ABSTRACT
Objective
To evaluate whether the fetal fraction of cell-free DNA at the first and second trimesters is associated with spontaneous preterm birth.
Methods
This was a retrospective cohort study with singleton pregnancies who underwent noninvasive prenatal testing. According to pregnancy outcome, eligible patients were divided into a delivery group ≥37 weeks of pregnancy (term group) and <37 weeks of pregnancy (spontaneous preterm group). Stepwise linear regression was used to identify maternal characteristics associated with the fetal fraction of cell‐free DNA. Logistic regression analysis was performed to evaluate the association between the fetal fraction of cell-free DNA and spontaneous preterm birth, adjusted for confounding factors.
Results
14,020 cases were included in the study, 13292 cases (94.81%) in the term group and 728 cases (5.19%) in the spontaneous preterm group. The cell-free fraction of fetal DNA was inversely correlated with maternal age and body mass index. Positively correlated with gestational age, fertility, and assisted reproductive technology. After adjusting for the covariates, logistic regression analysis revealed no statistically significant association between the fetal fraction of cell-free DNA and spontaneous preterm birth.
Conclusion
In our original study, we found no association between the fetal fraction on NIPT and subsequent spontaneous preterm birth.
1. Introduction
Preterm birth (PTB), defined as delivery before 37 weeks of gestation, is the primary cause of neonatal morbidity and mortality, which is also the main factor that affects perinatal prognosis seriously [Citation1]. About 1/4 of the surviving premature infants are complicated with severe or refractory diseases, which cause a heavy social and family burden, but the pathogenesis is unknown [Citation2]. Spontaneous preterm birth (SPTB) accounts for 70% of preterm births, and the incidence of SPTB is rising globally year by year. There are no effective therapies or markers to predict SPTB [Citation3].
Since the first detection of cell-free fetal DNA (cffDNA) by Lo et al, in maternal plasma in 1997 [Citation4], noninvasive prenatal testing (NIPT)has been widely used to screen for common fetal aneuploidies based on next-generation sequencing technology with high sensitivity and specificity [Citation5,Citation6]. Existing evidence suggests that cffDNA is mainly derived from the apoptosis and/or necrosis of trophoblast cells during placental maturation and senescence [Citation7]. It can be measured in the maternal circulation from the 5th week of pregnancy and increases with gestational age. It is unique to pregnancy as it is eliminated within hours of placental delivery [Citation8]. Available evidence suggests that parturition is initiated by an inflammatory cascade. Inflammatory cells and pro-inflammatory cytokines are found in both maternal and fetal tissues during labor [Citation9–11]. Pro-inflammatory cytokines trigger the production of multiple inflammatory mediators, including matrix metalloproteinases and prostaglandins, leading to cervical dilation, rupture of membranes, and uterine contractions [Citation9,Citation10]. Therefore, it has been suggested that increased cffDNA may be pro-inflammatory and stimulate parturition [Citation12–16]. Previous research has shown that cffDNA levels are significantly increased in pregnant women with SPTB, which seems to confirm this hypothesis to some extent [Citation17].
Recent studies have shown that elevated levels of cffDNA in maternal blood are associated with pregnancy complications, including premature loss of pregnancy, preeclampsia, fetal growth retardation, threatened premature birth, and premature birth [Citation18–22]. This has led to research into the potential for cffDNA concentrations to be used as a potential predictive marker of pregnancy complications [Citation19,Citation23–25]. Preliminary studies have found no association between the fetal cffDNA levels in the first trimester and SPTB [Citation26–28], whereas there is conflicting evidence as to whether the elevated cffDNA in the second trimester is associated with SPTB [Citation17,Citation22,Citation29–32]. The present study aimed to further explore the relationship between fetal cffDNA fraction and SPTB in the first and second trimesters using a large amount of data.
