ABSTRACT
Background
Villin is a protein of the brush border of epithelial cells, which is used as an immunohistochemical marker for colorectal and gastrointestinal neoplasms. However, other tumor entities can also express villin.
Methods
To comprehensively determine villin expression, tissue microarrays containing 14,398 samples from 118 different tumor types as well as 608 samples of 76 different normal tissues were analyzed by immunohistochemistry.
Results
Villin was found in 54 of 118 tumor categories, including 36 tumor categories with strong staining. Villin expression was frequent in colorectal (60-100%), upper gastrointestinal tract (61-100%), pancreatobiliary (25-86%), and renal tumors (≤18%) as well as in mucinous ovarian cancers (67%), yolk sac tumors (76%) and in neuroendocrine neoplasms (22-41%). Reduced villin expression was linked to advanced pT stage, lymph vessel invasion, and microsatellite instability (p ≤ 0.0006) in colorectal adenocarcinoma.
Conclusion
Our data support a high utility of villin immunohistochemistry for the identification of tumors with gastrointestinal, pancreatobiliary, and yolk sac tumor origin. However, considering that at least a weak villin positivity in some tumor cells occurred in 54 different tumor categories, villin immunohistochemistry should be applied as a part of a marker panel rather than as a stand-alone marker.
1. Introduction
Villin is a 92.5 kDa calcium-regulated actin-binding protein, coded by the VIL1 gene at 2q35 [Citation1,Citation2]. Villin was first isolated from the microvilli of intestinal epithelium which resulted in the naming of the protein [Citation3]. Microvilli are located on the apical membrane of polarized epithelial cells (brush border) and increase the surface area for the absorption of nutrients. Villin protein represents a dominant part of the brush border cytoskeleton that functions in the capping, severing, and bundling of actin filaments [Citation4,Citation5]. Elevated expression of villin results in changes in actin distribution, cell morphology, cell migration, and cell death [Citation4,Citation6].
Normal tissue analyses identified villin expression in only a limited number of epithelial cell types of the gastrointestinal and the biliary tract as well as proximal tubules in the kidney [Citation7]. Because of its tissue specificity, villin has been suggested as an immunohistochemical marker for the classification of metastatic cancer tissues of unknown origin. Several studies have indeed suggested high rates of villin positivity in gastrointestinal [Citation8,Citation9], biliary tract [Citation9], and kidney cancers [Citation9,Citation10], but the published data on the prevalence of villin immunostaining are discrepant for many tumor entities. For example, villin positivity has been found in 6–100% of colorectal [Citation11–16], 0–75% of esophageal [Citation17–22], and 7.6–100% of gastric adenocarcinomas [Citation8,Citation9,Citation23–26], 0–100% of cholangiocarcinomas [Citation9,Citation27–29], 0–69% of renal cell carcinomas [Citation9,Citation10,Citation30], 0–65% of urothelial carcinomas [Citation9,Citation15,Citation20,Citation23,Citation31], 0–76.9% of adenocarcinomas of the lung [Citation8,Citation9,Citation20,Citation23,Citation32–37], and 0–36% of endometrioid carcinomas of the endometrium [Citation1,Citation9,Citation23,Citation38]. These conflicting data are probably caused by the use of different antibodies, immunostaining protocols, and criteria to define villin positivity in these studies.
To better understand the prevalence and diagnostic utility of villin expression in cancer, more than 14,000 tumor tissue samples from 118 different tumor types and subtypes, as well as 76 non-neoplastic tissue categories, were analyzed by immunohistochemistry (IHC) under highly standardized conditions in a tissue microarray (TMA) format in this study.
