) [Citation160], Bahrain (October 2020) [Citation161] and Kuwait (July 2020) [Citation162] have validated the comparability of saliva samples (sensitivity 73–83%) to nasopharyngeal swabs for SARS-CoV-2 diagnostics, with additional research teams in Lebanon [Citation163], Qatar [Citation164], and a collaboration between Saudi Arabia and Pakistan [Citation165] recognizing the reliability of saliva as a sample type. Researchers in Turkey are working on the development of an electrochemical immunoassay that detects the SARS-CoV-2 spike S1 protein (March 2022), adding another saliva-based diagnostic tool for point-of-care testing [Citation166]. Saliva-based PCR testing programs have been implemented for screening programs at primary schools in Qatar (September 2020) [Citation167], Abu Dhabi (December 2020) [Citation159], and Israel (October 2021) [Citation168], driving the authorization and strong uptake in some regions.
In the private sector, scientists in Israel [Citation169] and Iran [Citation170] have developed and brought to market saliva-based tests for the detection of SARS-CoV-2. In Turkey, a nanotechnology-based diagnostic system that detects SARS-CoV-2 from oral cavity swabs was developed in January 2021 [Citation171], producing results in as little as 30 seconds [Citation171,Citation172]. It has since received approval from Turkish authorities (June 2021) and seen increased demand globally [Citation172]. In Israel, a simple, inexpensive mouth-washing (10–20 second gargle) method, previously evaluated for influenza, was adapted for SARS-CoV-2 detection [Citation173].
In North Africa, 30-minute saliva rapid diagnostic tests are commercially available and could help increase testing accessibility as they do not require mobile technology [Citation174]. In August 2021, Morocco shifted from requiring medical settings for diagnostic testing to permitting the sale of rapid saliva tests at pharmacies across the nation [Citation175].
2.9. Sub-Saharan Africa
In February 2021, the Africa Centres for Disease Control and Prevention (CDC) acknowledged saliva as a reliable sample type for COVID-19 testing. Even so, few African nations have officially authorized saliva-based SARS-CoV-2 tests as many do not have central regulatory agencies for the approval of diagnostic tools [Citation176]. In Nigeria (December 2019), a cross-sectional survey of healthcare workers highlighted that, despite participants being aware of the merits of saliva as a sample type for disease detection, few countries applied saliva-based methods in clinical settings [Citation177]. Wider use of saliva was most often impeded by methods with lower testing sensitivity, portability, speed, or automation. In addition, the fee-for-service model in many of these countries proved a barrier for those in need of testing [Citation176].
However, commercial saliva testing is offered in certain African nations. Saliva-based PCR is offered in Burundi, Sudan, the Democratic Republic of the Congo, and Seychelles [Citation178,Citation179]. Scientists in Nigeria are pushing for the government to fund SalivaDirectTM PCR testing at the national level based on its proven success and ability to cut costs and turnaround time [Citation180]. Saliva is also used as a sample type for rapid tests as part of Uganda’s surveillance program [Citation181] and for entry to Djibouti via national airports [Citation182].
A multitude of scientific publications further support the potential for saliva-based COVID-19 tests in this region. In South Africa, a randomized-controlled trial began in May 2021 to develop regionally-appropriate protocols for saliva self-collection (in place of self-administered nasal swabs) for SARS-CoV-2 detection [Citation183]. In Ghana (December 2020) [Citation184] and Ethiopia (November 2021) [Citation185], the high viral load and prolonged viral shedding of saliva was found to be more advantageous than nasopharyngeal swabs for diagnostics, and an ideal sample type for settings with limited diagnostic resources. Crucially, saliva was identified in South Africa (December 2021) to be the most reliable sample type for detection of the Omicron variant due to higher viral shedding in saliva than mid-turbinate swabs (PPA: saliva = 100%, mid-turbinate swabs = 86%) [Citation186].
While saliva is often used as a sample type for more traditional and widely-accepted PCR, LAMP, or antigen testing technology, researchers in Nigeria (April 2021) have considered training African Giant Rats, native to Sub-Saharan Africa, to detect SARS-CoV-2 olfactorily in saliva samples [Citation187].
2.10. Europe
In September 2021, the European Center for Disease Prevention and Control (ECDC) reiterated that saliva-based RT-PCR tests generate similar sensitivities to nasopharyngeal swabs for symptomatic cases and attributed differences to variation in populations tested, sampling techniques, and time of sample collection [Citation188,Citation189]. The ECDC initially recommended saliva as a sample option only if nasopharyngeal swabs are not accessible, as they desired further clinical validation data prior to suggesting saliva as a primary sample type [Citation190]. However, in August 2020, the ECDC endorsed saliva as an option to increase testing acceptance in younger populations [Citation191]. As of October 2021, saliva was not yet validated for rapid antigen tests by the ECDC [Citation192]. Nevertheless, an abundance of findings on saliva as a versatile sample type, for detection using various platforms on numerous populations, have emerged rapidly across Europe.
2.11. Central/Eastern Europe
Following robust validations, saliva-based SARS-CoV-2 testing is available in laboratories and pharmacies across much of Central and Eastern Europe including Montenegro [Citation193], Georgia [Citation194], Slovakia [Citation195], Slovenia [Citation196], Latvia [Citation197] and Ukraine [Citation198]In Romania, school students are required to perform self-administered, at-home saliva tests under parental observation twice a week [Citation199]. In Poland (January 2021), saliva-based LAMP has proved efficient and reliable [Citation200]. Russia also sought to quickly utilize saliva as a diagnostic sample type. The Russian biotechnology company, Sistema-Biotech, federally registered a saliva-based rapid test in early 2020 [Citation201,Citation202].
Additional assays across Central and Eastern Europe have demonstrated success. The saliva-based Advanta Dx SARS-CoV-2 RT-PCR assay (Greece, December 2020) reported high sensitivity in both diagnostic (88.5%) and screening (98.1%) samples [Citation203]. Good agreement was found between the saliva-based laboratory kit, Diagnolita (Lithuania. March 2021) and nasopharyngeal swabs (PPA = 98.28%, NPA = 98.11%) with a moderately strong correlation between the Ct values of the two samples (R = 0.53) [Citation204]. In Slovakia, LAMP methods that include or bypass the RNA extraction step were evaluated to reduce cost and processing time [Citation205,Citation206]. In Slovenia (October 2021), saliva-based RT-qPCR testing demonstrated sensitivity of ≥ 95% in people with subclinical infection, outperforming RT-LAMP (≥ 70% sensitivity) [Citation207]. Additionally, passive drool saliva demonstrated higher diagnostic sensitivity (95%) compared to oral cavity swab (87%) [Citation207].
2.12. Western Europe
Across Western Europe, numerous developments and applications of saliva-based assays for SARS-CoV-2 detection have emerged. Studies conducted in Italy (April 2020) [Citation208], Switzerland (January 2021) [Citation209], Finland (March and August 2021) [Citation210,Citation211], Sweden (October 2021) [Citation212], Belgium (December 2021) [Citation213], and the United Kingdom (UK; January 2022) [Citation214] have found saliva to perform with comparable or greater sensitivity than nasal-based swabs. Moreover, RNA-extraction free approaches have been validated in Sweden (May 2020) [Citation215], Spain (October 2020) [Citation216], Ireland (March 2021) [Citation217], Finland (December 2021) [Citation218], England (February 2022) [Citation219], and the Netherlands (May 2022) [Citation220]. Pooled saliva testing in France (October 2020) [Citation221], Portugal (October 2021) [Citation222] and Finland (2021) [Citation218] has demonstrated high sensitivity while preserving resources and increasing mass testing capacity. When evaluated as a screening tool for nursing home staff in Belgium (December 2020), saliva-based RT-PCR testing led to decreases in COVID-19 prevalence (from 34.4% to 13.4%) and deaths among nursing home residents [Citation223]. In Italy (April 2020), saliva swabs consistently tested positive throughout infection while nasopharyngeal swabs tested negative for some patients over the course of infection, indicating that saliva may reveal the clinical evolution of COVID-19 more reliably than nasal samples [Citation208]. The Irish government has urged staff and students to participate in research to explore potential surveillance systems on college campuses, including saliva-based testing solutions [Citation224]. Furthermore, a collaborative study (UniCOV) across four Irish universities aims to determine appropriate surveillance systems for COVID-19, evaluating both nasal antigen tests and saliva PCR tests [Citation225]. In Spain (December 2020), saliva and oral swabs were the sample types of choice for a study on pandemic wave trends [Citation226]. Researchers in the UK (January 2022) used saliva samples to validate SARS-CoV-2 CRISPR-Cas detection technology for point-of-care testing.
LAMP tests have also proven successful in Western Europe. In the United Kingdom (August 2021), following a large-scale study evaluating its potential [Citation227], the National Health Service is offering saliva-based LAMP testing to healthcare workers in addition to rapid antigen tests [Citation228]. However, other researchers from the UK evaluated the use of RT-LAMP to test saliva samples of asymptomatic individuals (March 2022) and while the specificity was 100%, the sensitivity (40.91%) was significantly lower than when testing the same saliva samples with RT-PCR [Citation229]. Additionally, Trinity College in Dublin, Ireland implemented saliva-based LAMP testing for its students (November 2021) [Citation230]. In France, a 40-minute rapid saliva-based LAMP test that can be performed entirely outside of a laboratory was designed to allow for mass testing [Citation231].
An alternative approach – an electrochemical immunoassay – was developed in Italy (October 2020) to rapidly detect SARS-CoV-2 in saliva promising high potential for market entry, being portable, non-invasive, and highly sensitive [Citation232].