2. Methods
2.1. Study population
We performed a retrospective cohort study of women with singleton pregnancies who underwent NIPT at 12 to 27 weeks of gestation at the Prenatal Diagnosis Center of Southwest Hospital, Army Medical University, Chongqing, China, between October 2017 and December 2020. The clinical data including the fetal fraction of cell‐free DNA, body mass index (BMI), maternal age, gestational age, parity, methods of conception (natural pregnancy or assisted reproductive pregnancy), ultrasound information, and follow-up data were collected for analysis. This study was approved by the institutional review board of the Southwest Hospital, Army Medical University (NO. KY2021175). All patients received pretest counseling and were given informed consent by genetic counselors before testing for NIPT. Written informed consent was provided by each participant. All the data downloaded for analysis was anonymous.
Pregnant women were excluded if they had the following conditions: chromosomal abnormality in either of the couple; or the mother had received an allogeneic blood transfusion, organ transplantation, cell therapy, or immunotherapy within 1 year; maternal malignancy during pregnancy; positive cffDNA test results and elective termination of pregnancy, fetal demise, or miscarriage, twin pregnancy, or loss to follow-up. Fetuses with structural abnormalities on ultrasound were also excluded, and invasive testing (chromosomal microarray and karyotype analysis) was immediately recommended (). Maternal characteristics, including age, height, weight, gestation age, medical history, method of conception, and FF values were recorded from the hospital’s prenatal screening database for our study.
2.2. Plasma cffDNA extraction and sequencing
5 ml of peripheral blood was collected from each subject into an ethylene diamine tetra-acetic (EDTA) tube. The plasma was separated within 8 hours by a two-step centrifugation protocol as described previously [Citation33,Citation34]. Briefly, the plasma was isolated by double centrifugation for 10 min at 1,600 g and then 10 min at 16,000 g, and cffDNA was extracted from the plasma by BGISP-300 (BGI, Shenzhen, China) and Nucleic Acid Extraction Kit (BGI, Shenzhen, China).
The extracted cffDNA was end‐repaired and then ligated with adapters for multiplex sequencing following a commercial NIPT protocol in the laboratory of Southwest Hospital of Army Medical University. The ligated products were added to the ABI 9700 hot start-ready master mix and subjected to 12 cycles of amplification. The DNA amplification products were then quality controlled and pooled by Qubit 2.0 (Invitrogen, US) using QubitTMdsDNA Assay Kit (Invitrogen, US) [Citation35]. Briefly, the PCR products were heat-denatured and incubated at 37°C to form single-stranded DNA (ssDNA) circles. The ssDNA circles are subjected to rolling circle amplification (RCA) to generate DNA nanoballs (DNBs). After RCA and the formation of DNBs, the final product was quantified by a QubitTMssDNA Assay Kit (Invitrogen, US). The DNBs were loaded onto chips and sequenced on the BGISEQ-500 sequencing platform (BGI, Shenzhen, China) [Citation35,Citation36].
2.3. Calculation of fetal fraction
The fetal fraction (FF) refers to the percentage of cffDNA in maternal plasma from the placenta [FF = fetal cffDNA/(fetal cffDNA + maternal cffDNA)]. Therefore, this is related to the levels of maternal and fetal cffDNA in maternal plasma. The fetal DNA is placental [Citation37]. During 10 to 20 weeks of gestation, the average FF is 10% to 15% [Citation38–40].
FF measurement is an important part of sample quality control and statistical certainty. Measurement of FF ensures that sufficient placental cffDNA is detected in the maternal plasma for meaningful results [Citation41]. Different fetal fraction calculation methods may be used in different testing platforms or laboratories. In the present study, the estimation method of the FF in NIPT depends on the proportion of Y‐chromosomal sequences in maternal plasma (male fetuses) or estimated by maternal plasma DNA size analysis (female fetuses) [Citation42]. Fetal fraction data for this study were provided by the laboratory, as this information is not usually included in laboratory reports.
2.4. Pregnancy outcome follow-up
Pregnancy outcomes were collected by clinical investigators based on medical records and telephone interviews three months after birth as described previously [Citation34]. Including gestational age at delivery, neonatal physical examination (including physical appearance and facial examination, heart inspection, intelligence and development evaluation, newborn disease screening, and so on), and genetic testing results. For women who lose to follow up via phone but give birth in our hospital, the delivery information is obtained from the medical record system.