2. Materials and methods
2.1 Tissue microarrays (TMAs)
Our normal tissue TMA was composed of eight samples from eight different donors each from 76 different normal tissue types (608 samples on one slide). The cancer TMAs contained a total of 14,398 primary tumors from 118 tumor types and subtypes. Detailed histopathological and molecular data were available for cancers of the colon (n = 1,784), stomach (327), pancreas (598), endometrium (259), and kidney (1,157). Clinical follow-up data were accessible from 850 kidney cancer patients with a median follow-up time of 48 months (range 1–250). The composition of normal and cancer TMAs is described in the results section. All samples were retrieved from the archives of the Institutes of Pathology, University Hospital of Hamburg, Germany, the Institute of Pathology, Clinical Center Osnabrueck, Germany, and Department of Pathology, Academic Hospital Fuerth, Germany. Tissues were fixed in 4% buffered formalin and then embedded in paraffin. The TMA manufacturing process was described earlier in detail [Citation39–41]. In brief, one tissue spot (diameter: 0.6 mm) was transmitted from a cancer containing tumor block in an empty recipient paraffin block. The use of archived remnants of diagnostic tissues for TMA manufacturing, their analysis for research purposes, and use of anonymized patient data are in accordance with local laws (HmbKHG, §12), and had been approved by the local ethics committee (Ethics commission Hamburg, WF-049/09). All work has been carried out in compliance with the Helsinki Declaration.
2.2. Immunohistochemistry
Freshly cut TMA sections were manually immunostained in 1 day and in one experiment. Slides were deparaffinized and exposed to heat-induced antigen retrieval for 5 min in an autoclave at 121°C in a pH 7.8 buffer. Primary antibody specific for villin (rabbit recombinant, MSVA-459 R, MS Validated Antibodies, GmbH, Hamburg, Germany) was applied at 37°C for 60 minutes at a dilution of 1:250. Bound antibody was then visualized using the EnVision Kit (Agilent, Santa Clara, CA, USA) according to the manufacturer’s directions. One pathologist (SS) scored all stained TMAs. From samples with questionable results, images were taken and discussed with other pathologists. For normal tissues, villin staining intensity was rated as negative (no visible staining in any cell type) or positive staining, including weak (staining of 1+ intensity), moderate (2+), and strong (3+) staining. For tumor tissues, the percentage of positive neoplastic cells was estimated, and the staining intensity was semiquantitatively recorded (0, 1+, 2+, 3+). For statistical analyses, the staining results were categorized into four groups. Tumors without any staining were considered negative. Cancers with 1+ staining intensity in ≤70% of tumor cells or 2+ intensity in ≤30% of tumor cells were considered weakly positive. Tumors with 1+ staining intensity in >70% of tumor cells, 2+ intensity in 31–70%, or 3+ intensity in ≤30% of tumor cells were considered moderately positive. Tumors with 2+ intensity in >70% or 3+ intensity in >30% of tumor cells were considered strongly positive. For the purpose of antibody validation, the normal tissue TMA was also analyzed with the CE-IVD marked anti-villin clone 1D2 C3 (Agilent #IR07661-2, RTU) on an Agilent Autostainer Link 48 according to the manufacturers’ directions.
2.3. Statistics
Statistical calculations were performed with JMP 14 software (SAS Institute Inc., NC, USA). Contingency tables and the chi2-test were performed to search for associations between villin immunostaining and tumor phenotype. Bonferroni correction was applied for multiple testing, and the alpha-level was set to 0.0016 for significant differences. Survival curves were plotted according to Kaplan–Meier. The Log-Rank test was applied to detect significant differences between groups.
