ABSTRACT
Introduction
The advance of diagnostics and treatments has greatly improved the prognosis of non-small cell lung cancer (NSCLC) patients. However, relapse and metastasis are still common problems encountered by NSCLC patients who have achieved complete remission. Therefore, overcoming the challenge of relapse and metastasis is particularly important for improving the prognosis of NSCLC patients. Research has shown that minimal residual disease (MRD) was a potential source of tumor relapse and metastasis, and circulating tumor DNA (ctDNA) MRD has obvious advantages in predicting the relapse and metastasis of NSCLC and evaluating treatment effectiveness. Therefore, dynamic monitoring of MRD is of great significance for NSCLC patient management strategies.
Areas covered
We have reviewed articles related to NSCLC MRD included in PubMed and describes the biological significance and historical context of MRD research, reasons for using ctDNA to evaluate MRD, and potential value and challenges of ctDNA MRD in assessing relapse and metastasis of NSCLC, ultimately guiding clinical therapeutic strategies and management.
Expert opinion
The standardized scope of ctDNA MRD detection for NSCLC requires more clinical research evidence to minimize study differences, making it possible to include in the clinical staging as a reliable indicator.
1. Introduction
In 2020, there were 2.2 million new cases of lung cancer and 1.76 million patients died of this disease worldwide [Citation1]. Non-small cell lung cancer (NSCLC) accounts for approximately 80–85% lung cancers [Citation2]. Targeted therapy has increased the 5-year overall survival rates of patients with localized and advanced NSCLC to 55% and 21%, respectively [Citation3]. Immunotherapy has also greatly improved the survival of patients with advanced NSCLC; however, only ~ 20% of NSCLC patients can benefit from this treatment [Citation4]. Most patients developed resistance during the treatment phase [Citation5]. Therefore, dynamic monitoring and timely intervention of patients is the cornerstone to improve the prognosis of NSCLC. The mechanism and precise and timely estimation of relapse and metastasis of NSCLC remains an urgent problem in the academic community.
Minimal residual disease (MRD) refers to the small number of cancer cells remaining in the body after treatment that do not respond or are resistant to treatment and can be detected by liquid biopsy technology [Citation6]. These residual tumor cells or molecular abnormalities derived from tumor cells are also known as molecular residual diseases or measurable residual diseases [Citation7]. Although the number of these residual cancer cells was small and could not cause any symptoms at the moment, they represent the clinical progression potential of cancer [Citation8]. When cells are necrotic or apoptotic, some 80–200 bp stable DNA fragments are released into the circulating blood, called circulating cell-free DNA (cfDNA) [Citation9,Citation10]. DNA fragments derived from tumor cells, called circulating tumor DNA (ctDNA), account for < 1% of cfDNA [Citation11,Citation12]. Since cfDNA derived from normal somatic cells usually has no tumor-specific genetic alterations, ctDNA can be distinguished from cfDNA. Thus, plasma DNA with tumor-specific genetic alterations is more likely to be ctDNA [Citation13], which is also considered an important marker of MRD. At present, an increasing number of studies have focused on the role of ctDNA MRD in prognosis assessment and efficacy prediction of NSCLC, suggesting the powerful potential of ctDNA MRD as a novel cancer marker.
2. MRD
2.1. Biological significance
In the early stages of cancer, tumor cells may spread [Citation14]. Cancer cells are released from the primary tumor and injected into the blood to form circulating tumor cells (CTCs), which become disseminated tumor cells (DTCs) after successful colonization of distant organs [Citation15]. These static DTCs usually have typical ‘cancer stem cell’-like features of self-renewal and proliferation [Citation15], and may be dormant and asymptomatic [Citation16,Citation17]; thus, resistant to drug or radiation therapy [Citation15,Citation18–22]. After treatment, the number of primary drug-resistant CTCs increases [Citation23,Citation24], and some secondary drug-resistant mutant cells are generated [Citation25]. These dormant or drug-resistant residual cells or cell clusters eventually lead to tumor relapse and metastasis [Citation25]. Therefore, tumor MRD is an insidious tumor burden that persists in patients after radical treatment [Citation26]. Specifically, this refers to isolated tumor cells or cell clusters that exist in patients without clinical symptoms of cancer and cannot be detected by traditional methods. Such MRD represents a potential source of relapse and distant metastasis [Citation27,Citation28].
2.2. Historical context of MRD research in NSCLC
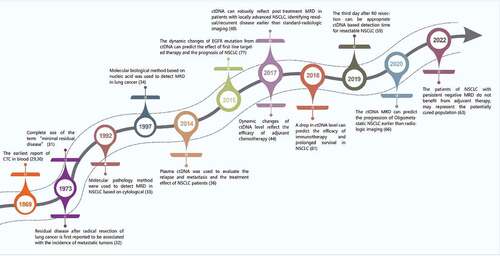
In 1869, Thomas Ashworth reported tumor-derived CTCs in the blood during autopsy () [Citation29,Citation30]. In 1973, Mathe reported that the term ‘minimal residual disease’ was used in the experiment of Bacillus Calmette-Guerin in the treatment of leukemia [Citation31]. That same year, Matthews reported that residual disease in patients undergoing radical surgical resection of lung cancer was associated with the incidence of metastatic tumors [Citation32], initiating the study of minimal residual lesions in lung cancer. In 1992, Chen detected keratins, carcinoembryonic antigen, and human milk fat globule 2 using immunohistochemical methods, and found occult single tumor cells or small clusters of tumor cells in the lymph nodes of patients with NSCLC [Citation33], which established the foundation for the detection of MRD in NSCLC by molecular pathology. In 1997, Marchetti et al. used enriched single-strand conformational polymorphism, an extremely sensitive polymerase chain reaction method to detect occult tumor cells, which could diagnose lung cancer in advance and screen lung cancer patients with occult micrometastases [Citation34], laying the foundation for detecting NSCLC MRD based on molecular biology technology. In 2001, Anker et al. found that plasma ctDNA could predict disease recurrence and response to treatment [Citation35]. Subsequently, a highly sensitive plasma ctDNA assay was developed to evaluate the recurrence, metastasis, and the treatment effect in NSCLC patients [Citation36], which paved the way for applying ctDNA to evaluate MRD in NSCLC.