Gestational age was determined by early ultrasound. According to the gestational age of delivery, eligible patients were divided into the pregnancy with spontaneous delivery ≥37 weeks (term group) and the SPTB group with pregnancy <37 weeks (spontaneous preterm group). Our research focused on SPTB, including pregnancies in spontaneous labor and premature rupture of membranes, excluding iatrogenic premature birth.
2.5. Statistical processing
In this study, SPSS 21.0 statistical software was applied for statistical analysis. The continuous data are presented as medians (interquartile ranges, IQR), and categorical data are presented as values or percentages. Spearman correlation analysis was used to test the correlation between the FF and maternal characteristics. Stepwise linear regression was performed to identify maternal characteristics associated with fetal fraction by a birth group.
We used the Mann-Whitney U test for continuous variables, and the χ2 test or Fisher exact test for categorical variables. The number of multiple comparisons was corrected using a Bonferroni correction (adjusted P < 0.025). Adjusted covariates included maternal age, gestational age, BMI, gravidity, and parity. In addition, logistic regression was used to determine the association between the fetal fraction of cell-free DNA and spontaneous preterm birth.
3. Results
During the study period, cffDNA testing was performed in 16,483 singleton pregnancies with a live fetus at 12 to 27 weeks of gestation. We excluded 2,463 (14.9%) cases owing to positive cffDNA test results (n = 89); twin pregnancy (n = 554); elective termination of pregnancy, miscarriage, and fetal demise (n = 182); loss to follow-up or incomplete data on pregnancy outcome (n = 1,599), and samples with the no-call result (n = 39). After all exclusions, 14020 women were included in this study. Details regarding the excluded cases are shown in .
Among them, 13292 (94.81%) constituted the team group (delivered at ≥37 weeks of gestation), and 728 (5.19%) constituted the spontaneous preterm group (delivered at<37 weeks of gestation). The gestational age in the first trimester (12–13 weeks) was 1,021 (7.28%), and in the second trimester (14–27 weeks) is 12,999 (92.72%). The characteristics of the study population are shown in , the advanced maternal age 1,796 (12.81%), ultrasonographic soft markers 1,177 (8.4%), Nullipatous 4,904 (34.98%), and assisted reproduction 617 (4.4%). The baseline characteristics of each outcome group are shown in .
Table 1. Baseline characteristics of the study population.
Table 2. Characteristics of the study population.
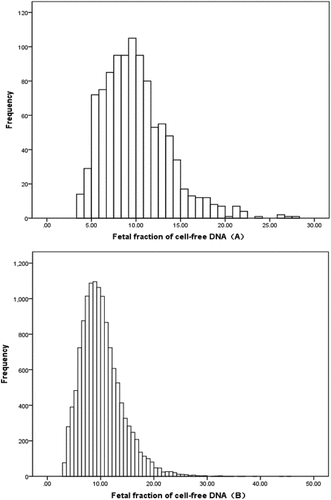
The distribution of the FF of cell‐free DNA between 12–13 weeks and 14–27 weeks gestation is shown in . A normality test demonstrated that the data were not normally distributed whether in the first trimester or the second trimester.
Figure 2. Fetal fraction of cell‐free DNA distribution in the total study group.
In addition, the relationship between the FF of cell-free DNA and maternal characteristics is presented in . Stepwise linear regression analysis indicated that the FF of cell‐free DNA was negatively correlated with maternal age(regression coefficient = −0.032, P<0.001) and BMI (regression coefficient = −0.142, P<0.001); positively correlated with gestational age (regression coefficient = 0.292, P<0.001), nulliparity (regression coefficient = 0.346, P<0.001), and assisted reproduction (regression coefficient = 0.495, P<0.01).
Table 3. The maternal characteristics that contributed to the fetal fraction of cff-DNA as identified by stepwise linear regression analysis of the term group.