3. Results
3.1. Normal tissue distribution
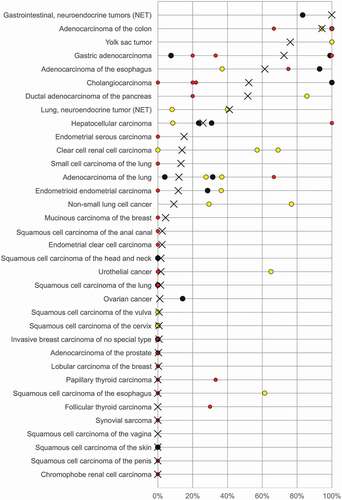
Villin immunostaining was predominantly membranous but also cytoplasmic and often showed a strong focus on the apical/luminal membranes (brush border). A strong villin expression was seen in all epithelial cells of the colorectum, appendix, small intestine, duodenum, pancreatic excretory ducts, gallbladder, bile ducts, and in proximal tubuli of the kidney. A weak-to-moderate villin expression was observed at the surface epithelium of the stomach, where the intensity gradually declined toward the base of the glands (which were villin negative), in ciliated columnar cells of the cauda epididymis, a fraction of neuroendocrine cells of islets of Langerhans, and at the luminal membranes of hepatocytes and of pancreatic acinar cells. Representative images of villin-positive normal tissues are shown in , and an overview of the stained normal tissue array is given in Supplemental Figure 1. Tabulated data on villin-positive normal tissues and a list of villin-negative normal tissues are given in Supplemental . All findings observed by MSVA-459 R were also detected by clone 1D2 C3. All tumor analyses were then only performed by MSVA-459 R because of a favorable signal-to-noise ratio and lower costs per staining (1.19 Euro per analysis) as compared to 1D2 C3 (6.93 Euro per analysis for Agilent IR07661-2).
Figure 1. Villin immunostaining in normal tissues. The panels show a strong villin positivity of epithelial cells of the ileum (a), appendix (b), and the gallbladder (c) as well as in proximal tubuli of the kidney where the staining predominates at the luminal membrane (d). A somewhat weaker villin staining occurs in a fraction of epithelial cells of the caput epididymis (e) and at apical membranes of hepatocytes (f). In the pancreas, a strong staining occurs in the epithelium of excretory ducts, while the positivity is less intense at the luminal membranes of acinar cells and the membranes of a fraction of islet cells (g). Villin immunostaining is lacking in urinary bladder urothelium (h).
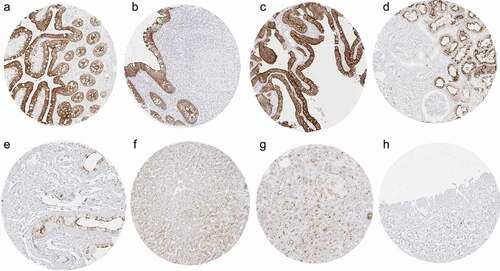
Table 1. Villin immunostaining in human tumors.
3.2. Villin in cancer
Villin immunostaining was found in 2,747 (22.1%) of the 12,429 analyzable tumors, including 462 (3.7%) with weak, 1,062 (8.5%) with moderate, and 1,223 (9.8%) with strong immunostaining. Overall, 54 (46%) of 118 tumor categories showed detectable villin expression with 36 (31%) tumor categories including at least one case with strong positivity (). Representative images of villin-positive tumors are shown in . The highest rate of positive villin staining and the highest levels of expression were found in adenocarcinomas and neuroendocrine tumors of the colorectum (60–100%), upper gastrointestinal tract (61–100%), pancreatobiliary system (25–86%), yolk sac tumors (76%), and in mucinous carcinomas of the ovary (67%). Villin positivity was also common in various categories of extra-gastrointestinal neuroendocrine neoplasms (22–41%), kidney tumors (up to 18%), hepatocellular carcinomas (25%), endometrioid carcinomas of the ovary (20%) and of the endometrium (12%), as well as in adenocarcinomas of the lung (12%). A graphical representation of the ranking order of villin-positive and strongly positive cancers is given in . In colorectal adenocarcinomas, a reduced villin expression was significantly linked to unfavorable tumor phenotype and microsatellite instability and the association with high pT (p < 0.0001) was also retained in 1,050 microsatellite stable cancers (). Similar findings were also made in clear cell renal cell carcinomas, where low villin expression was linked to high grade (p < 0.04), advanced pT stage (p = 0.0044), and distant metastatic cancers (p = 0.0072), although these associations did not reach statistical significance after Bonferroni correction for multiple testing. Villin immunostaining was unrelated to features of tumor aggressiveness in clear cell renal cell carcinoma, endometrial, pancreatic, and gastric adenocarcinomas ().