Across Western Europe, there have been varying levels of commercial uptake of saliva-based COVID-19 testing. Saliva testing for the detection of SARS-CoV-2 was introduced in Sweden in June 2020 [Citation233]. Labs located in cities such as Stockholm and Gothenburg monitor individuals using a saliva-based antigen test that meets the WHO’s recommended quality requirements [Citation234]. Saliva-based PCR testing has been implemented as a screening tool for students and staff across Western Europe, at the University of Liège in Belgium[Citation235], primary schools in Milan, Italy [Citation236], and primary schools and universities in Monaco and Norway [Citation237,Citation238]. It is also being used extensively in Germany in homeless shelters, schools, and citywide [Citation239–242]. The Netherlands created the Testing for Entry program to offer free testing for events, activities, or hospitality, which utilizes a combination of nasal swabs and throat swabs for antigen tests [Citation243]. In December of 2021, the Federal Council in Switzerland announced they would cover the costs for certain pooled saliva testing for those requiring a COVID-19 certificate [Citation244]. In the executive summary on the Conference Towards the Deployment of a Global and Collaborative Diagnostic Arsenal to Detect and Fight Against Pandemics that was hosted by Monaco in December 2021, saliva as a sample type is highlighted as a most useful strategy in detecting COVID-19 [Citation245]. The UK has approved comparatively few saliva-based assays that have gained traction [Citation246]. The lack of saliva testing across the UK may be in part due to the government’s prior emphasis on increasing access to rapid antigen tests [Citation247]. In Norway, one expert noted that the country is currently hesitant to use saliva as a sample type for COVID-19 detection but that with additional data there is potential to reconsider the adoption of saliva [Citation248].
3. Discussion
While nations differ in their public health capacity and implementation of saliva-based COVID-19 testing, every region, globally, has demonstrated success in using saliva as a sample type for SARS-CoV-2 detection. The extensive data that emerged from East Asian countries in conjunction with rapid action by their governments to deploy PCR saliva testing has led to the incorporation of saliva as a fundamental component in their pandemic response strategies. The USA’s saliva testing response has also been high-reaching, with interventions accessible through both the public and private sector (following federal authorization [Citation13]) and for diverse populations, including asymptomatic and symptomatic children, adults, and healthcare workers. Similarly, uptake of saliva-based detection has spanned across Europe, including large-scale surveillance for schools, universities, and the general public, and involving PCR, LAMP, and rapid antigen methods [Citation191]. In Oceania, testing has predominantly occurred in the high-income island nations of Australia and New Zealand, where the goal of safeguarding their borders has resulted in aviation, maritime, and quarantine hotel workers being the target groups for saliva surveillance. All regions have reported finding value in the non-invasive approach offered through saliva sampling for SARS-CoV-2 detection, whether in clinical diagnostic settings or for screening and surveillance testing.
The emergence of saliva as a sample type that is comparable in caliber and sensitivity to swab-based methods for SARS-CoV-2 detection offers a scalable and affordable solution to testing, warranting more widespread adoption. Though numerous studies cite concerns regarding saliva’s ability to match or outperform nasopharyngeal swabs, the efficacy of saliva-based COVID-19 testing has been demonstrated time and again. Poorer findings that resulted from less robust methods of this previously non-traditional sample type may account for some of the hesitancy by governments to broadly implement saliva-based diagnostics. As nasopharyngeal swabs were the default sample type at the start of the pandemic, unfamiliarity with saliva sampling may have led to apprehensions about switching collection methods at testing sites later on.
Saliva sampling can increase testing acceptability as it is less invasive than nasopharyngeal and oropharyngeal swabbing, and has been found to be the preferred method by patients. Almost two thirds of one sampled population reported that they would prefer a saliva-based test [Citation81]. Furthermore, 76% would be willing to repeat a saliva test for confirmation of their COVID-19 status, whereas only 63% would repeat a nasopharyngeal or oropharyngeal swab test [Citation81]. Saliva is also suitable for community settings comprising populations that may be more challenging to collect nasal samples from, including children and the elderly [Citation151]. Thus, the minimally-invasive nature of saliva collection makes it ideal for mass testing, as more individuals are likely to participate in screening programs [Citation249,Citation250]. Additionally, saliva collection has a lower staffing requirement than swabbing, reducing transmission risk for healthcare workers while enabling scalable sampling even in resource-limited settings [Citation251,Citation252].
Greater focus on resource-limited settings (including low- and middle-income countries) is warranted, as many countries in Africa, Central America, the Caribbean, and the Middle East have yet to validate and implement saliva testing. Nations should seek to replicate aspects of the successful applications demonstrated across various populations globally, while remaining cognizant of barriers they may face. This is of utmost importance in narrowing testing gaps between high- and low-income nations. The cost of saliva sample collection can be a fraction of the cost of nasopharyngeal swabs, permitting a greater increase to testing accessibility [Citation253]. Test costs can also be reduced with RNA-extraction-free approaches [Citation254,Citation255], requiring fewer materials and less labor while increasing efficiency. Moreover, saliva may prove a useful specimen type for occupied territories and conflict zones. Palestine, for example, has endured a restricted flow of necessary COVID-19 supplies to the region [Citation256], and in May 2021, an Israeli airstrike damaged Gaza’s only COVID-19 diagnostic laboratory [Citation257]. In Palestine, saliva-based antigen tests could increase diagnostic testing capacity, given the relatively low cost of production in the context of Palestine’s limited laboratory infrastructure and occupation-related resource constraints. Understanding the most useful tools for detecting SARS-CoV-2 in conflict zones continues to be relevant particularly as the conflict in Ukraine unfolds.
Additionally, the use of saliva for diagnostic testing has a history that precedes the COVID-19 pandemic. This includes salivary detection of norovirus in Peru [Citation258]; tuberculosis in Ecuador [Citation83]; Kaposi’s Sarcoma-Associated Herpesvirus and Epstein-Barr virus in Cameroon[Citation259]; HIV in Uganda[Citation260], Tanzania[Citation261], Myanmar [Citation262], and Jamaica [Citation76]; HPV in Australia [Citation263]; malaria in Cameroon [Citation264,Citation265] and the Philippines [Citation266]; Leishmania DNA in Thailand [Citation267]; and both Zika virus [Citation268] and Chikungunya [Citation269] in French Polynesia. The familiarity and versatility of saliva for the detection of numerous infectious diseases, especially in low-income nations, demonstrates its likely acceptability and feasibility for official government adoption by all regions for SARS-CoV-2 detection. Importantly, SARS-CoV-2 has been successfully sequenced from saliva by multiple groups around the world [Citation270–272], further elevating its status as compared to nasal swabs. One method, SARSeq (Austria; May 2021) also allows for the concurrent detection of other respiratory RNA viruses, including influenza A and B and human rhinoviruses [Citation271].
A plethora of global findings demonstrate the application of saliva as a sensitive, affordable, and sustainable sample type for SARS-CoV-2 detection. Expanding research around the world has led to government action, innovations in the private sectors, and implementation in a variety of populations. With great intra-regional disparities in the availability and uptake of COVID-19 testing, coupled with novel threats of new COVID-19 variants and low vaccine availability in some areas, the mass implementation of sustainable testing practices is imperative to enable the mitigation of COVID-19 burdens across all continents. In countries that may be under-resourced and unable to provide wide-scale oropharyngeal or nasopharyngeal swab testing, saliva testing is a strong candidate to augment existing efforts through the introduction of an easy, affordable, and comfortable testing option. Ultimately, international solidarity among research scientists, policymakers, and government agencies to standardize the most robust saliva testing methods and implementation practices will enable saliva to be a mainstream diagnostic sample type for the foreseeable future.
4. Expert opinion
Over two years into the pandemic, the term ‘gold-standard’ is still too often applied to the nasopharyngeal swab. This outdated terminology must be revised as it continues to fuel controversy within the scientific community and leads to skepticism of alternative sample types within the general public. It also does not reflect the utility of other sample types, such as saliva, which continues to demonstrate equivalent or greater efficacy to swabs. In line with this, saliva was even proposed as a potential new ‘gold-standard’ sample type after numerous international studies in 2020 already demonstrated strong performance and implementation success of saliva-based PCR testing [Citation4]. The misconception surrounding the sensitivity of saliva is one of the biggest obstacles to mass implementation of saliva-based PCR diagnostics. This global review of saliva as a robust sample type for SARS-CoV-2 diagnostics is the first piece to summarize findings of multinational studies and implementation efforts, while elucidating innovative saliva-based tools that generated effective protection to communities. Together, these success stories from diverse global contexts of the SARS-CoV-2 pandemic exemplify the need for further evaluation of and support for saliva as a sample type for respiratory infections from the scientific community. This action is necessary to increase awareness of the validity of saliva and the opportunities this awareness can provide by expanding a range of accepted sample types for clinical testing.
Throughout the pandemic, we have witnessed disparities in access to testing all around the world. However, in many cases, saliva-based tests decrease cost and increase access. Moreover, we have also witnessed the consequences of delayed response and lack of access to testing. We need to be ready for future surges, new variants of concern, reduced vaccine effectiveness in the face of these variants, and waning population immunity. Following the trends of earlier variants [Citation273], the further shift in SARS-CoV-2 infection profile reported for Omicron, resulted in dramatically poorer detection with nasal swabs and far more sensitive and earlier detection using saliva [Citation186,Citation274]. Here, the superiority of saliva for early and asymptomatic detection is essential to limiting virus transmission and ultimately, mitigating the potential for new variants to arise in the near future. Thus, we must remain flexible in our response and be open to the use of alternative sample types when the need arises and when they can better serve public health responses. Importantly, with many SARS-CoV-2 tests around the world only permitted for use under the current pandemic emergency response, formal approval of these diagnostic tools will be imperative to ensure they are available beyond this pandemic to provide reliable, easy testing for the long-term.