Since assisted reproduction was not an independent risk factor for preterm birth, we adjusted it as a confounding factor in the final logistic regression analysis. However, after adjusting for assisted reproductive technology, additional logistic regression analysis revealed no statistically significant associations between the FF of cell‐free DNA and SPTB in any of the preterm groups (). For fetal fraction drawn 12–13 weeks: the adjusted odds ratio (aOR) for SPTB delivery before 37 weeks was 0.950, 95% CI: 0.865–1.043); for fetal fraction drawn 14–27 weeks: aOR for SPTB delivery before 37 weeks was 0.998, 95% CI: 0.979–1.016 (). The FF of cell‐free DNA in the first and second trimesters for women who went on to deliver at various gestational ages was presented in .
Table 4. Relationship between the fetal fraction of cell‐free DNA and spontaneous preterm birth.
Table 5. Fetal fraction of cffDNA in the first and second trimester for women who went on to deliver at the various gestational ages.
4. Discussion
This is the largest cohort study linking maternal plasma FF of cffDNA on NIPT with SPTB in a Chinese population. In this retrospective cohort study, we found no association between the FF of cell‐free DNA in NIPT and subsequent SPTB. Therefore, we speculate that the FF on NIPT both in the first and second trimesters is unlikely to be useful for predicting SPTB.
To the best of our knowledge, the sample size of this study was much larger than that of previous studies (12,999 term cases and 1,021 SPTB cases). Given the large variability in cffDNA levels in the normal first and second trimesters, the use of a larger data set increases the power of our conclusions.
In this study, there were significant differences in maternal age, BMI, gestational age, and indications for cffDNA testing between the first and second-trimester populations (P < 0.05). The FF of NIPT was negatively correlated with maternal age and BMI, and positively correlated with gestational age, consistent with previous studies [Citation27,Citation32,Citation43]. Furthermore, we found that FF was positively associated with assisted reproduction, which is inconsistent with Yuan’s study reported that FF was negatively associated with assisted reproduction in NIPT [Citation43]. But the study by Ashoor et al. showed that there is no association between FF on NIPT and the method of conception [Citation39]. There may be two reasons for this discrepancy. First, the FF in NIPT was significantly influenced by the gestational age [Citation27]. Ashoor’s study focused on the first trimester (11–13 weeks gestation), and Yuan’s study focused on the second trimester (13–26 weeks gestation), whereas our study measured the time from the first trimester to the second trimester (12–27 weeks gestation). Second, a previous study showed that there is a negative correlation between FF on NIPT and South Asian racial origin, as well as an assisted conception [Citation26]. The observational population in the study by Ashoor et al. included multiple races (Caucasian mainly), while our study and Guo’s report were conducted on the Chinese Han Population. Similar results were obtained regarding the relationship between assisted conception and FF on NIPT [Citation32].
Preterm birth is a syndrome with multiple etiologies and phenotypic features. There is a biological plausibility between increased fetal fraction and preterm birth [Citation44,Citation45]. Placental injury leads to decreased placental perfusion, placental ischemia, and hypoxia, leading to increased apoptosis and necrosis of the trophoblast, resulting in increased fetal cffDNA content in maternal plasma. Therefore, it is assumed that the fetal fraction increased at 14.1–20 weeks may be associated with reduced deep myometrial invasion of spiral arteries [Citation17]. In normal pregnancy, the invasion of endovascular trophoblast into the deciduas of spiral arteries occurs around 8 and 12 weeks of gestation, while the invasion of deeper trophoblast into the muscular spiral arteries occurs around 14 weeks of gestation. Impaired endovascular trophoblast migration may lead to increased resistance in the spiral arteries, thereby reducing maternal blood supply to the placenta, leading to hypoperfusion, hypoxic-reperfusion injury, and oxidative stress [Citation46]. Elevated levels of circulating cffDNA can induce an inflammatory response, which may trigger preterm labor [Citation47]. Several studies have reported that women who are naturally at risk of spontaneous preterm birth due to premature rupture of membranes, or preterm birth have higher levels of fetal DNA than women who are not at risk of preterm birth. Two of these studies focused on pregnancies with SPTB symptoms in the second trimester and found significantly higher levels of cffDNA in SPTB [Citation17,Citation48]. One study found a highly significant association between cffDNA levels above the 95th percentile and SPTB in women at 25 weeks of gestation with no symptoms of labor. Other reports suggest that elevated fetal DNA levels at 14.1 to 20.0 weeks of gestation were significantly associated with an increased incidence of preterm birth [Citation22]. However, four previous studies that focused on the first trimester were consistent with our finding that there was no association between cffDNA and subsequent SPTB [Citation22,Citation26–28]. Three other studies that focused on the second trimester reached the same conclusion as ours [Citation22,Citation32,Citation43].