2.3. ctDNA analysis is an important means to evaluate MRD of solid tumors
Compared to hematological malignancies, the number of CTCs in plasma after surgery for solid tumors is very small, which limits the role of CTCs in MRD estimation for solid tumors. Generally, ctDNA is a single- or double-stranded DNA fragment released into the systemic circulation by tumor cells [Citation37]. The ctDNA contains mutations, deletions, insertions, rearrangements, copy number abnormalities, methylation, and other related gene mutations and epigenetic information, which is similar to that of the primary tumor and can simulate the characteristics of the primary tumor [Citation38]. It has been reported that the mutation consistency between plasma ctDNA and tumor tissue DNA was 68.4% [Citation39], indicating that plasma ctDNA mutations can reflect tissue DNA mutations. The positive rate of plasma ctDNA (63.2%) was higher than that of serum tumor protein markers (49.3%) [Citation39], reflecting the higher sensitivity of plasma ctDNA, which makes it potentially capable of evaluating MRD in solid tumors. In a study by Chaudhuri et al, ctDNA was detected in the first plasma sample collected after treatment, and 94% of patients relapsed, indicating some reliability of ctDNA in evaluating MRD [Citation40]. The sensitivity of MRD assessment can be improved by monitoring multiple mutations in ctDNA [Citation36]. These results suggested that plasma ctDNA analysis may reflect tumor MRD. In colorectal cancer, breast cancer, myoinvasive urothelial carcinoma, and other cancers, MRD-positive plasma ctDNA after surgery can predict tumor relapse and metastasis earlier than imaging () [Citation40–44].
Table 1. ctDNA MRD evaluation of resectable NSCLC.
3. Detection of ctDNA MRD
Although many ctDNA detection technologies exist [Citation45], droplet digital PCR (ddPCR) and Next-Generation Sequencing (NGS) are the two main platforms for ctDNA detection in plasma, and are widely used in MRD evaluation of solid tumors. High detection sensitivity is an advantage of ddPCR, up to 0.001% [Citation46], but it can only detect a limited number of known variants. High throughput is the advantage of traditional NGS detection platform and can detect unknown mutations, but its sensitivity is lower than ddPCR [Citation47]. With the development of the technology, the detection sensitivity of new NGS platform can also reach 0.01% [Citation48], such as Safe Sequencing System (Safe-Seq) and Cancer Personalized Profiling by Deep Sequencing (CAPP-seq). In summary, ctDNA MRD detection based on NGS platform is mainly divided into two strategies: Tumor-naïve assays and Tumor-informed assays. Tumor-naïve assays are panels composed of several or even hundreds of common driving genes for specific tumors, which do not need to detect the whole genome or exon sequencing of patient tissues in advance. Therefore, it has the advantage of short time and easy commercialization [Citation49]. Tumor-informed assays need to screen the mutations panel with high tumor relevance based on the sequencing results of the patient’s tumor tissue, and design specific ctDNA probes and primers for this patient in combination with known drug sensitivity and drug resistance gene mutations. Therefore, it has high sensitivity and high correlation with tumor, which is conducive to the evaluation of MRD [Citation50]. However, due to the heterogeneity of tumor tissue and the clonal variation of tumor tissue during the treatment process, the initial specific tumor tissue genome cannot represent the genomic variation of the whole tumor tissue and the new tumor clonal variation. There are still controversial about the selection of the Tumor-naïve assays and Tumor-informed assays. In recent years, the research has continuously improved the ctDNA detection method in order to improve the sensitivity and accuracy of the evaluation of ctDNA MRD [Citation45,Citation51,Citation52]. (). Studies have shown that every stage of NSCLC has different detection rates of ctDNA. In stage 1 patients the detection rate is 42%-57.9%, and in stage 2–4 patients is 54%-100% [Citation36,Citation39,Citation61,Citation62]. Therefore, more sensitive detection methods are needed to improve the detection rate of ctDNA in patients with stage 1 of ctDNA.
Table 2. Main detection techniques of ctDNA.
4. Significance of ctDNA MRD evaluation in NSCLC
Accurate staging and classification of NSCLC patients with different biological and clinical characteristics is the key to achieve individualized treatment and improve prognosis. The existing TNM staging of NSCLC is based on the evaluation of classical imaging methods of tumor invasive lesions, lymph node invasion, and distant metastasis. This staging system plays an important role in the risk stratification and clinical treatment of NSCLC [Citation63]. However, owing to the extremely heterogeneous and complex evolution of NSCLC, existing staging and detection methods cannot fully simulate and measure the biological and clinical characteristics of the tumor. ctDNA MRD has significant advantages for estimating lung cancer recurrence, metastasis, and drug sensitivity, and if it is incorporated into the staging system as a new indicator, it is expected to guide the precision medicine for patients with NSCLC.
4.1. Predicting relapse and prognosis
4.1.1. Resectable NSCLC
Most patients with stage I, II, and IIIa NSCLC have resectable disease, and radical surgical resection is the best treatment option. However, approximately 30–50% of patients experience recurrence and metastasis after radical resection [Citation64]. Accurate and timely recurrence monitoring and prognosis assessment are key to achieving optimal clinical management. Thus, ctDNA MRD may play an important role in clinical management.
First, ctDNA can effectively assess the recurrence probability of resectable NSCLC, and patients with ctDNA positivity have a high recurrence probability. Studies have shown that patients who are ctDNA MRD-positive have a higher risk of relapse than patients who are ctDNA MRD-negative, and ctDNA MRD-positive can predict relapse earlier than radiography [Citation44,Citation65,Citation66]. In a study of TRACERx, 13 of 14 NSCLC patients with confirmed relapse were ctDNA positivity, with a median time of 70 days before radiographically confirmed relapse [Citation44]. Peng et al. found that 63.3% (19/30) of ctDNA-positive patients relapsed, and the median time before radiographic confirmation of recurrence was 12.6 months [Citation65]. Kuang et al. found that only two of 20 patients with negative ctDNA levels before and after chemotherapy had a relapse [Citation66].