The demands of the pandemic spurred rapid development in diagnostic tools. Innovative saliva-based approaches are continuing to emerge with great potential for further creative applications that diagnostic test developers and public health decision-makers can pursue beyond COVID-19. This is particularly true for multiplexed approaches that enable non-invasive, routine screening for early warnings of multiple diseases in a single assay. Implementation science will be key to translating many of these diagnostic innovations into practice as on-the-ground barriers arise. These barriers will be especially prominent in resource-limited settings where frequent testing is still of great urgency yet great disparities exist in access to testing. Consideration should also be given to the optimal specimen type for point-of-care and rapid tests across a range of diseases for low- or middle- income countries that may not have adequate laboratory infrastructure to use PCR assays. To help accommodate affordability and convenience, methods such as pooled sample testing can be implemented, which has demonstrated success in settings such as workplaces and schools.
Countries all around the world are demonstrating how saliva-based sampling can increase the acceptance of clinical testing. With programs in place at schools, workplaces, older living facilities, prisons, healthcare centers, etc., there are numerous, robust examples that can serve as a framework for the quick implementation of scalable testing. These examples can act as a playbook for other communities, or even entire countries, to rapidly implement in response to new public health threats. The need for earlier detection and more accurate diagnostics becomes greater as COVID-19 treatments become increasingly available (as highlighted in the USA with The White House’s current Test to Treat initiative [Citation275]). With many treatments most effective when administered early in the course of infection, test results must be returned in an actionable time frame for early treatment and better recovery.
Looking forward, these accurate and timely diagnoses have a larger role to play in protecting public health. As another major public health crisis looms with increasing antibiotic resistance, more accessible diagnostics can also promote antibiotic stewardship. While stewardship initiatives better equip clinicians to optimize the appropriate treatment of infections, this also reduces the risk of adverse events associated with antibiotic use. Thus, global solidarity is warranted to most effectively apply the learnings of saliva-based diagnostics as a way to expand diagnostic options, access, and efficiency. Through our review, we call upon these stakeholders to collaborate internationally and continue driving affordable, sustainable testing interventions. Actioning this compilation of implementation successes can subsequently enable saliva to transition nations into a safer tomorrow.
Article highlights
The ‘gold standard’ sample types for SARS-CoV-2 detection defaulted early in the pandemic to nasopharyngeal or oropharyngeal swabs, but there is a growing body of evidence demonstrating saliva as a suitable sample type for SARS-CoV-2 detection
Misinformation and skepticism generated by the debate surrounding saliva as a reliable sample type have impeded the implementation and expansion of saliva testing programs globally.
This review highlights the best practices employed by successful testing operations, including effective methodologies and the use of low-cost materials, while addressing barriers and challenges that should be considered when developing or implementing salivary diagnostic programs.
Global solidarity is warranted to most effectively apply the learnings of saliva-based diagnostics as a way to expand diagnostic options, access, and efficiency.
Saliva testing is a strong candidate for countries that may be under-resourced and unable to provide wide-scale oropharyngeal or nasopharyngeal swab testing, to augment existing efforts through the introduction of an easy, affordable, and comfortable testing option.
Declaration of interest
AL Wyllie has received research support from the Rockefeller Foundation, Flambeau Dx, Tempus Labs, Pfizer and Merck. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- To KKW, Yip CCY, Lai CYW, et al. Saliva as a diagnostic specimen for testing respiratory virus by a point-of-care molecular assay: a diagnostic validity study. Clin Microbiol Infect. 2019;25:372–378. DOI:https://doi.org/10.1016/j.cmi.2018.06.009
- To KKW, Tsang OT-Y, Yip CC-Y, et al. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. 2020;71:841–843.
- Khurshid Z, Asiri FYI, Al Wadaani H. Human saliva: non-invasive fluid for detecting novel coronavirus (2019-nCoV). Int J Environ Res Public Health. 2020;17:2225.
- Tan SH, Allicock O, Armstrong-Hough M, et al. Saliva as a gold-standard sample for SARS-CoV-2 detection. Lancet Respir Med. 2021;9:562–564.
- Savela ES, Winnett A, and Romano AE, et al. Quantitative SARS-CoV-2 viral-load curves in paired saliva and nasal swabs inform appropriate respiratory sampling site and analytical test sensitivity required for earliest viral detection. J Clin Microbiol JCM0178521.16;60(2):e0178521.
- Smith RL, Gibson LL, Martinez PP, et al. Longitudinal assessment of diagnostic test performance over the course of acute SARS-CoV-2 infection. J Infect Dis. 2021;224:976–982.
- Lai J, German J, Hong F, et al. Comparison of saliva and midturbinate swabs for detection of SARS-CoV-2. Microbiol Spectr. 2022;10:e0012822.
- Zimba R, Kulkarni S, Berry A, et al. SARS-CoV-2 testing service preferences of adults in the United States: discrete choice experiment. JMIR Public Health Surveill. 2020;6:e25546.
- Acceptance of saliva-based specimen collection for SARS-CoV-2 testing among K-12 students, teachers, and staff. Public Health Rep.2022;433021:333549221074395.
- Sabino-Silva R, Jardim ACG, Siqueira WL. Coronavirus COVID-19 impacts to dentistry and potential salivary diagnosis. Clin Oral Investig. 2020;24:1619–1621.
- Public Health Agency of Canada. Testing for COVID-19: increasing testing supply. Government of Canada; 2020. Available from: https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/symptoms/testing/increased-supply.html
- Goldfarb DM, Tilley P, Al-Rawahi GN, et al. Self-collected saline gargle samples as an alternative to health care worker-collected nasopharyngeal swabs for COVID-19 diagnosis in outpatients. J Clin Microbiol. 2021;59. DOI:https://doi.org/10.1128/JCM.02427-20.
- Center for Devices & Radiological Health. In vitro diagnostics EUAs - molecular diagnostic tests for SARS-CoV-2. 2021. Available from: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-molecular-diagnostic-tests-sars-cov-2
- U.S. Food & Drug Administration. Emergency Use Authorization (EUA) summary SARS-CoV-2 assay (Rutgers clinical genomics laboratory). Available from: https://www.fda.gov/media/136875/download.
- New Rutgers saliva test for coronavirus gets FDA approval. Rutgers Today; 2021. Available from: https://www.rutgers.edu/news/new-rutgers-saliva-test-coronavirus-gets-fda-approval
- U.S. Food & Drug Administration. Emergency Use Authorization (EUA) summary SARS-CoV-2. RT-PCR Assay (SalivaDirect). https://www.fda.gov/media/141192/download.
- SHIELD. University of Illinois system. https://www.uillinois.edu/shield
- Vander Schaaf NA, Fund AJ, Munnich BV, et al. Routine, cost-effective SARS-CoV-2 surveillance testing using pooled saliva limits viral spread on a residential college campus. Microbiol Spectr. 2021;9:e0108921.
- Ohio state to begin processing COVID-19 tests on campus. Ohio State News; 2020. https://news.osu.edu/ohio-state-to-begin-processing-covid-19-tests-on-campus/.
- UO COVID-19 testing program switching to saliva tests. Around the O; 2021. Available from: https://around.uoregon.edu/content/uo-covid-19-testing-program-switching-saliva-tests
- Pace M University expands student COVID-19 testing. The Well; 2020. Available from: https://thewell.unc.edu/2020/09/28/university-expands-student-covid-19-testing/
- Be SAFE saliva testing. University of Virginia Available from: https://besafe.virginia.edu/.
- UCI. COVID-19 response center: testing. Irvine: University of California. Available from: https://uci.edu/coronavirus/testing-response/testing.php
- Plumb EV, Ham RE, Napolitano JM, et al. Implementation of a rural community diagnostic testing strategy for SARS-CoV-2 in upstate South Carolina. Front Public Health. 2022;10:858421.
- Ehrenberg AJ, Moehle EA, Brook CE, et al. Launching a saliva-based SARS-CoV-2 surveillance testing program on a university campus. PLoS One. 2021;16:e0251296.
- Kowalsky RH, Fine S, Eisenberg MA. Exclusion of SARS-COV-2 from two Maine overnight camps July-August 2020. Disaster Med Public Health Prep. 2021;1–3. DOI:https://doi.org/10.1017/dmp.2021.86
- Ioannidis JPA. COVID-19 vaccination in children and university students. Eur J Clin Invest. 2021;51:e13678.
- Mendoza RP, Bi C, and Cheng H-T, et al., Implementation of a pooled surveillance testing program for asymptomatic SARS-CoV-2 infections in K-12 schools and universities. EClinicalMedicine. 2021;38(101028):101028.
- U.S. Food & Drug Administration. Emergency Use Authorization (EUA) summary SARS-CoV-2 RT-PCR assay (Gravity diagnostics). Available from: https://www.fda.gov/media/143935/download.
- Co-diagnostics, Inc receives CE marking for direct saliva SARS-CoV-2 test. Newsroom | Co-Diagnostics, Inc Availabel from: https://news.codiagnostics.com/2021-06-18-Co-Diagnostics,-Inc-Receives-CE-Marking-for-Direct-Saliva-SARS-CoV-2-Test.
- U.S. Food & Drug Administration. Letter of Emergency Use Authorization (EUA) (Life Technologies Corporation). 2021. Available from: https://www.fda.gov/media/151208/download
- Open qPCR machine: your personal real-time PCR machine. Available from: https://www.chaibio.com/openqpcr.