Figure 2. Villin immunostaining in cancer. The panels show a strong villin staining in a colorectal adenocarcinoma (a), a gastric adenocarcinoma (b), a mucinous ovarian carcinoma (c), and a muscle-invasive urothelial carcinoma (d). A focal positivity is seen in an adenocarcinoma of the lung (90% of tumor cells; e) and a papillary renal cell carcinoma (50% of tumor cells; f). A strong villin staining also occurs in Yolk sac tumor components of a mixed germ cell tumor also containing villin-negative embryonal carcinoma (g). Villin staining is absent in an invasive breast cancer of no special type (h).
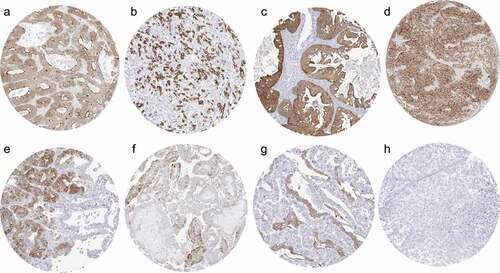
Figure 3. Ranking order of villin immunostaining in cancers. Both the frequency of positive cases (blue dots) and the frequency of strongly positive cases (orange dots) are shown.
Table 2. Villin immunostaining and tumor phenotype in colon, kidney, endometrial, ovarian, pancreatic, and stomach cancers.
4. Discussion
Given the controversial data on villin expression in previous studies and the large scale of our study, the reagents and protocols used for this project were extensively validated. The International Working Group for Antibody Validation (IWGAV) has proposed that antibody validation for immunohistochemistry on formalin-fixed tissues should include either a comparison of the findings obtained by two different independent antibodies or a comparison with expression data obtained by another independent method [Citation42]. Considering both recommendations, we compared our normal tissue staining with known villin RNA expression data derived from three independent RNA screening studies, including the Human Protein Atlas (HPA) RNA-seq tissue dataset [Citation43], the FANTOM5 project [Citation44,Citation45], and the Genotype-Tissue Expression (GTEx) project [Citation46], and also compared the staining patterns with a second independent antibody (1D2 C3). Normal tissues are optimal for antibody validation because they typically show characteristic organ-specific expression profiles with little interindividual variability. To as much as possible ensure that any antibody cross reactivity would be detected in our validation experiment, 76 different normal tissue categories were analyzed. Using MSVA-059 R, villin immunostaining was detected in all organs with documented RNA expression including duodenum, small intestine, colon, rectum, appendix, liver, pancreas, and the kidney. In addition, villin immunostaining was seen in stomach surface epithelium and the epididymis which were both villin negative by RNA analysis. Most likely, this discrepancy is because the villin-positive cell populations constitute too small subsets of the total amount of cells in these organs for becoming detectable in RNA analyses. Identical staining patterns found by using the independent anti-villin antibody 1D2 C3 (Supplemental Figure 2) confirmed true villin expression in these tissues. Western blot analysis showing one band at the expected molecular weight around 93 kDa further supported antibody specificity (Supplemental Figure 3).
The successful analysis of 12,429 cancers revealed that villin expression strongly predominated in tumors derived from villin-positive normal precursor cells. A total of 16 of the 18 tumor categories with more than 50% positive cases, and 21 of the 31 tumor categories with more than 10% positive cases, had originated from villin expressing tissues. These included colorectal tumors (7 categories), upper gastrointestinal tract tumors (4), pancreatobiliary tumors (8), and renal tumors (2). Tumors that were not obviously derived from villin-positive normal tissues but showed particularly frequent and high-level villin expression included mucinous ovarian cancer, yolk sac tumors of the testis as well as several neuroendocrine neoplasms from various extra-gastrointestinal sites. Possible explanations for these findings include the physiological expression of villin in the yolk sac of the normal embryo [Citation47]. At this stage of development, the yolk sac takes over the function of the liver and the intestine for the fetus and expresses multiple gastrointestinal-type proteins [Citation48,Citation49]. A particularly high prevalence of villin positivity in neuroendocrine tumors is also supported by data from earlier studies describing villin expression in 12% and 40% of neuroendocrine tumors of the lung [Citation50,Citation51]. The role of villin in normal neuroendocrine cells and the reason for the frequent villin positivity of neuroendocrine neoplasms is unclear. It is of note, however, that a fraction of the neuroendocrine cells of the islets of Langerhans in the pancreas, the only organ where neuroendocrine cells occur in a noticeable cluster, showed a distinct membranous villin positivity. The cell of origin of the rare mucinous carcinomas of the ovary is unknown. It is well conceivable and supported by our data that these – possibly metaplastic – cells, which could for example be derived from Brenner tumors or the fallopian tube-peritoneal junction, may express villin [Citation52]. Intestinal metaplasia has been described to occur in adenocarcinomas from different locations [Citation23]. This may explain the occurrence of villin positivity in various types of adenocarcinomas in this study.