Furthermore, ctDNA can be used to effectively assess the survival time of patients with resectable NSCLC. Several studies have shown the RFS and OS of patients with ctDNA positivity are shorter [Citation66–70]. Therefore, ctDNA-positive patients have worse prognosis than ctDNA-negative patients. Zhang et al. believed that no matter what stage patients were in and how adjuvant therapy was, if MRD could not be detected continuously, it may represent a potentially cured population [Citation71]. Therefore, ctDNA MRD may be used as an indicator for postoperative relapse risk stratification of NSCLC, which can be combined with clinical staging to develop more accurate adjuvant therapy. Thus, the MRD may be a reliable prognostic indicator for NSCLC in the future.
4.1.2. Oligometastatic NSCLC
In 1995, Weichselbaum and Hellman proposed an intermediate metastatic state between local and widespread metastatic diseases, namely the ‘oligometric’ disease state, which represents diseases with limited transmission potential [Citation72]. At present, the choice of treatment and monitoring of oligometastatic (OMD) NSCLC after resection is still uncertain, and there are no clear predictive markers to help determine whether patients with OMD can benefit from postoperative adjuvant therapy.
The molecular progress of OMD patients may occur earlier than the imaging progress, and the ctDNA index may reflect the tumor burden during treatment. A prospective clinical study, NCT01725165, randomly divided 49 patients with OMD into a local treatment group or maintenance treatment group in a 1:1 ratio [Citation73]. Dynamic ctDNA monitoring was performed in 21 patients (10 of which were in the local treatment group). Baseline ctDNA index was not associated with progression-free survival (PFS) or overall survival (OS). In all five patients with radiographic progression at the last follow-up, ctDNA measurements were higher than that at baseline, and the time to first detection of increased mutation was 6.7 months (2.9 to 17.9 months) before the median time to radiographic confirmation of progression [Citation74]. Therefore, MRD is also expected to be used as an indicator for monitoring disease progression and treatment selection in patients with oligometastatic NSCLC.
4.1.3. Unresectable NSCLC
Stereotactic radiotherapy is an effective treatment for patients with early stage unresectable NSCLC [Citation75]. The 2- and 5-year local control rates of patients with early stage unresectable NSCLC after radiotherapy were 97.6% and 93%, respectively, and the 3- and 5-year survival rates were 55% and 40.0%, respectively [Citation76]. Stage IIIc and most stage IIIB are classified as unresectable NSCLC, and the treatment is mainly radical concurrent chemoradiotherapy, whereas patients with stage IV unresectable NSCLC choose appropriate systemic treatment [Citation64]. ctDNA MRD can still be used to assess recurrence and prognosis and shows more advantages than classical methods ().
Table 3. ctDNA MRD evaluation of unresectable NSCLC.
Chaudhuri et al. found that the 3-year freedom from progression (FFP) of ctDNA-positive and ctDNA-negative patients was 0% and 93%, respectively, after the landmark of MRD. Analysis of disease-specific survival (DSS) and overall survival (OS) revealed similar results, with patients with undetectable ctDNA at the MRD landmark experiencing significantly better long-term survival than those with detectable ctDNA. Only 1 patient who ultimately recurred had undetectable ctDNA at the landmark of MRD, but turned positive 8 months later [Citation40]. Therefore, ctDNA-positive patients have worse prognosis than ctDNA-negative patients in Stage I-III unresectable NSCLC.
Moding et al. divided 65 patients with stage IIB-IIIB unresectable NSCLC into a chemoradiation therapy (CRT) group and CRT combined with immune checkpoint inhibitors (ICIs) group. There were eight patients who had positive ctDNA before or at the early stage of consolidation treatment with ICI, and all of them (100%) progressed. This molecular progress was on average 4.1 months ahead of that confirmed by imaging [Citation77]. Therefore, ctDNA MRD can be used to estimate the risk of relapse in patients with stage IIB-IIIB unresectable NSCLC treated with ICI consolidation therapy in advance. In the CRT group, ctDNA-positive and ctDNA-negative patients had 0% and 100% FFP, respectively, 24 months after CRT initiation [Citation77]. In the CRT combined with ICI group, six (86%) of the seven ctDNA-positive patients in the early stage of treatment progressed, whereas two (13%) of the 15 ctDNA-negative patients progressed, and the FFP of ctDNA-positive and ctDNA-negative patients was 0% and 87.5%, respectively [Citation77]. These results showed that after CRT treatment of stage IIB-IIIB unresectable NSCLC, ctDNA-negative patients had a good prognosis regardless of ICI consolidation therapy. Therefore, ctDNA MRD can estimate the prognosis of stage IIB-IIIB unresectable NSCLC patients in advance.
Similarly, ctDNA MRD was effective in predicting the risk and prognosis of patients with stage IV unresectable NSCLC. Xia et al. analyzed ctDNA methylation and maximum allele fraction (maxAF) in eight patients with stage IV lung adenocarcinoma and found that methylation levels were increased in six patients and maxAF level was increased in five patients. The mean time of progression was 3.0 months and 1.9 months earlier than the radiographic confirmation, respectively [Citation78]. Hellmann et al. performed ctDNA analysis of the plasma of stage IV NSCLC patients treated continuously with PD-(L)1 and found that four ctDNA-positive patients eventually progressed, with a median time of 4.4 months before the progression was confirmed from imaging, while 25 (93%) of the 27 ctDNA-negative patients did not progress [Citation79]. The above studies indicate that positive plasma ctDNA in patients with stage IV unresectable NSCLC after treatment can predict disease progression earlier than imaging and indicate a poor prognosis.
4.2. Guide treatment and reflect therapeutic effect
4.2.1. Adjuvant chemotherapy
Adjuvant chemotherapy (ACT) is the most widely used type of adjuvant therapy. Owing to the large side effects of chemotherapy drugs, the 5-year survival rate has increased by approximately 5% with ACT [Citation82]. ctDNA MRD can reflect the effect of ACT, to modify the treatment plan of patients who are not sensitive to chemotherapy and avoid unnecessary toxicity of ACT.