There may be several reasons for this discrepancy. First, a different study population was used. Another research group used by Jakobsen et al. whose participants were women screened for fetal RHD genotype [Citation17], and in our study and Dugoff et al. [Citation22] the population consisted primarily of women who were screened for aneuploidy. This is the main reason for cffDNA screening at present. Second, different measurement methods for FF quantity used by different testing platforms may differ. Many studies have compared different FF calculation methods, showing significant differences in the FF among them [Citation49]. The study by Jakobsen et al, [Citation17] estimated the amount of cffDNA in a routine RHD analysis using the Ct value (geq/mL plasma, genome equivalents per mL of plasma). In the study by Dugoff et al, they estimated FF by sex-independent quantification and polymorphisms in cffDNA isolated from maternal plasma using multiple methylation-based approaches [Citation22]. The studies by our and Guo have determined FF based on the proportion of Y‐chromosome sequences in maternal plasma (male fetuses) or estimated from maternal plasma DNA size analysis (female fetuses) [Citation32,Citation42]. Third, different bioinformatics algorithms will affect the calculation methods of the FF [Citation50]. Moreover, the difference could be explained by the differential fitting of confounders. This may also partly explain why some studies come to different conclusions.
The present study has several strengths and limitations. The strengths were as follows: (1) All cffDNA screening tests were performed using the same sequencing platform and settings; (2) Potential confounding factors such as fetuses or patients with abnormalities were excluded before analysis, and logistic regression analysis was used to correct NIPT. Known confounders such as maternal age, parity, BMI, and gestational age. (3) Given the large variability in cffDNA levels in the second trimester of normal pregnancy, we suspected that conclusions based on a larger dataset (13,292 term birth cases and 728 cases with SPTB) might be more convincing. The limitations of the study were as follows: (1) This is a historical observational study, which means that the statistical analysis may be biased due to measuring fetal fraction to missing data and/or factors not measured in the database. (2) The method for measuring fetal fraction in this study was different for female and male fetuses. It is not clear what the correlation is between these two methods. While I am not aware of any evidence indicating an association between sex and SPTB. (3) This study is a single-center retrospective study and further multi-center prospective studies based on the general population are needed to verify the conclusions. (4) Due to the different fluctuation ranges of cffDNA in maternal circulation at different gestational weeks, the large sample size of the included studies, the large number of comorbidities and complications, and the large heterogeneity, all confounding factors should be classified, and then the cffDNA in maternal blood should be classified. In addition, more testing and analysis to determine whether it has clinical value in predicting preterm birth.
5. Conclusion
This study shows that, after adjustment for covariates including maternal age, gestational age, BMI, gravidity, and parity, the FF on NIPT at the first and second trimesters is not associated with SPTB. However, SPTB is a syndrome with multiple etiological factors and phenotypic features, and different pathological causes of SPTB may lead to different performances at the cffDNA level. Further research into the relationship between cffDNA levels and different preterm birth phenotypes in large prospective cohort studies may be worthwhile.