That 23 additional tumor entities showed villin immunostaining in a fraction of cases limits the diagnostic utility of villin immunohistochemistry to some extent. However, most villin-positive cancers derived from villin-negative precursor cells showed only weak staining often occurring in only a small subset of cells. Such findings may be caused by a random involvement of the villin gene in genomic alterations which typically occur in genetically instable neoplastic cells and lead to a deregulation of the expression of many genes. From a diagnostic point of view, these data suggest that especially low level and focal villin immunostaining should be interpreted with caution.
The large number of tumors analyzed enabled a survey of the potential biological and clinical impact of villin expression levels in several tumor types. That a loss of villin expression was linked to advanced tumor stage, nodal metastasis, and microsatellite instability in colorectal cancer is consistent with data from Arango et al. reporting similar associations in a cohort of 577 tumors [Citation53]. These authors excluded coding sequence mutations, epigenetic inactivation, or promoter mutation as possible causes for the progressive loss of villin expression during cancer progression. A similar finding – although at lower expression levels, and although not statistically secured by Bonferroni correction – was observed for clear cell kidney cancer in this study. Even though villin staining was generally lower in these cancers and resulted in positivity rates of only 15% in clear cell carcinomas, low villin immunostaining was linked to prognostically unfavorable tumor features such as low grade, low pT category, and distant metastasis. Clinico-pathological associations have so far not been evaluated in kidney cancer by other authors. The gradual loss of villin expression during tumor progression of colorectal and kidney cancers may reflect cellular dedifferentiation resulting in a loss of normally expressed proteins that are no longer vital for the cancer cells such as proteins that are relevant for the function of brush borders. The absence of unequivocal significant statistical associations between villin expression and parameters of tumor aggressiveness in clear cell renal cell cancers, endometrial, pancreatic, and gastric adenocarcinomas further argues against a pivotal direct role of villin for cancer cell behavior which also cannot be expected based on the known function of the protein.
Our data provide a ranking order of human tumors according to the prevalence and intensity of villin immunostaining. The comparison with previously published data in the literature demonstrates that this information cannot easily be obtained from the literature given the highly discrepant findings reported on many tumor entities (). While the prevalence rates described in this study are specific to the reagents and the protocol used in our laboratory, it can be expected that the standardized use of other specific villin antibodies and/or other protocols might result in different absolute numbers and prevalence rates but a comparable ranking order.
5. Conclusion
In summary, our data support a high utility of villin immunohistochemistry for the identification of gastrointestinal, pancreatobiliary, and yolk sac tumor origin. However, considering that at least a weak villin positivity in some tumor cells occurred in 54 different tumor categories, villin immunohistochemistry should be applied as a part of a marker panel rather than as a stand-alone marker.
Declaration of interest
The villin (rabbit recombinant, MSVA-459R) was provided from MS Validated Antibodies GmbH (owned by a family member of GS). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewers disclosure
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Author’s contributions
DD, PL, RS, and GS contributed to conception, design, data collection, data analysis and manuscript writing.
AM, SiM, SW, ML, AML, EB, TSC, WW, CB, TK, and SS participated in pathology data analysis and data interpretation.
KM, CF, RU, FJ, LH, and SM contributed to the immunohistochemistry and Western blot analysis.