Studies have shown that resectable NSCLC patients with ctDNA MRD positive are sensitive to chemotherapy, and the relapse-free survival (RFS) of patients receiving chemotherapy is significantly improved [Citation67,Citation68,Citation83], while the RFS of patients with ctDNA MRD-negative tumors after chemotherapy is lower than that of patients without chemotherapy [Citation83]. These results suggest that patients positive for ctDNA are more likely to benefit from ACT than those negative for ctDNA. Therefore, ctDNA MRD testing, when used in combination with AJCC staging, can provide relatively precise risk stratification, which is helpful for precise personalized treatment of patients and avoids unnecessary chemotherapy toxicities. Negative ctDNA or decreased single-nucleotide variants (SNVs) number after ACT in NSCLC may indicate the sensitivity of patients to ACT, while persistently positive ctDNA or increased SNV number may indicate the need for ACT [Citation44,Citation66]. In studies based on sequential liquid biopsies, ACT will be discontinued in patients who are ctDNA MRD negative, and ACT will be administered to patients with persistently positive ctDNA, and targeted therapy can be added based on newly identified ctDNA mutations [Citation84]. Therefore, ctDNA dynamic monitoring of patients can predict the effect of ACT to modify the treatment strategy for patients who are not sensitive to ACT.
4.2.2. Targeted therapy
Targeted therapy is an important treatment option for patients with advanced lung cancer. Compared with traditional chemotherapy, it can significantly improve the prognosis of patients with advanced lung cancer [Citation85]. Previous studies on targeted therapy have mainly focused on patients with advanced NSCLC, but recent studies have found that targeted therapy targeting EGFR mutations also plays an important role in adjuvant therapy for patients with early and mid-stage NSCLC after complete tumor resection [Citation86]. ctDNA MRD provides an important indication for patients with NSCLC to benefit from targeted therapy as early as possible. Tony Mok et al. detected changes in EGFR mutations from ctDNA to predict the survival outcomes of patients with NSCLC treated with first-line target therapy [Citation87].
Continuous ctDNA MRD monitoring of patients with NSCLC can identify candidates for targeted therapy and adjust the treatment plan, which can achieve precise personalized treatment. Chaudhuri et al. found that 53% of patients had ctDNA mutation profiles associated with tyrosine kinase inhibitors, and proposed if these patients were candidates for these inhibitors, they could benefit from first-line targeted therapy [Citation40]. In addition, a combination of MRD dynamic monitoring and imaging can more comprehensively evaluate the therapeutic effect of targeted therapy in patients with advanced NSCLC. In the AURA17 phase II trial, decreased methylation and maxAF levels predicted sensitivity to osimertinib, whereas increased methylation and maxAF levels predicted resistance to osimertinib [Citation78]. The ongoing clinical study NCT05079022 is evaluating the feasibility and efficacy of adjuvant therapy with furmonertinib in patients with EGFR mutation-positive stage I lung adenocarcinoma by using ctDNA MRD.
4.2.3. Immunotherapy
Immunotherapy has expanded treatment options for patients with advanced NSCLC. ICIs have been approved as first- or second-line therapies for advanced NSCLC [Citation88,Citation89]. However, not all patients benefited from immunotherapy. Patients using different immunosuppressants achieve an objective response rate of 20% [Citation4,Citation90], but the median response time is 1–2 years, and most patients develop drug resistance [Citation5]. ctDNA MRD can reflect the effect of immunotherapy and patient prognosis in a timely and effective manner.
Dynamic monitoring of ctDNA levels can effectively evaluate the efficacy of immunotherapy and can be used as an indicator of disease progression risk stratification and prognosis after receiving ICI treatment. Patients with advanced NSCLC who are treated with immunotherapy can interfere with the evaluation of the treatment efficacy because the tumor typically shrinks slowly or expands transiently due to inflammation. By quantifying ctDNA levels and monitoring tumor cell death in real time, the effect of immunotherapy can be evaluated at the early stage of treatment. Goldberg et al. conducted dynamic monitoring of ctDNA levels and imaging tumor size in ICI-treated metastatic NSCLC patients and analyzed the relationship between ctDNA response and survival outcome. The ctDNA response was defined as a reduction in the mutated allele fraction by > 50% from baseline [Citation80]. A strong consistency was found between ctDNA and radiological responses, and ctDNA responders had significantly longer treatment times than non-responders [Citation80]. Jia et al. studied 10 patients with stage IIIB/IV NSCLC who received durvalumab monotherapy or combinatorial therapy and analyzed their ctDNA status before and after treatment. Eight weeks after the first infusion, patients who had an objective response to ICI showed a greater decrease in ctDNA levels than those who did not [Citation81]. A significant decrease in plasma ctDNA abundance at an early stage (≥50% decrease at eight weeks) predicted prolonged PFS after ICI administration in nine patients [Citation81]. These results suggested that ctDNA MRD can effectively predict the effects of immunotherapy.
Ongoing clinical studies have evaluated the personalized therapeutic effect of immunotherapy combined with chemotherapy or immune monotherapy by the dynamic monitoring of ctDNA MRD in patients [Citation51] (). The clinical trial MERMAID-1 (NCT04385368) compared durvalumab plus platinum-based chemotherapy with placebo plus platinum-based chemotherapy in MRD-positive patients. The clinical trials MERMAID-2 (NCT04642469) and NCT04585477 evaluated the efficacy of durvalumab monotherapy in MRD-positive patients. The clinical trial SCION (NCT04944173) evaluated whether ctDNA MRD can predict patients who may benefit from long-term treatment with duvaluzumab. In the NCT04585490 study, patients with stage III unresectable NSCLC with MRD-positive ctDNA after CRT received durvalumab plus four cycles of platinum dual chemotherapy using the AVENIO ctDNA detection platform. Patients with no detectable ctDNA MRD after CRT received the standard treatment with durvalumab for consolidation. These studies collect indicators of patient survival status, changes in ctDNA levels, treatment effect, and ultimately point out that changes in ctDNA levels can serve as indicators for evaluating drug efficacy.
Table 4. Ongoing studies utilizing ctDNA MRD monitoring in NSCLC.
5. The challenges of ctDNA MRD application in clinical practice
Although several studies have shown that ctDNA MRD can be used as a prognostic indicator after NSCLC treatment, it is important in adjuvant therapy and whole-course management of patients. However, there are still some challenges in the application of ctDNA MRD in the clinical setting of NSCLC.