Declaration of interest
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Ethics approval statement
This study was approved by the institutional review board of the Southwest Hospital, Army Medical University (NO. KY2021175). All patients received pretest counseling and were given informed consent by genetic counselors before testing for NIPT. Written informed consent was provided by each participant. All methods in this study were performed by the relevant guidelines and regulations.
Data availability statement
The data used to support the findings of this study are available from the corresponding authors upon resonable request.
Additional information
Funding
References
- van Boeckel SR, Davidson DJ, Norman JE, et al. Cell-free fetal DNA and spontaneous preterm birth. Reproduction. 2018;155(3):R137–145. DOI: 10.1530/REP-17-0619
- Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162–2172. DOI:10.1016/S0140-6736(12)60820-4
- Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. DOI: 10.1016/S0140-6736(08)60074-4
- Lo YMD, Corbetta N, Chamberlain PF, et al. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350(9076):485–487. DOI:10.1016/S0140-6736(97)02174-0
- Norton ME, Jacobsson B, Swamy GK, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372(17):1589–1597. DOI:10.1056/NEJMoa1407349
- Chen YP, He ZQ, Shi Y, et al. Not all chromosome aberrations can be detected by NIPT in women at advanced maternal age: a multicenter retrospective study. Clin Chim Acta. 2018;486:232–236. DOI: 10.1016/j.cca.2018.08.018
- Bianchi DW. Circulating fetal DNA: its origin and diagnostic potential-a review. Placenta. 2004;25(Suppl A):S93–101. DOI: 10.1016/j.placenta.2004.01.005
- Wang E, Batey A, Struble C, et al. Gestational age and maternal weight effects on fetal cell-free DNA in maternal plasma. Prenat Diagn. 2013;33(7):662–666. DOI: 10.1002/pd.4119
- Christiaens I, Zaragoza DB, Guilbert L, et al. Inflammatory processes in preterm and term parturition. J Reprod Immunol. 2008;79(1):50–57. DOI: 10.1016/j.jri.2008.04.002
- Bollopragada S, Youssef R, Jordan F, et al. Term labor is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. Am J Obstet Gynecol. 2009;200(1):104 e101–111. DOI: 10.1016/j.ajog.2008.08.032
- Cappelletti M, Della Bella S, Ferrazzi E, et al. Inflammation and preterm birth. J Leukoc Biol. 2016;99(1):67–78. DOI: 10.1189/jlb.3MR0615-272RR
- Phillippe M, Phimister EG. Cell-free fetal DNA — a trigger for parturition. N Engl J Med. 2014;370(26):2534–2536. DOI: 10.1056/NEJMcibr1404324
- Nadeau-Vallee M, Obari D, Palacios J, et al. Sterile inflammation and pregnancy complications: a review. Reproduction. 2016;152(6):R277–292. DOI:10.1530/REP-16-0453
- Herrera CA, Stoerker J, Carlquist J, et al. Cell-free DNA, inflammation, and the initiation of spontaneous term labor. Am J Obstet Gynecol. 2017;217(5):581–588. DOI:10.1016/j.ajog.2017.05.027
- Phillippe M. The link between cell-free DNA, inflammation and the initiation of spontaneous labor at term. Am J Obstet Gynecol. 2017;217(5):501–502. DOI: 10.1016/j.ajog.2017.09.003
- Phillippe M. Cell-free fetal DNA, telomeres, and the spontaneous onset of parturition. Reproductive Sciences. 2015;22(10):1186–1201. DOI: 10.1177/1933719115592714
- Jakobsen TR, Clausen FB, Rode L, et al. High levels of fetal DNA are associated with increased risk of spontaneous preterm delivery. Prenat Diagn. 2012;32(9):840–845. DOI:10.1002/pd.3917