AHM and TK contributed to conception and design, collection of samples.
CHM, MK and RS performed statistical analysis.
DD, PL, WW, RS, and GS contributed to study supervision.
All authors agree to be accountable for the content of the work.
Ethical statement
The usage of archived diagnostic leftover tissues for manufacturing of TMAs and their analysis for research purposes, as well as anonymized patient data analysis, has been approved by local laws (HmbKHG, §12,1) and by the local ethics committee (Ethics commission Hamburg, WF-049/09). All work has been carried out in compliance with the Helsinki Declaration.
Supplemental Material
Download Zip (732.1 KB)Acknowledgments
We are grateful to Melanie Witt, Inge Brandt, Laura Behm, Maren Eisenberg, and Sünje Seekamp for excellent technical assistance.
Supplementary material
Supplemental data for this article can be accessed here
Data Availability statement
Raw data are available upon reasonable request. All data relevant to the study are included in the article.
Additional information
Funding
References
- Nakamura E, Iwakawa M, Furuta R, et al. Villin1, a novel diagnostic marker for cervical adenocarcinoma. Cancer Biol Ther. 2009;8(12):1146–1153.
- George SP, Wang Y, Mathew S, et al. Dimerization and actin-bundling properties of villin and its role in the assembly of epithelial cell brush borders. J Biol Chem. 2007;282(36):26528–26541.
- Bretscher A, Weber K. Villin is a major protein of the microvillus cytoskeleton which binds both G and F actin in a calcium-dependent manner. Cell. 1980;20(3):839–847.
- Delacour D, Salomon J, Robine S, et al. Plasticity of the brush border - the yin and yang of intestinal homeostasis. Nat Rev Gastroenterol Hepatol. 2016;13(3):161–174.
- Grone HJ, Weber K, Helmchen U, et al. Villin–a marker of brush border differentiation and cellular origin in human renal cell carcinoma. Am J Pathol. 1986;124(2):294–302.
- Wang Y, George SP, Roy S, et al. Both the anti- and pro-apoptotic functions of villin regulate cell turnover and intestinal homeostasis. Sci Rep. 2016;6:35491.
- Robine S, Huet C, Moll R, et al. Can villin be used to identify malignant and undifferentiated normal digestive epithelial cells? Proc Natl Acad Sci U S A. 1985;82(24):8488–8492.
- Merchant SH, Amin MB, Tamboli P, et al. Primary signet-ring cell carcinoma of lung: immunohistochemical study and comparison with non-pulmonary signet-ring cell carcinomas. Am J Surg Pathol. 2001;25(12):1515–1519.
- Moll R, Robine S, Dudouet B, et al. Villin: a cytoskeletal protein and a differentiation marker expressed in some human adenocarcinomas. Virchows Arch B Cell Pathol Incl Mol Pathol. 1987;54(3):155–169.
- Droz D, Zachar D, Charbit L, et al. Expression of the human nephron differentiation molecules in renal cell carcinomas. Am J Pathol. 1990;137(4):895–905.
- Lin F, Shi J, Zhu S, et al. Cadherin-17 and SATB2 are sensitive and specific immunomarkers for medullary carcinoma of the large intestine. Arch Pathol Lab Med. 2014;138(8):1015–1026.
- Al-Maghrabi J, Gomaa W, Buhmeida A, et al. Loss of villin immunoexpression in colorectal carcinoma is associated with poor differentiation and survival. ISRN Gastroenterol. 2013;2013:679724.
- Zhang MQ, Lin F, Hui P, et al. Expression of mucins, SIMA, villin, and CDX2 in small-intestinal adenocarcinoma. Am J Clin Pathol. 2007;128(5):808–816.
- Mizoshita T, Tsukamoto T, Inada KI, et al. Loss of MUC2 expression correlates with progression along the adenoma-carcinoma sequence pathway as well as de novo carcinogenesis in the colon. Histol Histopathol. 2007;22(3):251–260.