- U.S. Food & Drug Administration. Letter of Emergency Use Authorization (EUA) (rheonix). 2020. Available from: https://www.fda.gov/media/137490/download
- MicroGEM granted emergency use authorization from FDA for fast point of care PCR COVID-19 saliva test. MicroGEM; 2022. Available from: https://microgembio.com/2022/04/microgem-granted-emergency-use-authorization-from-fda-for-fast-point-of-care-pcr-covid-19-saliva-test/
- U.S. Food & Drug Administration. Letter of Emergency Use Authorization (EUA) (PerkinElmer); 2020. Available from: https://www.fda.gov/media/136407/download
- ProPhase labs begins advanced, saliva-based viral PCR multiplex-testing for COVID-19, influenza A & B, and other viruses after FDA confirmation of two emergency use authorizations. ProPhase Labs, Inc Available form https://ir.prophaselabs.com/news-events/press-releases/detail/113/prophase-labs-begins-advanced-saliva-based-viral-pcr.
- Pisanic N, Randad PR, Kruczynski K, et al. COVID-19 serology at population scale: SARS-CoV-2-specific antibody responses in saliva. J Clin Microbiol. 2020;59. DOI:https://doi.org/10.1128/JCM.02204-20.
- COVID-19 testing. Howard County Maryland Available from: https://www.howardcountymd.gov/covid-19-testing.
- COVID-19 testing sites. Minneapolis-St. Paul Airport Available from: https://www.mspairport.com/airport/covid-19-testing-site.
- Awsumb K, Pretzel E COVID-19 vault saliva testing at home. Minnesota Dept. of Health https://www.health.state.mn.us/diseases/coronavirus/testsites/athome.html.
- U.S. Food and Drug Administration. Letter of Emergency Use Authorization (EUA). (Infinity BiologiX); 2021. Available from: https://www.fda.gov/media/137773/download
- Infinity BiologiX’s RT-PCR saliva test to be provided free to New Jerseyans for at-home testing. IBX; 2021. Available from: https://ibx.bio/infinity-biologixs-rt-pcr-saliva-test-to-be-provided-free-to-new-jerseyans-for-at-home-testing/
- Schoening E, Grimaldi LA. Airlines tighten mask restrictions, partner with COVID-19 testing providers. 2020.
- Bringing you safer travel. Vault Health Available from: https://learn.vaulthealth.com/hawaiian/.
- JetBlue and Vault Health partner to make At-home COVID-19 tests more widely available to customers. JetBlue Available from: http://blueir.investproductions.com/investor-relations/press-releases/2020/09-28-2020-194516403
- Huang X, Tang G, Ismail N, et al. Developing RT-LAMP assays for rapid diagnosis of SARS-CoV-2 in saliva. EBioMedicine. 2021;75:103736.
- Newman CM, Ramuta MD, McLaughlin MT, et al. Initial evaluation of a mobile SARS-CoV-2 RT-LAMP testing strategy. J Biomol Tech. 2021;32:137–147. DOI:https://doi.org/10.7171/jbt.21-32-03-009
- Development and implementation of a simple and rapid extraction-free saliva SARS-CoV-2 RT-LAMP workflow for workplace surveillance. medRxiv. 2022;2022:03.11.22272282.
- Zhang Y, Tanner NA. Development of multiplexed reverse-transcription loop-mediated isothermal amplification for detection of SARS-CoV-2 and influenza viral RNA. Biotechniques. 2021;70:167–174.
- Li E, Larson A, Kothari A, et al. Handyfuge-LAMP: low-cost and electricity-free centrifugation for isothermal SARS-CoV-2 detection in saliva. bioRxiv. 2020. DOI:https://doi.org/10.1101/2020.06.30.20143255
- Diaz LM, Johnson BE, Jenkins DM. Real-time optical analysis of a colorimetric LAMP assay for SARS-CoV-2 in saliva with a handheld instrument improves accuracy compared with endpoint assessment. J Biomol Tech. 2021;32:158–171.
- Yang Y, Murray J, Haverstick J, et al. Silver nanotriangle array based LSPR sensor for rapid coronavirus detection. Sens Actuators B Chem. 2022;359:131604.
- Akarapipad P, Kaarj K, Breshears LE, et al. Smartphone-based sensitive detection of SARS-CoV-2 from saline gargle samples via flow profile analysis on a paper microfluidic chip. Biosens Bioelectron. 2022;207:114192.
- Miranda-Ortiz H, Fernández-Figueroa EA, Ruíz-García EB, et al. Development of an alternative saliva test for diagnosis of SARS-CoV-2 using TRIzol: adapting to countries with lower incomes looking for a large-scale detection program. PLoS One. 2021;16:e0255807.
- Martinez-Cuazitl A, Vazquez-Zapien GJ, Sanchez-Brito M, et al. ATR-FTIR spectrum analysis of saliva samples from COVID-19 positive patients. Sci Rep. 2021;11:19980.
- López-Martínez B, Guzmán-Ortiz AL, Nevárez-Ramírez AJ, et al. Saliva as a promising biofluid for SARS-CoV-2 detection during the early stages of infection. Bol Med Hosp Infant Mex. 2020;77:228–233.
- Valdés VJ 2021.
- Girón-Pérez DA, Ruiz-Manzano RA, Benitez-Trinidad AB, et al. Saliva pooling strategy for the large-scale detection of SARS-CoV-2, through working-groups testing of asymptomatic subjects for potential applications in different workplaces. J Occup Environ Med. 2021;63:541–547.
- Herrera LA, Hidalgo-Miranda A, Reynoso-Noverón N, et al. Saliva is a reliable and accessible source for the detection of SARS-CoV-2. Int J Infect Dis. 2021;105:83–90.
- Urgent reforms needed to boost growth and prevent another lost decade in Latin America and the Caribbean. World Bank Available from: https://www.worldbank.org/en/news/press-release/2021/10/05/urgent-reforms-needed-to-boost-growth-and-prevent-another-lost-decade-in-latin-america-and-the-caribbean.
- Henriquez-Marquez KI, Lainez‐Murillo DC, Sierra M, et al. High impact of SARS-CoV-2 or COVID-19 in the Honduran health personnel. J Med Virol. 2021;93:1885–1887.
- PAHO calls for countries to prioritize rapid tests for those with COVID-19 symptoms. Pan American Health Organization (PAHO); 2022. https://www.paho.org/en/news/19-1-2022-paho-calls-countries-prioritize-rapid-tests-those-covid-19-symptoms.
- Recommendations for national SARS-CoV-2 testing strategies and diagnostic capacities: interim guidance; 2021. Available from: https://www.paho.org/en/documents/recommendations-national-sars-cov-2-testing-strategies-and-diagnostic-capacities-interim
- Zúñiga A SJO airport in Costa Rica to offer on-site covid testing. The Tico Times | Costa Rica News | Travel https://ticotimes.net/2021/05/13/sjo-airport-in-costa-rica-to-offer-on-site-covid-testing 2021.
- Coronavirus information for the Dominican Republic. U.S. Embassy in the Dominican Republic; 2022. Available from: https://do.usembassy.gov/u-s-citizen-services/covid-19-information/
- Find and schedule a COVID test in bonaire. AZOVA Available from: https://www.azova.com/delta/bonaire/.
- Find and schedule a COVID test in Aruba. AZOVA Available from: https://www.azova.com/delta/aruba/.
- Find and schedule a COVID test in the Dominican Republic. AZOVA Available from: https://www.azova.com/delta/dominicanrepublic/.
- Huete-Pérez JA, Cabezas-Robelo C, Páiz-Medina L, et al. First report on prevalence of SARS-CoV-2 infection among health-care workers in Nicaragua. PLoS One. 2021;16:e0246084.
- Clinical testing for medical professionals, employees, insured and uninsured. Clinical Laboratory. Available from: https://www.stcroixlab.com/
- Jones R Jr. Govt. to expand testing protocols with SalivaDirect pilot program. Eyewitness News; 2020. Available from: https://ewnews.com/govt-to-expand-testing-protocols-with-salivadirect-pilot-program
- Clinical laboratory in Puerto Rico confirms availability of PCR tests using saliva to detect the COVID-19. Puerto Rico Science, Technology & Research Trust; 2021. https://prsciencetrust.org/clinical-laboratory-in-puerto-rico-confirms-availability-of-pcr-tests-using-saliva-to-detect-the-covid-19/.
- CorePlus Servicios Clínicos y Patológicos, LLC. Available from: https://corepluspr.com/#/
- Latest travel advisory. Antigua & Barbuda Available from: https://visitantiguabarbuda.com/travel-advisory/
- Escobar I. El caso del viajero al que no le aceptaron un test PCR de saliva en Guatemala y qué responde Salud. Prensa Libre; 2021. Available from: https://www.prensalibre.com/guatemala/comunitario/el-caso-del-viajero-al-que-no-le-aceptaron-un-test-pcr-de-saliva-en-guatemala-y-que-responde-salud/
- King SD, Wynter SH, Bain BC, et al. Comparison of testing saliva and serum for detection of antibody to human immunodeficiency virus in Jamaica, West Indies. J Clin Virol. 2000;19:157–161.
- Escobar DF, Díaz P, Díaz-Dinamarca D, et al. Validation of a methodology for the detection of severe acute respiratory syndrome coronavirus 2 in saliva by real-time reverse transcriptase-PCR. Front Public Health. 2021;9:743300.