- Lim JH, Kim MH, Han YJ, et al. Cell-free fetal DNA and cell-free total DNA levels in spontaneous abortion with fetal chromosomal aneuploidy. PLoS ONE. 2013;8(2):e56787. DOI:10.1371/journal.pone.0056787
- Contro E, Bernabini D, Farina A. Cell-free fetal DNA for the prediction of pre-eclampsia at the first and second trimesters: a systematic review and meta-analysis. Mol Diagn Ther. 2017;21(2):125–135. DOI: 10.1007/s40291-016-0245-9
- Hahn S, Huppertz B, Holzgreve W. Fetal cells and cell free fetal nucleic acids in maternal blood: new tools to study abnormal placentation? Placenta. 2005;26(7):515–526. DOI: 10.1016/j.placenta.2004.10.017
- Sifakis S, Koukou Z, Spandidos DA. Cell-free fetal DNA and pregnancy-related complications (review). Mol Med Rep. 2015;11(4):2367–2372. DOI: 10.3892/mmr.2014.3118
- Dugoff L, Barberio A, Whittaker PG, et al. Cell-free DNA fetal fraction and preterm birth. Am J Obstet Gynecol. 2016;215(2):231 e231–237. DOI:10.1016/j.ajog.2016.02.009
- Alberry MS, Maddocks DG, Hadi MA, et al. Quantification of cell free fetal DNA in maternal plasma in normal pregnancies and in pregnancies with placental dysfunction. Am J Obstet Gynecol. 2009;200(1):91–96. DOI:10.1016/j.ajog.2008.07.063
- Rolnik DL, da Silva Costa F, Lee TJ, et al. Association between fetal fraction on cell-free DNA testing and first-trimester markers for pre-eclampsia. Ultrasound Obstet Gynecol. 2018;52(6):722–727. DOI: 10.1002/uog.18993
- Morgan TK. Role of the placenta in preterm birth: a review. Am J Perinatol. 2016;33(3):258–266. DOI: 10.1055/s-0035-1570379
- Quezada MS, Francisco C, Dumitrascu-Biris D, et al. Fetal fraction of cell-free DNA in maternal plasma in the prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol. 2015;45(1):101–105. DOI: 10.1002/uog.14666
- Poon LC, Musci T, Song K, et al. Maternal plasma cell-free fetal and maternal DNA at 11-13 weeks’ gestation: relation to fetal and maternal characteristics and pregnancy outcomes. Fetal Diagn Ther. 2013;33(4):215–223. DOI: 10.1159/000346806
- Thurik FF, Lamain-de Ruiter M, Javadi A, et al. Absolute first trimester cell-free DNA levels and their associations with adverse pregnancy outcomes. Prenat Diagn. 2016;36(12):1104–1111. DOI:10.1002/pd.4940
- Bauer M, Hutterer G, Eder M, et al. A prospective analysis of cell-free fetal DNA concentration in maternal plasma as an indicator for adverse pregnancy outcome. Prenat Diagn. 2006;26(9):831–836. DOI:10.1002/pd.1513
- Illanes S, Gomez R, Fornes R, et al. Free fetal DNA levels in patients at risk of preterm labour. Prenat Diagn. 2011;31(11):1082–1085. DOI:10.1002/pd.2838
- Stein W, Muller S, Gutensohn K, et al. Cell-free fetal DNA and adverse outcome in low risk pregnancies. Eur J Obstet Gynecol Reprod Biol. 2013;166(1):10–13. DOI: 10.1016/j.ejogrb.2012.09.006
- Guo FF, Yang JX, Huang YL, et al. Association between fetal fraction at the second trimester and subsequent spontaneous preterm birth. Prenat Diagn. 2019;39(13):1191–1197. DOI:10.1002/pd.5566
- Yao H, Jiang F, Hu H, et al. Detection of fetal sex chromosome aneuploidy by massively parallel sequencing of maternal plasma DNA: initial experience in a Chinese hospital. Ultrasound Obstet Gynecol. 2014;44(1):17–24. DOI:10.1002/uog.13361