5.1. The application of ctDNA MRD is limited in stage I NSCLC
Both preoperative and postoperative plasma ctDNA positivity can be used as prognostic indicators for NSCLC [Citation65,Citation83]. However, the positivity rate of plasma ctDNA MRD is low in patients with stage I NSCLC [Citation65]. The frequency of plasma ctDNA mutations in patients with stage I NSCLC after surgery is significantly lower than that before [Citation39,Citation91], suggesting that preoperative plasma ctDNA is more suitable for MRD evaluation than postoperative plasma ctDNA. However, several studies have shown that postoperative plasma ctDNA positivity is associated with lower RFS and OS in patients with NSCLC [Citation66–70], while preoperative ctDNA positivity is not associated with RFS in NSCLC patients [Citation70]. These contradictions limit the application of ctDNA MRD in stage I NSCLC. Therefore, further improving the ability of ctDNA MRD detection appears to be an effective way to overcome this problem.
5.2. Sensitivity and specificity
In a study by Waldeck et al., four patients with relapse confirmed by imaging were consistently negative for ctDNA during follow-up, while two patients with ctDNA positivity in single plasma samples had no radiographically confirmed disease progression [Citation92]. This suggests that ctDNA MRD has certain false-negative and false-positive probabilities. Clonal hematopoiesis of undetermined potential (CHIP) is the accumulation of mutations in hematopoietic progenitor cells during aging,which are generally different from solid tumor mutations but have some overlap, such as TP53. CHIP is the main factor affecting the specificity and sensitivity of ctDNA MRD detection. Evidence has shown that the frequency of CHIP mutation in patients ~60 years old is 92% [Citation93]. Therefore, it may interfere with ctDNA test results and reduce the specificity and sensitivity of ctDNA MRD assessments. In addition, not all detected plasma mutations are associated with cancer processes. For example, BRAF V600E is the driver mutation for melanoma and NSCLC, but it is more common in benign nevus tissue specimens [Citation94]. This can lead to reduced specificity for ctDNA MRD assessment. Due to concern over CHIP variants contaminating the ctDNA dataset, the strategies for CHIP correction are available such as performed in IMpower150 trial in NSCLC [Citation95].
5.3. Selection of optimal detection time
The optimal time point for ctDNA MRD detection in NSCLC has yet to be standardized. Post-treatment MRD evaluation based on ctDNA test results at a single time point is significantly different from that based on multiple time points [Citation93]. Therefore, ctDNA MRD evaluation of surgically resectable and non-resectable NSCLC patients is significantly different from integrated ctDNA test results at multiple time points. It remains controversial whether to judge by ctDNA test results at a single time point or integrate test results at multiple time points. At present, it is still uncertain whether preoperative or postoperative plasma ctDNA positivity can predict the prognosis of patients. Some studies have suggested that plasma ctDNA positivity before and after surgery can be used as a prognostic indicator for NSCLC [Citation65,Citation83]. However, most studies have shown that plasma ctDNA positivity in NSCLC patients after surgery is associated with lower RFS and OS [Citation66–69]. Ohara et al. reported that preoperative plasma ctDNA positivity was not associated with RFS in patients with NSCLC, but postoperative ctDNA positivity was significantly correlated with histological grade and could predict shorter RFS [Citation70]. Therefore, postoperative ctDNA positivity is more likely to reflect MRD.
6. Conclusion
Although minimal residual disease (MRD) has shown significant advantages in predicting the ability of clinical recurrence and metastasis in patients with NSCLC and evaluating the treatment effect, due to the sensitivity and specificity of MRD detection, as well as the diversity of MRD indicators, there are still some limitations in the clinical application of MRD for patients with NSCLC. More clinical studies are required to prove its application value. In this study, we analyzed the potential value and challenges of MRD in guiding the clinical treatment of NSCLC with a focus on the definition, development history, and application of MRD in NSCLC and highlight its potential to be integrated into the NSCLC staging system as an accurate biological indicator.
7. Expert opinion
Although multiple clinical studies on the relationship between MRD and drug response in NSCLC have been report, the application of MRD in NSCLC is still immature. At present, there are still lack of standardized scope for ctDNA MRD detection in non-small cell lung cancer, such as the selection of blood collection vessels, blood collection time, plasma extraction and storage conditions, DNA extraction and quantification, pre analysis and analysis conditions, and the standardized for definition of MRD ‘positive’ and provisions for defining ‘recurrence’ and ‘progression’ or ‘response’ during longitudinal monitoring, which can minimize differences between studies. Therefore, it is essential to provide reliable and consistent evidence for clinical practice.
In previous and ongoing clinical studies, ctDNA MRD has made some progress as an indicator for evaluating the therapeutic effect of NSCLC. However, current studies primarily evaluate MRD by ctDNA detection, mainly because ctDNA contains more tumor information, and the development of next-generation sequencing technology has improved the analytical performance of ctDNA detection. Although ctDNA MRD has shown great potential in predicting clinical relapse ability and therapeutic effect in NSCLC patients, its application in MRD evaluation of stage I NSCLC is limited due to the extremely low blood abundance of ctDNA. Molecular abnormalities of various cancer sources can be used as MRD detection indicators. Compared with ctDNA, the abundance of non-coding RNA (ncRNA) in plasma is much higher than that of ctDNA (12). ncRNA can be considered a marker to reflect tumor MRD level, which is helpful in optimizing the application of MRD in stage I NSCLC.
Tumor heterogeneity significant affect the effectiveness of treatment. Tumor microenvironment is an important factor of tumor heterogeneity. Therefore, judging the composition of the tumor microenvironment and delicately dividing the nature of the tumor are crucial for formulating accurate and effective treatment plans. In the future, high-throughput and omics detection methods will be used to analyze the blood composition and evaluate the tumor microenvironment of patients, and provide the best treatment plan, which is expected to truly achieve precise personalized treatment. Therefore, ctDNA combined with other molecular testing can contribute to the development of more effective MRD assessment indicators and has significant potential to improve the whole-course management of patients with NSCLC.