- Garrido-Ayala JD, Chavez-Valverde AO, Diaz-Campos JS, et al. ¿Saliva como prueba diagnóstica de COVID-19? Rev Estomatol Hered. 2021;31:150–151.
- INS validó la metodología de pruebas PCR de covid-19 solo con saliva hechas en Santander. Semana: Últimas Noticias de Colombia y el Mundo; 2021. Available from: https://www.semana.com/coronavirus/articulo/ins-dio-visto-bueno-a-pruebas-pcr-de-covid-19-solo-con-saliva-hechas-en-santander/202108/
- Echavarria M, Reyes NS, Rodriguez PE, et al. Self-collected saliva for SARS-CoV-2 detection: a prospective study in the emergency room. J Med Virol. 2021;93:3268–3272.
- Nacher M, Mergeay-Fabre M, Blanchet D, et al. Diagnostic accuracy and acceptability of molecular diagnosis of COVID-19 on saliva samples relative to nasopharyngeal swabs in tropical hospital and extra-hospital contexts: the COVISAL study. PLoS One. 2021;16:e0257169.
- SARS-CoV-2 infections in households in a peri-urban community of Lima, Peru: a prospective cohort study. Influenza Other Respi Viruses. 2021. DOI:https://doi.org/10.1111/irv.12952.
- Echeverría G, Espinoza W, de Waard JH. How TB and COVID-19 compare: an opportunity to integrate both control programmes. Int J Tuberc Lung Dis. 2020;24:971–974.
- Zerbinati RM, Palmieri M, Schwab G, et al. Use of saliva and RT-PCR screening for SARS-CoV-2 variants of concern: surveillance and monitoring. J Med Virol. 2022. DOI:https://doi.org/10.1002/jmv.27839.
- de Oliveira CM, Brochi L, Scarpelli LC, et al. SARS-CoV-2 saliva testing is a useful tool for covid-19 diagnosis. J Virol Methods. 2021;296:114241.
- Ziegler MF. Two new COVID-19 tests developed in Brazil are patented. Agência FAPESP. 2021. Available from: https://agencia.fapesp.br/two-new-covid-19-tests-developed-in-brazil-are-patented/36279/
- Teste Molecular RT-PCR Saliva. Sabin: Medicina Diagnóstica. Available from: https://loja.sabin.com.br/teste-molecular-rt-pcr-saliva?___store=brasilia_df
- Barra GB, Santa Rita TH, Mesquita PG, et al. Overcoming supply shortage for SARS-CoV-2 Detection by RT-qPCR. Genes (Basel). 2021;12:90.
- Chile announces new form of testing as COVID-19 cases rise above 122,000. The Santiago Times.
- COVID-19. Nuevopudahel | Aeropuerto de Santiago Available from: https://www.nuevopudahuel.cl/covid-19-en?language=en.
- Clinical validation of RCSMS: a rapid and sensitive CRISPR-Cas12a test for the molecular detection of SARS-CoV-2 from saliva. bioRxiv. 2021. DOI:https://doi.org/10.1101/2021.04.26.21256081.
- Desai KT, Alfaro K, Mendoza L, et al. Multisite clinical validation of isothermal amplification-based SARS-CoV-2 detection assays using different sampling strategies. Microbiol Spectr. 2021;9:e0084621.
- Micronesia (federated states of). WHO coronavirus disease (COVID-19) dashboard with vaccination data. Available from: https://covid19.who.int/region/wpro/country/fm.
- COVID-19 data explorer. Our World In Data https://ourworldindata.org/explorers/coronavirus-data-explorer?tab=table&zoomToSelection=true&time=2020-03-01.latest&facet=none&pickerSort=asc&pickerMetric=location&Metric=Confirmed+cases&Interval=7-day+rolling+average&Relative+to+Population=true&Align+outbreaks=false&country=USA~GBR~CAN~DEU~ITA~IND~FJI.
- Williams E, Bond K, Zhang B, et al. Saliva as a noninvasive specimen for detection of SARS-CoV-2. J Clin Microbiol. 2020;58. DOI:https://doi.org/10.1128/JCM.00776-20
- Williams E, Isles N, and Chong B, et al. Detection of SARS-CoV-2 in saliva: implications for specimen transport and storage. J Med Microbiol 2021;70. DOI:https://doi.org/10.1099/jmm.0.001285.
- Oliver J, Tosif S, Lee L-Y, et al. Adding saliva testing to oropharyngeal and deep nasal swab testing increases PCR detection of SARS-CoV-2 in primary care and children. Med J Aust. 2021;215:273–278.
- Australian Government Department of Health. Testing Framework for COVID-19 in Australia; 2021. Available from: https://www.health.gov.au/resources/publications/coronavirus-covid-19-testing-framework-for-covid-19-in-australia
- Graham M, Ballard SA, Pasricha S, et al. Use of emerging testing technologies and approaches for SARS-CoV-2: review of literature and global experience in an Australian context. Pathology. 2021;53:689–699.
- Australian Government Department of Health. COVID-19 rapid antigen self-tests that are approved in Australia. Therapeutic Goods Administration (TGA); 2022. Available from: https://www.tga.gov.au/covid-19-rapid-antigen-self-tests-are-approved-australia
- Wood BR, Kochan K, Bedolla DE, et al. Infrared based saliva screening test for COVID-19. Angew Chem Int Ed Engl. 2021;60:17102–17107.
- Validation of a molecular assay to detect SARS-CoV-2 in saliva. N Z Med J. 2021;134:14-27.
- Hill Laboratories accredited for Covid testing. Avaialble from: https://www.hill-laboratories.com/about-us/news/hill-laboratories-accredited-for-covid-testing/.
- COVID-19 Public Health Response (Required Testing) Order; 2021. Avaialble from: https://www.legislation.govt.nz/regulation/public/2020/0230/latest/LMS533785.html
- Changes to saliva testing requirements; 2021. Avaialbel from: https://www.health.govt.nz/news-media/news-items/changes-saliva-testing-requirements
- Designated places for saliva testing - 2021-go3860 - New Zealand gazette. Avaialble from: https://gazette.govt.nz/notice/id/2021-go3860.
- About. Rako Science Available from: https://www.rakoscience.com/about.
- USFDA. SalivaDirect assay EUA Summary; 2021. Available from: https://www.fda.gov/media/141192/download
- Yolda-Carr D, Thammavongsa DA, Vega N, et al. Evaluation of the liberty16 mobile real time PCR device for use with the salivadirect assay for SARS-CoV-2 testing. Front Cell Infect Microbiol. 2022;11. DOI:https://doi.org/10.3389/fcimb.2021.808773.
- Ubiquitome MRF Fast, reliable, on-site COVID testing now available for New Zealand businesses. New Zealand Doctor; 2022. Available from:https://www.nzdoctor.co.nz/article/undoctored/fast-reliable-site-covid-testing-now-available-new-zealand-businesses
- Fang Z, Zhang Y, Hang C, et al. Comparisons of viral shedding time of SARS-CoV-2 of different samples in ICU and non-ICU patients. J Infect. 2020;81:147–178.
- Chow FW-N, Chan TT-Y, Tam AR, et al. A rapid, simple, inexpensive, and mobile colorimetric assay COVID-19-LAMP for mass on-site screening of COVID-19. Int J Mol Sci. 2020;21:5380.
- Lai CK, Lui GC, Chen Z, et al. Comparison of self-collected mouth gargle with deep-throat saliva samples for the diagnosis of COVID-19: mouth gargle for diagnosis of COVID-19. J Infect. 2021;83:496–522.
- Early Testing and Detection. The Government of Hong Kong Special Administrative Region Availablr from: https://www.coronavirus.gov.hk/eng/early-testing.html.
- Hung K-F, Sun Y-C, Chen B-H, et al. New COVID-19 saliva-based test: how good is it compared with the current nasopharyngeal or throat swab test? J Chin Med Assoc. 2020;83:891–894.
- Jeong HW, Kim S-M, Kim H-S, et al. Viable SARS-CoV-2 in various specimens from COVID-19 patients. Clin Microbiol Infect. 2020;26:1520–1524.
- Smith J, Shin H. South Korea to boost testing as coronavirus surge threatens medical collapse. Reuters. 2020.
- Hong KH, Kim GJ, Roh KH, et al. Update of guidelines for laboratory diagnosis of COVID-19 in Korea. Ann Lab Med. 2022;42:391–397.
- Fukumoto T, Iwasaki S, Fujisawa S, et al. Efficacy of a novel SARS-CoV-2 detection kit without RNA extraction and purification. Int J Infect Dis. 2020;98:16–17.
- Iwasaki S, Fujisawa S, Nakakubo S, et al. Comparison of SARS-CoV-2 detection in nasopharyngeal swab and saliva. J Infect. 2020;81:e145–e147.
- Yokota I, Hattori T, Shane PY, et al. Equivalent SARS-CoV-2 viral loads by PCR between nasopharyngeal swab and saliva in symptomatic patients. Sci Rep. 2021;11:4500.
- Yokota I, Shane PY, Okada K, et al. Mass screening of asymptomatic persons for severe acute respiratory syndrome coronavirus 2 using saliva. Clin Infect Dis. 2021;73:e559–e565.
- Okamura S, Akamatsu N, Kitajima T, et al. Screening of COVID −19 polymerase chain reaction tests using saliva for pregnant women and their partners in Himeji city. J Obstet Gynaecol Res. 2021;47:1253–1255.