- Suh N, Yang XJ, Tretiakova MS, et al. Value of CDX2, villin, and alpha-methylacyl coenzyme A racemase immunostains in the distinction between primary adenocarcinoma of the bladder and secondary colorectal adenocarcinoma. Mod Pathol. 2005;18(9):1217–1222.
- Nishizuka S, Chen S-T, Gwadry FG, et al. Diagnostic markers that distinguish colon and ovarian adenocarcinomas: identification by genomic, proteomic, and tissue array profiling. Cancer Res. 2003;63(17):5243–5250.
- Cao HH, Zhang S-Y, Shen J-H, et al. A three-protein signature and clinical outcome in esophageal squamous cell carcinoma. Oncotarget. 2015;6(7):5435–5448.
- Peng ZM, Yu W, Xie Y, et al. A four actin-binding protein signature model for poor prognosis of patients with esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2014;7(9):5950–5959.
- Weimann A, Rieger A, Zimmermann M, et al. Comparison of six immunohistochemical markers for the histologic diagnosis of neoplasia in Barrett’s esophagus. Virchows Arch. 2010;457(5):537–545.
- Pereira TC, Share SM, and Magalhães AV, et al. Can we tell the site of origin of metastatic squamous cell carcinoma? An immunohistochemical tissue microarray study of 194 cases. Appl Immunohistochem Mol Morphol. 2011;19(1):10–14.
- Anders M, Sarbia M, Grotzinger C, et al. Expression of EpCam and villin in Barrett’s esophagus and in gastric cardia. Dis Markers. 2008;24(6):287–292.
- Regalado SP, Nambu Y, and Iannettoni MD, et al. Abundant expression of the intestinal protein villin in Barrett’s metaplasia and esophageal adenocarcinomas. Mol Carcinog. 1998;22(3):182–189.
- Seipel AH, Samaratunga H, Delahunt B, et al. Immunohistochemistry of ductal adenocarcinoma of the prostate and adenocarcinomas of non-prostatic origin: a comparative study. APMIS. 2016;124(4):263–270.
- Gushima R, Yao T, Kurisaki-Arakawa A, et al. Expression of adipophilin in gastric epithelial neoplasia is associated with intestinal differentiation and discriminates between adenoma and adenocarcinoma. Virchows Arch. 2016;468(2):169–177.
- Ran X, Xu X, and Yang Y, et al. A quantitative proteomics study on olfactomedin 4 in the development of gastric cancer. Int J Oncol. 2015;47(5):1932–1944.
- Tian MM, Zhao AL, and Li ZW, et al. Phenotypic classification of gastric signet ring cell carcinoma and its relationship with clinicopathologic parameters and prognosis. World J Gastroenterol. 2007;13(23):3189–3198.
- Yang Z. The utility of villin and mammaglobin in the differential diagnosis between intrahepatic cholangiocarcinoma and breast cancer. Appl Immunohistochem Mol Morphol. 2015;23(1):19–25.
- Al-Muhannadi N, Ansari N, Brahmi U, et al. Differential diagnosis of malignant epithelial tumours in the liver: an immunohistochemical study on liver biopsy material. Ann Hepatol. 2011;10(4):508–515.
- Lau SK, Prakash S, and Geller SA, et al. Comparative immunohistochemical profile of hepatocellular carcinoma, cholangiocarcinoma, and metastatic adenocarcinoma. Hum Pathol. 2002;33(12):1175–1181.
- Nogales FF, Prat J, Schuldt M, et al. Germ cell tumour growth patterns originating from clear cell carcinomas of the ovary and endometrium: a comparative immunohistochemical study favouring their origin from somatic stem cells. Histopathol. 2018;72(4):634–647.
- Roy S, Smith MA, Cieply KM, et al. Primary bladder adenocarcinoma versus metastatic colorectal adenocarcinoma: a persisting diagnostic challenge. Diagn Pathol. 2012;7:151.
- Bian T, Zhao J, Feng J, et al. Combination of cadherin-17 and SATB homeobox 2 serves as potential optimal makers for the differential diagnosis of pulmonary enteric adenocarcinoma and metastatic colorectal adenocarcinoma. Oncotarget. 2017;8(38):63442–63452.