Due to its excellent prognostic performance, MRD is expected to be integrated into the NSCLC staging system as an accurate biological indicator, enabling more accurate clinical staging for patients and contributing to the realization of individualized treatment. MRD monitoring can be used as a good tool for patient whole-process management, efficacy evaluation, and treatment plan selection and optimization. MRD negative patients have the characteristics of low risk of relapse, good prognosis, and poor benefit with adjuvant therapy. Furthermore, no matter what stage patients were in and how adjuvant therapy was, if MRD could not be detected continuously, it may represent a potentially cured population. Therefore, the scale of adjuvant therapy for MRD negative patients should be reduced. MRD positive patients have a high risk of relapse, poor prognosis, sensitivity to treatment, and may need more active postoperative adjuvant therapy. If MRD-positive patients turn negative after treatment, this indicates that they are sensitive to treatment and have sustainable benefits. If MRD continues to be positive after treatment, or dynamic monitoring changes from negative to positive, this indicates that treatment is not sensitive, and that the treatment plan needs to be adjusted. With the continuous progress of diagnosis, new drug development, and treatment strategies, NSCLC may no longer be scary in the future.
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewers disclosure
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Acknowledgments
We would like to thank every member of our research team for their help and Editage (www.editage.cn) for English language editing.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca A Cancer J Clinicians. 2021;71(3):209–249. doi: 10.3322/caac.21660
- Fruh M, De Ruysscher D, Popat S, et al. Small-cell lung cancer (SCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi99–105. doi: 10.1093/annonc/mdt178
- Wen S, Dai L, Wang L, et al. Genomic signature of driver genes identified by target next-generation sequencing in Chinese non-small cell lung cancer. Oncology. 2019;24(11):e1070–e1081. doi: 10.1634/theoncologist.2018-0572
- Champiat S, Dercle L, Ammari S, et al. Hyperprogressive disease is a New Pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res. 2017;23(8):1920–1928. doi: 10.1158/1078-0432.CCR-16-1741
- Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33(18):2004–2012. doi: 10.1200/JCO.2014.58.3708
- Guo H, Li W, Wang B, et al. Coexisting opportunities and challenges: in which scenarios can minimal/measurable residual disease play a role in advanced non-small cell lung cancer? Chin J Cancer Res. 2021;33(5):574–582. doi: 10.21147/j.issn.1000-9604.2021.05.04
- Benson AB, Venook AP, Al-Hawary MM, et al. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(3):329–359. doi: 10.6004/jnccn.2021.0012
- Pantel K, Alix-Panabieres C. Tumour microenvironment: informing on minimal residual disease in solid tumours. Nat Rev Clin Oncol. 2017;14(6):325–326. doi: 10.1038/nrclinonc.2017.53
- Beiter T, Fragasso A, Hudemann J, et al. Short-term treadmill running as a model for studying cell-free DNA kinetics in vivo. Clin Chem. 2011;57(4):633–636. doi: 10.1373/clinchem.2010.158030
- Snyder MW, Kircher M, Hill AJ, et al. Cell-free DNA comprises an in vivo nucleosome footprint that informs its tissues-of-origin. Cell. 2016;164(1–2):57–68. doi: 10.1016/j.cell.2015.11.050
- Cheng ML, Pectasides E, Hanna GJ, et al. Circulating tumor DNA in advanced solid tumors: clinical relevance and future directions. Ca A Cancer J Clinicians. 2021;71(2):176–190. doi: 10.3322/caac.21650
- Nagasaka M, Uddin MH, Al-Hallak MN, et al. Liquid biopsy for therapy monitoring in early-stage non-small cell lung cancer. Mol Cancer. 2021;20(1):82. doi: 10.1186/s12943-021-01371-1
- Diaz LA Jr., Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014;32(6):579–586. doi: 10.1200/JCO.2012.45.2011
- Hosseini H, Obradovic MMS, Hoffmann M, et al. Early dissemination seeds metastasis in breast cancer. Nature. 2016;540(7634):552–558. doi: 10.1038/nature20785
- Badia-Ramentol J, Linares J, Gomez-Llonin A, et al. Minimal residual disease, metastasis and immunity. Biomolecules. 2021;11(2):130. doi: 10.3390/biom11020130
- Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9(4):274–284. doi: 10.1038/nrc2622
- Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer. 2014;14(9):611–622. doi: 10.1038/nrc3793
- Borst P. Cancer drug pan-resistance: pumps, cancer stem cells, quiescence, epithelial to mesenchymal transition, blocked cell death pathways, persisters or what? Open Biol. 2012;2(5):120066. doi: 10.1098/rsob.120066
- Clevers H. The cancer stem cell: premises, promises and challenges. Nature Med. 2011;17(3):313–319. doi: 10.1038/nm.2304
- Klein CA. Framework models of tumor dormancy from patient-derived observations. Curr Opin Genet Dev. 2011;21(1):42–49. doi: 10.1016/j.gde.2010.10.011
- Goss PE, Chambers AF. Does tumour dormancy offer a therapeutic target? Nat Rev Cancer. 2010;10(12):871–877. doi: 10.1038/nrc2933
- Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7(11):834–846. doi: 10.1038/nrc2256
- Papadaki MA, Stoupis G, Theodoropoulos PA, et al. Circulating tumor cells with stemness and epithelial-to-mesenchymal transition features are chemoresistant and predictive of poor outcome in metastatic breast cancer. Mol Cancer Ther. 2019;18(2):437–447. doi: 10.1158/1535-7163.MCT-18-0584
- Ortiz-Otero N, Marshall JR, Lash B, et al. Chemotherapy-induced release of circulating-tumor cells into the bloodstream in collective migration units with cancer-associated fibroblasts in metastatic cancer patients. BMC Cancer. 2020;20(1):873. doi: 10.1186/s12885-020-07376-1
- Blatter S, Rottenberg S. Minimal residual disease in cancer therapy–small things make all the difference. Drug Resist Updat. 2015;21-22:1–10. doi: 10.1016/j.drup.2015.08.003
- Abbosh C, Birkbak NJ, Swanton C. Early stage NSCLC - challenges to implementing ctDNA-based screening and MRD detection. Nat Rev Clin Oncol. 2018;15(9):577–586. doi: 10.1038/s41571-018-0058-3
- Frisone D, Friedlaender A, Addeo A. The role and impact of minimal residual disease in NSCLC. Curr Oncol Rep. 2021;23(12):136. doi: 10.1007/s11912-021-01131-w
- Chudacek J, Bohanes T, Klein J, et al. Detection of minimal residual disease in lung cancer. Biomed Pap Med Fac Univ Palacky Olomouc Czech. 2014;158(2):189–193. doi: 10.5507/bp.2013.019
- Racila E, Euhus D, Weiss AJ, et al. Detection and characterization of carcinoma cells in the blood. Proc Natl Acad Sci USA. 1998;95(8):4589–4594. doi: 10.1073/pnas.95.8.4589
- Coakley M, Garcia-Murillas I, Turner NC. Molecular residual disease and adjuvant trial design in solid tumors. Clin Cancer Res. 2019;25(20):6026–6034. doi: 10.1158/1078-0432.CCR-19-0152
- Mathe G, Weiner R, Pouillart P, et al. BCG in cancer immunotherapy: experimental and clinical trials of its use in treatment of leukemia minimal and or residual disease. Natl Cancer Inst Monogr. 1973;39:165–175.