- Bureau of Consular Affairs. Ministry of Labor announced to lift the entry ban on foreign migrant workers from Thailand; R.O.C. (Taiwan) overseas missions to process related visa applications; 2021. Available from: https://www.boca.gov.tw/fp-220-5081-c06dc-2.html
- Paulose AK, Huang C-C, Chen P-H, et al. A rapid detection of COVID-19 viral RNA in human saliva using electrical double layer-gated field-effect transistor-based biosensors. Adv Mater Technol. 2022;7:2100842.
- Chen PH, Huang CC, Wu CC, et al. Saliva-based COVID-19 detection: a rapid antigen test of SARS-CoV-2 nucleocapsid protein using an electrical-double-layer gated field-effect transistor-based biosensing system. Sens Actuators B Chem. 2022;357:131415.
- COVID-19 tests. Ministry of Health, Labour, and Welfare Available from: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000121431_00182.html.
- Allplex 2019-nCoV assay - letter of authorization. Available from: https://www.fda.gov/media/137179/.
- Chin J. EUA granted to iCare’s saliva-based COVID-19 test kit. Taipei Times 2021.
- Zhu J, Guo J, Xu Y, et al. Viral dynamics of SARS-CoV-2 in saliva from infected patients. J Infect. 2020;81:e48–e50.
- Han G, Lin Q, Yi J, et al. Isothermal gene amplification coupled MALDI-TOF MS for SARS-CoV-2 detection. Talanta. 2022;242:123297.
- Zhang Z, Jiang S, Wang X, et al. A novel enhanced substrate for label-free detection of SARS-CoV-2 based on surface-enhanced Raman scattering. Sensors and Actuators B: Chemical. 2022;359:131568.
- Zhang Z, Li D, Wang X, et al. Rapid detection of viruses: based on silver nanoparticles modified with bromine ions and acetonitrile. Chem Eng J. 2022;438:135589.
- Deng Y, Peng Y, Wang L, et al. Target-triggered cascade signal amplification for sensitive electrochemical detection of SARS-CoV-2 with clinical application. Anal Chim Acta. 2022;1208:339846.
- Kakimzhanova M. NU alumnus developed the test to detect the virus that causes COVID-19. Nazarbayev University; 2021.
- Batchimeg B Mongolian scholars developing diagnostic tool to detect coronavirus in saliva sample. Montsame: Mongolia News Agency 2021.
- Casas W. Saliva test finally begins. Manilastandard.net. 2021.
- Esposo J Saliva RT-PCR Test. Philippine Red Cross; 2021. Available from: https://redcross.org.ph/covid19/saliva-test/
- Dashlabs.ai. Philippine Red Cross Available from: https://shop.dashlabs.app/607a41404d14dc68461eb3be?searchText=&page=0&rowsPerPage=25&sortBy=createdAt&sortDirection=desc.
- Hernando-Malipot M. Saliva test of PH Red Cross now included in all official DOH laboratory results. Manila Bulletin 2021.
- Pasomsub E, Watcharananan SP, Boonyawat K, et al. Saliva sample as a non-invasive specimen for the diagnosis of coronavirus disease 2019: a cross-sectional study. Clin Microbiol Infect. 2021;27:285.e1–285.e4.
- Aegirbios salivtest godkänd av Thailands FDA. AegirBio Available from: https://www.aegirbio.com/news/aegirbios-salivtest-godkand-av-thailands-fda/.
- Thailand sandbox requirements. Sandbox Program; 2021. Available from: https://asq.in.th/thailand-sandbox-requirements
- Teo AKJ, Choudhury Y, Tan IB, et al. Validation of saliva and self-administered nasal swabs for COVID-19 testing. bioRxiv. 2020. DOI:https://doi.org/10.1101/2020.08.13.20173807.
- Ku CW, Shivani D, Kwan JQT, et al. Validation of self-collected buccal swab and saliva as a diagnostic tool for COVID-19. Int J Infect Dis. 2021;104:255–261.
- Auto H. Saliva testing available in Singapore for Covid-19 pre-departure tests of travellers. The Straits Times; 2021. Available from: https://www.straitstimes.com/singapore/saliva-testing-approved-in-singapore-for-covid-19-pre-departure-tests-of-travellers
- Singapore develops COVID-19 saliva amplified antigen rapid test. BioSpectrum Asia 2021.
- Van Vinh Chau N, Lam VT, Dung NT, et al. The natural history and transmission potential of asymptomatic severe acute respiratory syndrome coronavirus 2 infection. Clin Infect Dis. 2020;71:2679–2687.
- Mahendra C, Kaisar MMM, Vasandani SR, et al. Wide application of minimally processed saliva on multiple RT-qPCR kits for SARS-CoV-2 detection in Indonesia. Front Cell Infect Microbiol. 2021;11:691538.
- Giri AK, Rana DR. Charting the challenges behind the testing of COVID-19 in developing countries: Nepal as a case study. Biosaf Health. 2020;2:53–56.
- Uddin MKM, Shirin T, Hossain ME, et al. Diagnostic performance of self-collected saliva versus nasopharyngeal swab for the molecular detection of SARS-CoV-2 in the clinical setting. Microbiol Spectr. 2021;9:e0046821.
- Saeed U, Uppal SR, Piracha ZZ, et al. Evaluation of SARS-CoV-2 antigen-based rapid diagnostic kits in Pakistan: formulation of COVID-19 national testing strategy. Virol J. 2021;18(34). DOI:https://doi.org/10.1186/s12985-021-01505-3.
- Gupta A, Mittal A, Dhakad S, et al. Gargle lavage & saliva: feasible & cheaper alternatives to nasal & throat swabs for diagnosis of COVID-19. Ind J Med Res. 2021;153:665–670.
- Sasikala M, Sadhana Y, Vijayasarathy K, et al. Comparison of saliva with healthcare workers- and patient-collected swabs in the diagnosis of COVID-19 in a large cohort. BMC Infect Dis. 2021;21:648.
- Azmi I, Faizan MI, Kumar R, et al. A saliva-based RNA extraction-free workflow integrated with Cas13a for SARS-CoV-2 detection. Front Cell Infect Microbiol. 2021;11:632646.
- Clinical resources. India Covid SOS Available from: https://www.indiacovidsos.org/clinical-resources.
- Pandey A. Startup aims to replace nasal swab with ‘lollipop-like device’ which collects saliva for Covid-19 testing. India Today 2021.
- Kent C Coronavirus company news summary - intelligent fingerprinting creates saliva-based test - cue health gets approval for point-of-care test in India - verdict medical devices. Medical Device Network; 2021. Available from: https://www.medicaldevice-network.com/uncategorised/coronavirus-company-news-summary-intelligent-fingerprinting-creates-saliva-based-test-cue-health-gets-approval-for-point-of-care-test-in-india/
- Virji A, Al Hamiz A, and Al Hajeri O, et al. Feasibility of a saliva-based covid-19 screening program in Abu Dhabi primary schools. J Clin Outcomes Manag. 2021;28. DOI:https://doi.org/10.12788/jcom.0062.
- Senok A, Alsuwaidi H, Atrah Y, et al. Saliva as an alternative specimen for molecular COVID-19 testing in community settings and population-based screening. Infect Drug Resist. 2020;13:3393–3399.
- AbdulRahman A, AlBastaki A, AlAwadhi A, et al. Diagnostic and monitoring utilities of saliva for SARS-CoV-2. bioRxiv. 2020. DOI:https://doi.org/10.1101/2020.12.07.20244681
- Altawalah H, AlHuraish F, Alkandari WA, et al. Saliva specimens for detection of severe acute respiratory syndrome coronavirus 2 in Kuwait: a cross-sectional study. J Clin Virol. 2020;132:104652.
- Saleh FA, Sleem A. COVID-19: test, test and test. Med Sci (Basel). 2020;9. DOI:https://doi.org/10.3390/medsci9010001
- Varma A, Abubaker M, Dergaa I. Extensive Saliva based COVID-19 testing - the way forward to curtail the global pandemic?he Journal of Medical Research. 2020;6(6):309-310.
- Hamid H, Khurshid Z, Adanir N, et al. COVID-19 pandemic and role of human saliva as a testing biofluid in point-of-care technology. Eur J Dent. 2020;14:S123–S129.
- Erdem A, Senturk H, Yildiz E, et al. Amperometric immunosensor developed for sensitive detection of SARS-CoV-2 spike S1 protein in combined with portable device. Talanta. 2022;244:123422.
- MOPH announces plans to conduct saliva-based COVID-19 tests for children. Hukoomi Qatar e-Government.
- TOI Staff. Israel starts pilot program for fast, swab-less PCR tests. 2021. The Times of Israel.
- Israel’s BATM launches no-swab saliva-based COVID-19 test. BioSpectrum Asia 2021.
- Xinhua. Iran produces advanced test kits to detect COVID-19 in saliva: report. Xinhua Net News 2020.
- Töre Ö New COVID-19 test from turkey can be a game-changer. FTN News 2021.
- Turkish scientists’ 10-second COVID diagnosis test gets approval. Daily Sabah 2021.
- Jaffe-Hoffman M. Tired of nose swabs? Israeli research: test for COVID-19 in mouthwash. The Jerusalem Post 2021.
- C4Diagnostics announces CE marking of C4Covid-19 HUMANTM: a covid-19 saliva-based rapid diagnostic test. BioSpace; 2021. Available from https://www.biospace.com/article/c4diagnostics-announces-ce-marking-of-c4covid-19-humantm-a-covid-19-saliva-based-rapid-diagnostic-test/
- Hamann J Morocco permits sales of rapid COVID-19 tests at pharmacies. Morocco World News 2021.