- Calio A, Lever V, Rossi A, et al. Increased frequency of bronchiolar histotypes in lung carcinomas associated with idiopathic pulmonary fibrosis. Histopathol. 2017;71(5):725–735.
- Wang CX, Liu B, Wang Y-F, et al. Pulmonary enteric adenocarcinoma: a study of the clinicopathologic and molecular status of nine cases. Int J Clin Exp Pathol. 2014;7(3):1266–1274.
- Goldstein NS, Thomas M. Mucinous and nonmucinous bronchioloalveolar adenocarcinomas have distinct staining patterns with thyroid transcription factor and cytokeratin 20 antibodies. Am J Clin Pathol. 2001;116(3):319–325.
- Nambu Y, Iannettoni MD, Orringer MB, et al. Unique expression patterns and alterations in the intestinal protein villin in primary and metastatic pulmonary adenocarcinomas. Mol Carcinog. 1998;23(4):234–242.
- Tan J, Sidhu G, Greco MA, et al. Villin, cytokeratin 7, and cytokeratin 20 expression in pulmonary adenocarcinoma with ultrastructural evidence of microvilli with rootlets. Hum Pathol. 1998;29(4):390–396.
- Nakamura E, Satoh T, Iwakawa M, et al. Villin1, a diagnostic marker for endometrial adenocarcinoma with high grade nuclear atypia. Cancer Biol Ther. 2011;12(3):181–190.
- Dancau AM, Simon R, and Mirlacher M, et al. Tissue microarrays. Methods Mol Biol. 2016;1381:53–65.
- Mirlacher M, Simon R. Recipient block TMA technique. Methods Mol Biol. 2010;664:37–44.
- Kononen J, Bubendorf L, and Kallioniemie A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4(7):844–847.
- Uhlen M, Bandrowski A, Carr S, et al. A proposal for validation of antibodies. Nat Methods. 2016;13(10):823–827.
- Uhlén M, Fagerbeg L, and Hallström BM , et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. DOI:10.1126/science.1260419.
- Lizio M, Abugessaisa I, and Noguchi S , et al. Update of the FANTOM web resource: expansion to provide additional transcriptome atlases. Nucleic Acids Res. 2019;47(D1):D752–D758.
- Lizio M, Harshbarger J, Shimoji H, et al. Gateways to the FANTOM5 promoter level mammalian expression atlas. Genome Biol. 2015;16:22.
- Consortium GT. The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013;45(6):580–585.
- Maunoury R, Robine S, Pringault E, et al. Villin expression in the visceral endoderm and in the gut anlage during early mouse embryogenesis. EMBO J. 1988;7(11):3321–3329.
- Nogales FF, Quiñonez E, López-Marín L, et al. A diagnostic immunohistochemical panel for yolk sac (primitive endodermal) tumours based on an immunohistochemical comparison with the human yolk sac. Histopathol. 2014;65(1):51–59.
- Nogales FF, Dulcey I. The secondary human yolk sac has an immunophenotype indicative of both hepatic and intestinal differentiation. Int J Dev Biol. 2012;56(9):755–760.
- Sun L, Sakurai S, Sano T, et al. High-grade neuroendocrine carcinoma of the lung: comparative clinicopathological study of large cell neuroendocrine carcinoma and small cell lung carcinoma. Pathol Int. 2009;59(8):522–529.
- Zhang PJ, Harris KR, and Alobeid B, et al. Immunoexpression of villin in neuroendocrine tumors and its diagnostic implications. Arch Pathol Lab Med. 1999;123(9):812–816.
- Seidman JD, Yemelyanova A, and Zaino RJ, et al. The fallopian tube-peritoneal junction: a potential site of carcinogenesis. Int J Gynecol Pathol. 2011;30(1):4–11.
- Arango D, Al-Obaidi S, and Williams DS, et al. Villin expression is frequently lost in poorly differentiated colon cancer. Am J Pathol. 2012;180(4):1509–1521.