- Matthews MJ, Kanhouwa S, Pickren J, et al. Frequency of residual and metastatic tumor in patients undergoing curative surgical resection for lung cancer. Cancer Chemother Rep Part. 1973;3(2):63–67.
- Chen ZL. An immunohistochemical study of occult micrometastases in regional lymph nodes of patients with stage I non-small cell lung carcinoma. Zhonghua Zhong Liu Za Zhi. 1992;14(6):423–426.
- Marchetti A, Buttitta F, Carnicelli V, et al. Enriched SSCP: a highly sensitive method for the detection of unknown mutations. Application to the molecular diagnosis of lung cancer in sputum samples. Diagn Mol Pathol: Am J Surg Pathol Part B. 1997;6(4):185–191. doi: 10.1097/00019606-199708000-00002
- Anker P, Stroun M. Tumor-related alterations in circulating DNA, potential for diagnosis, prognosis and detection of minimal residual disease. Leukemia. 2001;15(2):289–291. doi: 10.1038/sj.leu.2402016
- Newman AM, Bratman SV, To J, et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nature Med. 2014;20(5):548–554. doi: 10.1038/nm.3519
- Kazemifard N, Sadeghi A, Varaminian B, et al. Circulating tumor DNA applications in monitoring the treatment of metastatic colorectal cancer patients. Gastroenterol Hepatol Bed Bench. 2019;12(Suppl1):S14–S21.
- Amatu A, Schirripa M, Tosi F, et al. High circulating methylated DNA is a negative predictive and prognostic marker in metastatic colorectal cancer patients treated with Regorafenib. Front Oncol. 2019;9:622. doi: 10.3389/fonc.2019.00622
- Chen K, Zhang J, Guan T, et al. Comparison of plasma to tissue DNA mutations in surgical patients with non-small cell lung cancer. J Thorac Cardiovasc Surg. 2017;154(3):1123–31e2. doi: 10.1016/j.jtcvs.2017.04.073
- Chaudhuri AA, Chabon JJ, Lovejoy AF, et al. Early detection of molecular residual disease in localized lung cancer by circulating tumor DNA profiling. Cancer Discovery. 2017;7(12):1394–1403. doi: 10.1158/2159-8290.CD-17-0716
- Garcia-Murillas I, Schiavon G, Weigelt B, et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci, Trans Med. 2015;7(302):302ra133. doi: 10.1126/scitranslmed.aab0021
- Tie J, Wang Y, Tomasetti C, et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci, Trans Med. 2016;8(346):346ra92. doi: 10.1126/scitranslmed.aaf6219
- Powles T, Assaf ZJ, Davarpanah N, et al. ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature. 2021;595(7867):432–437. doi: 10.1038/s41586-021-03642-9
- Abbosh C, Birkbak NJ, Wilson GA, et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature. 2017;545(7655):446–451. doi: 10.1038/nature22364
- Fang X, Yu S, Jiang Y, et al. Circulating tumor DNA detection in MRD assessment and diagnosis and treatment of non-small cell lung cancer. Front Oncol. 2022;12:1027664. doi: 10.3389/fonc.2022.1027664
- Vendrell JA, Mazieres J, Senal R, et al. Ultra-sensitive EGFR (T790M) detection as an independent prognostic marker for lung cancer patients harboring EGFR (del19) mutations and treated with first-generation TKIs. Clin Cancer Res. 2019;25(14):4280–4289. doi: 10.1158/1078-0432.CCR-18-2683
- Bassan R, Spinelli O, Oldani E, et al. Improved risk classification for risk-specific therapy based on the molecular study of minimal residual disease (MRD) in adult acute lymphoblastic leukemia (ALL). Blood. 2009;113(18):4153–4162. doi: 10.1182/blood-2008-11-185132
- Chakrabarti S, Xie H, Urrutia R, et al. The promise of Circulating Tumor DNA (ctDNA) in the management of early-stage colon cancer: a critical review. Cancers. 2020;12(10):2808. doi: 10.3390/cancers12102808
- Dasari A, Morris VK, Allegra CJ, et al. ctDNA applications and integration in colorectal cancer: an NCI Colon and Rectal-Anal Task Forces whitepaper. Nat Rev Clin Oncol. 2020;17(12):757–770. doi: 10.1038/s41571-020-0392-0
- Cescon DW, Bratman SV, Chan SM, et al. Circulating tumor DNA and liquid biopsy in oncology. Nat Cancer. 2020;1(3):276–290. doi: 10.1038/s43018-020-0043-5
- Shields MD, Chen K, Dutcher G, et al. Making the rounds: exploring the role of circulating tumor DNA (ctDNA) in non-small cell lung cancer. Int J Mol Sci. 2022;23(16):9006. doi: 10.3390/ijms23169006
- Qin Z, Ljubimov VA, Zhou C, et al. Cell-free circulating tumor DNA in cancer. Chin J Cancer. 2016;35(1):36. doi: 10.1186/s40880-016-0092-4
- Little S. Amplification-refractory mutation system (ARMS) analysis of point mutations. Curr Protoc Hum Genet. 2001; Chapter 9:Unit 9 8. Chapter 9. doi: 10.1002/0471142905.hg0908s07
- Hindson CM, Chevillet JR, Briggs HA, et al. Absolute quantification by droplet digital PCR versus analog real-time PCR. Nat Methods. 2013;10(10):1003–1005. doi: 10.1038/nmeth.2633
- Garrido P, Paz-Ares L, Majem M, et al. LungBEAM: a prospective multicenter study to monitor stage IV NSCLC patients with EGFR mutations using BEAMing technology. Cancer Med. 2021;10(17):5878–5888. doi: 10.1002/cam4.4135
- Forshew T, Murtaza M, Parkinson C, et al. Noninvasive identification and monitoring of cancer mutations by targeted deep sequencing of plasma DNA. Sci, trans med. 2012;4(136):136ra68. doi: 10.1126/scitranslmed.3003726