- African Union Member States. COVID-19 Scientific and Public Health Policy Update; 2021. Available from: https://africacdc.org/download/covid-19-scientific-and-public-health-policy-update-02-february-2021/
- Lasisi TJ, Lawal FB. Preference of saliva over other body fluids as samples for clinical and laboratory investigations among healthcare workers in Ibadan, Nigeria. Pan Afr Med J. 2019;34:191.
- Hall H. Contipharma launches saliva-based Omicron detecting PCR test in Africa. Research & Development World 2021.
- Book your PCR or ANTIGEN test in Seychelles for your journey home. Seychelles Medical Services; 2021. Available from: https://seychellesmedicalservices.com/
- Adebowale N. COVID-19: why Nigeria should adopt saliva-based testing technology - experts. Premium Times. 2020.
- Testing & Surveillance: Uganda. Available from: https://www.exemplars.health/emerging-topics/epidemic-preparedness-and-response/testing-and-surveillance/uganda.
- UN Country Team in Djibouti. Republic of Djibouti: COVID-19 situation report. 2020. Available from: https://reliefweb.int/report/djibouti/republic-djibouti-covid-19-situation-report-15-28-august-10-september-2020.
- Majam M, Msolomba V, Scott L, et al. Self-sampling for SARS-CoV-2 diagnostic testing by using nasal and saliva specimens: protocol for usability and clinical evaluation. JMIR Res Protoc. 2021;10:e24811.
- Badu-Boatng E, Asante LS, Dompreh A, et al. A comparative study between nasopharyngeal/oropharyngeal, faecal and saliva viral shedding in Ghanaian COVID-19 patients attending Komfo Anokye teaching hospital (KATH), Kumasi from October – December, 2020. bioRxiv. 2021. DOI:https://doi.org/10.1101/2021.09.04.21262932.
- Beyene GT, Alemu F, Kebede ES, et al. Saliva is superior over nasopharyngeal swab for detecting SARS-CoV2 in COVID-19 patients. Sci Rep. 2021;11:22640.
- Marais G, Madzivhandila M, Makhado Z, et al. Saliva swabs are the preferred sample for omicron detection. bioRxiv. 2021. DOI:https://doi.org/10.1101/2021.12.22.21268246.
- Adekanmbi AJ, Olude MA. An alternate prospect in detecting presymptomatic and asymptomatic COVID-19 carriers through odor differentiation by HeroRATs. J Vet Behav. 2021;42:26–29.
- Countries in the EU and EEA. Gov.UK Available from: https://www.gov.uk/eu-eea.
- Diagnostic testing and screening for SARS-CoV-2; 2021. Available from: https://www.ecdc.europa.eu/en/covid-19/latest-evidence/diagnostic-testing
- Considerations for the Use of Saliva as Sample Material for COVID-19 Testing; 2021. Available from: https://www.ecdc.europa.eu/en/publications-data/considerations-use-saliva-sample-material-covid-19-testing
- Objectives for COVID-19 testing in school settings; 2020. Available from: https://www.ecdc.europa.eu/en/publications-data/objectives-covid-19-testing-school-settings
- Options for the use of rapid antigen tests for COVID-19 in the EU/EEA - first update; 2021. Available from: https://www.ecdc.europa.eu/en/publications-data/options-use-rapid-antigen-tests-covid-19-eueea-first-update
- Laboratory: the first COVID-19 laboratory in Montenegro. Moj Lab; 2021. Available from: https://mojlab.me/en/laboratory/
- ‘Covid-19’ diagnosis with saliva. Synevo; 2021. Available from: https://synevo.ge/en/news17-12-2021/
- PCR-RT, antigen test for covid-19 Bratislava, order, booking. RC; 2021. Available from: https://www.rychlotest-covid.sk/en/
- Free fast COVID-19 testing. nyd COVID Točka; 2021. Available from: https://testiraj.si/en/
- Tests for COVID-19 at synevo. Synevo; 2021. Available from: https://covid19.synevo.ua/?utm_source=site&utm_medium=alert&utm_campaign=covid19
- COVID-19 testing by ELISA, PCR, and Antigen tests. Synevo; 2021. Available from: https://covid19.synevo.ua/
- David G Romania: success in the negotiations for a vaccination agreement. European Trade Union Committee for Education; 2021. Available from: https://www.csee-etuce.org/en/policy-issues/covid-19/latest-updates/4679-romania-success-in-the-negotiations-for-a-vaccination-agreement.
- Products. Genomtec; 2021. Available from: https://genomtec.com/en/products/
- Sistema announces financial results for the first quarter 2020. 2020. https://sistema.com/press/pressreleases/sistema-announces-financial-results-for-the-first-quarter-2020.
- Russian company creates breakthrough rapid response test to detect COVID-19. TASS. 2020.
- Balaska S, Pilalas D, Takardaki A, et al. Evaluation of the advanta Dx SARS-CoV-2 RT-PCR assay, a high-throughput extraction-free diagnostic test for the detection of SARS-CoV-2 in saliva: a diagnostic accuracy study. Diagnostics (Basel). 2021;11. DOI:https://doi.org/10.3390/diagnostics11101766.
- Palikša S, Lopeta M, Belevičius J, et al. Saliva testing is a robust non-invasive method for SARS-CoV-2 RNA detection. Infect Drug Resist. 2021;14:2943–2951.
- Janíková M, Hodosy J, Boor P, et al. Loop-mediated isothermal amplification for the detection of SARS-CoV-2 in saliva. Microb Biotechnol. 2021;14:307–316.
- Garcia-Venzor A, Rueda-Zarazua B, Marquez-Garcia E, et al. SARS-CoV-2 direct detection without RNA isolation with Loop-Mediated Isothermal Amplification (LAMP) and CRISPR-Cas12. Front Med. 2021;8:627679.
- Rajh E, Šket T, Praznik A, et al. Robust saliva-based RNA extraction-free one-step nucleic acid amplification test for mass SARS-CoV-2 monitoring. Molecules. 2021;26:6617.
- Azzi L, Carcano G, Gianfagna F, et al. Saliva is a reliable tool to detect SARS-CoV-2. J Infect. 2020;81:e45–e50.
- Huber M, Schreiber PW, Scheier T, et al. High efficacy of saliva in detecting SARS-CoV-2 by RT-PCR in adults and children. Microorganisms. 2021;9:642.
- Kerimov D, Tamminen P, Viskari H, et al. Sampling site for SARS-CoV-2 RT-PCR-An intrapatient four-site comparison from Tampere, Finland. PLoS One. 2021;16:e0260184.
- Poukka E, Mäkelä H, Hagberg L, et al. Detection of SARS-CoV-2 infection in gargle, spit, and sputum specimens. Microbiol Spectr. 2021;9:e0003521.
- De Marinis Y, Pesola A-K, Söderlund Strand A, et al. Detection of SARS-CoV-2 by rapid antigen tests on saliva in hospitalized patients with COVID-19. Infect Ecol Epidemiol. 2021;11:1993535.
- Defêche J, Azarzar S, Mesdagh A, et al. In-depth longitudinal comparison of clinical specimens to detect SARS-CoV-2. Pathogens. 2021;10:1362.
- Connor MC, Copeland M, Curran T. Investigation of saliva, tongue swabs and buccal swabs as alternative specimen types to nasopharyngeal swabs for SARS-CoV-2 testing. J Clin Virol. 2022;146:105053.
- Smyrlaki I, Ekman M, Lentini A, et al. Massive and rapid COVID-19 testing is feasible by extraction-free SARS-CoV-2 RT-PCR. Nat Commun. 2020;11:4812.
- Brotons P, Perez-Argüello A, Launes C, et al. Validation and implementation of a direct RT-qPCR method for rapid screening of SARS-CoV-2 infection by using non-invasive saliva samples. Int J Infect Dis. 2021;110:363–370.
- De Santi C, Jacob B, Kroich P, et al. Concordance between PCR-based extraction-free saliva and nasopharyngeal swabs for SARS-CoV-2 testing. HRB Open Res. 2021;4:85.
- Rainey A, Pierce A, Deng X, et al. Validation and deployment of a direct saliva real-time RT-PCR test on pooled samples for COVID-19 surveillance testing. PLoS One. 2021;16:e0261956.
- Tarantini FS, Wu S, Jenkins H, et al. Direct RT-qPCR assay for the detection of SARS-CoV-2 in saliva samples. Meth Protocols. 2022;5:25.
- Schoeber JPH, Schlaghecke JM, Meuwissen BMJ, et al. Comprehensive analytical and clinical evaluation of a RNA extraction-free saliva-based molecular assay for SARS-CoV-2. PLoS One. 2022;17:e0268082.
- Migueres M, Vellas C, Abravanel F, et al. Testing individual and pooled saliva samples for sars-cov-2 nucleic acid: a prospective study. Diagn Microbiol Infect Dis. 2021;101:115478.
- Esteves E, Mendes AK, Barros M, et al. Population wide testing pooling strategy for SARS-CoV-2 detection using saliva. PLoS One. 2022;17:e0263033.
- Saegerman C, Donneau A-F, Speybroeck N, et al. Repetitive saliva-based mass screening as a tool for controlling SARS-CoV-2 transmission in nursing homes. Transbound Emerg Dis. 2021. DOI:https://doi.org/10.1111/tbed.14280.
- Minister Harris urges staff and students to join pilot rapid testing project on four college campuses; 2021. Available from: https://www.gov.ie/en/press-release/12772-minister-harris-urges-staff-and-students-to-join-pilot-rapid-testing-project-on-four-college-campuses/
- Casey J. Predicting pandemics: how Irish universities are building early warning covid systems. Irish Examiner. 2021.