- Luo Y, Hu H, Jiang L, et al. A retrospective analysis the clinic data and follow-up of non-invasive prenatal test in detection of fetal chromosomal aneuploidy in more than 40,000 cases in a single prenatal diagnosis center. Eur J Med Genet. 2020;63(9):104001. DOI:10.1016/j.ejmg.2020.104001
- Xu Y, Lin Z, Tang C, et al. A new massively parallel nanoball sequencing platform for whole exome research. BMC Bioinf. 2019;20(1):153. DOI:10.1186/s12859-019-2751-3
- Huang J, Liang X, Xuan Y, et al. A reference human genome dataset of the BGISEQ-500 sequencer. Gigascience. 2017;6(5):1–9. DOI:10.1093/gigascience/gix024
- Taglauer ES, Wilkins-Haug L, Bianchi DW. Review: cell-free fetal DNA in the maternal circulation as an indication of placental health and disease. Placenta. 2014;35(Suppl):S64–68. DOI: 10.1016/j.placenta.2013.11.014
- Hui L, Bianchi DW. Fetal fraction and noninvasive prenatal testing: what clinicians need to know. Prenat Diagn. 2020;40(2):155–163. DOI: 10.1002/pd.5620
- Ashoor G, Syngelaki A, Poon LC, et al. Fetal fraction in maternal plasma cell-free DNA at 11-13 weeks’ gestation: relation to maternal and fetal characteristics. Ultrasound Obstet Gynecol. 2013;41(1):26–32. DOI: 10.1002/uog.12331
- Palomaki GE, Kloza EM, Lambert-Messerlian GM, et al. DNA sequencing of maternal plasma to detect down syndrome: an international clinical validation study. Genet Med. 2011;13(11):913–920. DOI:10.1097/GIM.0b013e3182368a0e
- Canick JA, Palomaki GE, Kloza EM, et al. The impact of maternal plasma DNA fetal fraction on next generation sequencing tests for common fetal aneuploidies. Prenat Diagn. 2013;33(7):667–674. DOI: 10.1002/pd.4126
- Jiang F, Ren J, Chen F, et al. Noninvasive fetal trisomy (NIFTY) test: an advanced noninvasive prenatal diagnosis methodology for fetal autosomal and sex chromosomal aneuploidies. BMC Med Genomics. 2012;5(1):57. DOI:10.1186/1755-8794-5-57
- Yuan X, Zhou L, Zhang B, et al. Association between low fetal fraction of cell free DNA at the early second-trimester and adverse pregnancy outcomes. Pregnancy Hypertens. 2020;22:101–108. DOI: 10.1016/j.preghy.2020.07.015
- Kim YM, Chaiworapongsa T, Gomez R, et al. Failure of physiologic transformation of the spiral arteries in the placental bed in preterm premature rupture of membranes. Am J Obstet Gynecol. 2002;187(5):1137–1142. DOI:10.1067/mob.2002.127720
- Misra VK, Hobel CJ, Sing CF. Placental blood flow and the risk of preterm delivery. Placenta. 2009;30(7):619–624. DOI: 10.1016/j.placenta.2009.04.007
- Khong SL, Kane SC, Brennecke SP, et al. First-trimester uterine artery Doppler analysis in the prediction of later pregnancy complications. Dis Markers. 2015;2015:679730. DOI: 10.1155/2015/679730
- Scharfe-Nugent A, Corr SC, Carpenter SB, et al. TLR9 provokes inflammation in response to fetal DNA: mechanism for fetal loss in preterm birth and preeclampsia. J Immunol. 2012;188(11):5706–5712. DOI:10.4049/jimmunol.1103454
- Farina A, LeShane ES, Romero R, et al. High levels of fetal cell-free DNA in maternal serum: a risk factor for spontaneous preterm delivery. Am J Obstet Gynecol. 2005;193(2):421–425. DOI:10.1016/j.ajog.2004.12.023
- Deng C, Liu S. Factors affecting the fetal fraction in noninvasive prenatal screening: a review. Front Pediatr. 2022;10:812781. DOI: 10.3389/fped.2022.812781
- Peng XL, Jiang P. Bioinformatics approaches for fetal DNA fraction estimation in noninvasive prenatal testing. Int J Mol Sci. 2017;18(2):453. DOI: 10.3390/ijms18020453