- Soriano V, Ganado-Pinilla P, Sanchez-Santos M, et al. Main differences between the first and second waves of COVID-19 in Madrid, Spain. Int J Infect Dis. 2021;105:374–376.
- Kidd SP, Burns D, Armson B, et al. RT-LAMP has high accuracy for detecting SARS-CoV-2 in saliva and naso/oropharyngeal swabs from asymptomatic and symptomatic individuals. J Mol Diagn. 2022;24:320–336.
- LAMP (Saliva) testing. NHS: Royal Cornwall Hospitals. 2021. Available from https://www.royalcornwall.nhs.uk/wellbeing/regular-covid-testing-for-patient-facing-staff-self-test-kits/lamp-saliva-testing/
- Brown B, O’Hara RW, Guiver M, et al. Evaluation of a novel direct RT-LAMP assay for the detection of SARS-CoV-2 from saliva samples in asymptomatic individuals. J Clin Virol Plus. 2022;2:100074.
- TCD UniCoV Team. Update from TCD UniCoV Team to staff and students. 2021.
- EasyCOV, Covid-19 salivary rapid and easy molecular test. SkillCell | Alcen; Available from: https://www.skillcell-alcen.com/.
- Fabiani L, Saroglia M, Galatà G, et al. Magnetic beads combined with carbon black-based screen-printed electrodes for COVID-19: a reliable and miniaturized electrochemical immunosensor for SARS-CoV-2 detection in saliva. Biosens Bioelectron. 2021;171:112686.
- Ludvigsson JF. The first eight months of Sweden’s COVID-19 strategy and the key actions and actors that were involved. Acta Paediatr. 2020;109:2459–2471.
- PCR and antigen test for Covid-19 with travel certificates. Testmottagningen Available from: https://www.testmottagningen.se/en/.
- Renault V, Fontaine S, Mpouam SE, et al. Main determinants of the acceptance of COVID-19 control measures by the population: a first pilot survey at the University of Liege, Belgium. Transbound Emerg Dis. 2021. DOI:https://doi.org/10.1111/tbed.14410
- Carmagnola D, Pellegrini G, Canciani E, et al. Saliva molecular testing for SARS-CoV-2 surveillance in two Italian primary schools. Children. 2021;8:544.
- Saliva test. Gouvernement Princier Principauté de Monaco |; 2021. Available from: https://covid19.mc/en/fight-against-coronavirus/tout-sur-les-tests-diagnostics/saliva-test/
- Winje BA, Ofitserova TS, Brynildsrud OB, et al. Comprehensive contact tracing, testing and sequencing show limited transmission of SARS-CoV-2 between children in schools in Norway, August 2020 to May 2021. Microorganisms. 2021;9:2587.
- Introduction of lollipop tests for primary and special schools. City of Bonn; 2021 Available from: https://www.bonn.de/microsite/en/press-releases/mai-2021/Introduction-of-lollipop-tests-for-primary-and-special-schools.php.
- Lindner AK, Sarma N, Rust LM, et al. Monitoring for COVID-19 by universal testing in a homeless shelter in Germany: a prospective feasibility cohort study. BMC Infect Dis. 2021;21:1241.
- Oberste M, Pusch L-M, Roth R, et al. Protocol of the cologne corona surveillance (CoCoS) study- a prospective population-based cohort study. BMC Public Health. 2021;21:1295.
- Vogel S, von Both U, Nowak E, et al. SARS-CoV-2 saliva mass screening in primary schools: a 10-week sentinel surveillance study in Munich, Germany. Diagnostics. 2022;12:162.
- Out and about? get tested at test for access. Test for Access Available from: https://www.testenvoortoegang.org/.
- The Federal Council. Coronavirus: federal council introduces tighter measures. Confédération Suisse; 2021. Available from: https://www.admin.ch/gov/en/start/documentation/media-releases.msg-id-86544.html
- GVN and Monaco COVID-19 Diagnostic Conference: Executive Summary; 2021. Available from: https://gvn.org/gvn-and-monaco-covid-19-diagnostic-conference-executive-summary/
- Caswell M. UK government approves first saliva-based Day 2 and Day 8 tests, Business Traveller, 2021
- Robson S. What is operation moonshot? the government plan for coronavirus ‘freedom pass’ to be trialled in salford. Manchester Evening News 2020.
- Holm-Hansen C. 2021.
- Mudenda S. Coronavirus disease 2019 (COVID-19): the role of pharmacists in the fight against COVID-19 pandemic. Int J Pharm Pharmacol. 2020;4:1–3.
- Jacobs J, Kühne V, Lunguya O, et al. Implementing COVID-19 (SARS-CoV-2) rapid diagnostic tests in Sub-Saharan Africa: a review. Front Med. 2020;7:557797.
- Ro N, E B, I K, et al. The impact on public health and economy using lockdown as a tool against COVID-19 pandemic in Africa: a perspective. J Epidemiol Public Health Rev. 2020;5. DOI:https://doi.org/10.16966/2471-8211.188.
- Nachega JB, Grimwood A, Mahomed H, et al. From easing lockdowns to scaling up community-based coronavirus disease 2019 screening, testing, and contact tracing in Africa-shared approaches, innovations, and challenges to minimize morbidity and mortality. Clin Infect Dis. 2021;72:327–331.
- Vaz SN, Santana DSD, Netto EM, et al. Saliva is a reliable, non-invasive specimen for SARS-CoV-2 detection. Braz J Infect Dis. 2020;24:422–427.
- Yusuf L, Appeaning M, Amole TG, et al. Rapid, cheap, and effective COVID-19 diagnostics for Africa. Diagnostics (Basel). 2021;11. DOI:https://doi.org/10.3390/diagnostics11112105.
- Vogels CBF, Watkins AE, Harden CA, et al. SalivaDirect: a simplified and flexible platform to enhance SARS-CoV-2 testing capacity. Med. 2021;2:263–280. e6.
- Hawari Y In Palestine, COVID-19 meets the Israeli occupation. Al Shabaka: the Palestinian Policy Network 2020.
- Rasgon A An Israeli airstrike damaged Gaza’s only lab for processing coronavirus tests, officials said. The New York Times 2021.
- Pisanic N, Ballard S-B, Colquechagua FD, et al. Minimally invasive saliva testing to monitor norovirus infection in community settings. J Infect Dis. 2019;219:1234–1242.
- Labo N, Marshall V, Miley W, et al. Mutual detection of Kaposi’s sarcoma-associated herpesvirus and Epstein-Barr virus in blood and saliva of Cameroonians with and without Kaposi’s sarcoma. Int J Cancer. 2019;145:2468–2477.
- Nangendo J, Obuku EA, Kawooya I, et al. Diagnostic accuracy and acceptability of rapid HIV oral testing among adults attending an urban public health facility in Kampala, Uganda. PLoS One. 2017;12:e0182050.
- Holm-Hansen C, Nyombi B, Nyindo M. Saliva-based HIV testing among secondary school students in Tanzania using the OraQuick rapid HIV1/2 antibody assay. Ann N Y Acad Sci. 2007;1098:461–466.
- Hofman LF. Human saliva as a diagnostic specimen. J Nutr. 2001;131:1621S–5S.
- Jamieson LM, Antonsson A, Garvey G, et al. Prevalence of oral human papillomavirus infection among Australian indigenous adults. JAMA Network Open. 2020;3:e204951.
- Apinjoh TO, Ntasin VN, Tataw PCC, et al. Comparison of conventional and non-invasive diagnostic tools for detecting Plasmodium falciparum infection in southwestern Cameroon: a cross-sectional study. Infect Dis Poverty. 2021;10:75.
- Mfuh KO, Tassi Yunga S, Esemu LF, et al. Detection of Plasmodium falciparum DNA in saliva samples stored at room temperature: potential for a non-invasive saliva-based diagnostic test for malaria. Malar J. 2017;16:434.
- Fung AO, Damoiseaux R, Grundeen S, et al. Quantitative detection of PfHRP2 in saliva of malaria patients in the Philippines. Malar J. 2012;11:175.
- Pandey N, Siripattanapipong S, Leelayoova S, et al. Detection of Leishmania DNA in saliva among patients with HIV/AIDS in Trang Province, southern Thailand. Acta Trop. 2018;185:294–300.
- Musso D, Roche C, Nhan T-X, et al. Detection of Zika virus in saliva. J Clin Virol. 2015;68:53–55.
- Musso D, Teissier A, Rouault E, et al. Detection of chikungunya virus in saliva and urine. Virol J. 2016;13:102.
- Avendano C, Lilienfeld A, Rulli L, et al. SARS-CoV-2 variant tracking and mitigation during in-person learning at a Midwestern University in the 2020-2021 school year. JAMA Network Open. 2022;5:e2146805.
- Yelagandula R, Bykov A, Vogt A, et al. Multiplexed detection of SARS-CoV-2 and other respiratory infections in high throughput by SARSeq. Nat Commun. 2021;12:3132.
- Alpert T, Vogels CBF, Breban MI, et al. Sequencing SARS-CoV-2 genomes from saliva. Virus Evol. 2022;8. DOI:https://doi.org/10.1093/ve/veab098.
- King KL, Wilson S, Napolitano JM, et al. SARS-CoV-2 variants of concern alpha and delta show increased viral load in saliva. PLOS ONE. 2022;17:e0267750.
- Adamson B, Sikka R, Wyllie AL, et al. Discordant SARS-CoV-2 PCR and rapid antigen test results when infectious: a December 2021 occupational case series. bioRxiv. 2022. DOI:https://doi.org/10.1101/2022.01.04.22268770
- Test to treat. Available from: https://aspr.hhs.gov/TestToTreat/Pages/default.aspx