- Kinde I, Wu J, Papadopoulos N, et al. Detection and quantification of rare mutations with massively parallel sequencing. Proc Natl Acad Sci USA. 2011;108(23):9530–9535. doi: 10.1073/pnas.1105422108
- Newman AM, Lovejoy AF, Klass DM, et al. Integrated digital error suppression for improved detection of circulating tumor DNA. Nat Biotechnol. 2016;34(5):547–555. doi: 10.1038/nbt.3520
- Kurtz DM, Soo J, Co Ting Keh L, et al. Enhanced detection of minimal residual disease by targeted sequencing of phased variants in circulating tumor DNA. Nat Biotechnol. 2021;39(12):1537–1547. doi: 10.1038/s41587-021-00981-w
- Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nature Med. 2017;23(6):703–713. doi: 10.1038/nm.4333
- Chabon JJ, Hamilton EG, Kurtz DM, et al. Integrating genomic features for non-invasive early lung cancer detection. Nature. 2020;580(7802):245–251. doi: 10.1038/s41586-020-2140-0
- Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci, trans med. 2014;6(224):224ra24. doi: 10.1126/scitranslmed.3007094
- Ettinger DS, Wood DE, Aisner DL, et al. Non-small cell lung cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(5):497–530. doi: 10.6004/jnccn.2022.0025
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39–51. doi: 10.1016/j.jtho.2015.09.009
- Peng M, Huang Q, Yin W, et al. Circulating tumor DNA as a prognostic biomarker in localized non-small cell lung cancer. Front Oncol. 2020;10:561598.
- Kuang PP, Li N, Liu Z, et al. Circulating tumor DNA analyses as a potential marker of recurrence and effectiveness of adjuvant chemotherapy for resected non-small-cell lung cancer. Front Oncol. 2020;10:595650.
- Chen K, Zhao H, Shi Y, et al. Perioperative dynamic changes in circulating tumor DNA in patients with lung cancer (DYNAMIC). Clin Cancer Res. 2019;25(23):7058–7067. doi: 10.1158/1078-0432.CCR-19-1213
- Qiu B, Guo W, Zhang F, et al. Dynamic recurrence risk and adjuvant chemotherapy benefit prediction by ctDNA in resected NSCLC. Nat Commun. 2021;12(1):6770. doi: 10.1038/s41467-021-27022-z
- Zviran A, Schulman RC, Shah M, et al. Genome-wide cell-free DNA mutational integration enables ultra-sensitive cancer monitoring. Nature Med. 2020;26(7):1114–1124. doi: 10.1038/s41591-020-0915-3
- Ohara S, Suda K, Sakai K, et al. Prognostic implications of preoperative versus postoperative circulating tumor DNA in surgically resected lung cancer patients: a pilot study. Transl Lung Cancer Res. 2020;9(5):1915–1923. doi: 10.21037/tlcr-20-505
- Zhang JT, Liu SY, Gao W, et al. Longitudinal undetectable molecular residual disease defines potentially cured population in localized non-small cell lung cancer. Cancer Discovery. 2022;12(7):1690–1701. doi: 10.1158/2159-8290.CD-21-1486
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13(1):8–10. doi: 10.1200/JCO.1995.13.1.8
- Gomez DR, Tang C, Zhang J, et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol. 2019;37(18):1558–1565. doi: 10.1200/JCO.19.00201
- Tang C, Lee WC, Reuben A, et al. Immune and circulating tumor DNA profiling after radiation treatment for oligometastatic non-small cell lung cancer: translational correlatives from a mature randomized phase II trial. Int J Radiat Oncol Biol Phys. 2020;106(2):349–357. doi: 10.1016/j.ijrobp.2019.10.038
- Ma L, Xiang J. Clinical outcomes of video-assisted thoracic surgery and stereotactic body radiation therapy for early-stage non-small cell lung cancer: a meta-analysis. Thorac Cancer. 2016;7(4):442–451. doi: 10.1111/1759-7714.12352
- Verstegen NE, Lagerwaard FJ, Haasbeek CJ, et al. Outcomes of stereotactic ablative radiotherapy following a clinical diagnosis of stage I NSCLC: comparison with a contemporaneous cohort with pathologically proven disease. Radiother Oncol. 2011;101(2):250–425. doi: 10.1016/j.radonc.2011.09.017
- Moding EJ, Liu Y, Nabet BY, et al. Circulating tumor DNA dynamics predict benefit from consolidation immunotherapy in locally advanced non-small cell lung cancer. Nat Cancer. 2020;1(2):176–183. doi: 10.1038/s43018-019-0011-0
- Xia S, Ye J, Chen Y, et al. Parallel serial assessment of somatic mutation and methylation profile from circulating tumor DNA predicts treatment response and impending disease progression in osimertinib-treated lung adenocarcinoma patients. Transl Lung Cancer Res. 2019;8(6):1016–1028. doi: 10.21037/tlcr.2019.12.09
- Hellmann MD, Nabet BY, Rizvi H, et al. Circulating tumor DNA analysis to assess risk of progression after long-term response to PD-(L)1 blockade in NSCLC. Clin Cancer Res. 2020;26(12):2849–2858. doi: 10.1158/1078-0432.CCR-19-3418
- Goldberg SB, Narayan A, Kole AJ, et al. Early assessment of lung cancer immunotherapy response via circulating tumor DNA. Clin Cancer Res. 2018;24(8):1872–1880. doi: 10.1158/1078-0432.CCR-17-1341
- Jia Q, Chiu L, Wu S, et al. Tracking neoantigens by personalized circulating tumor DNA sequencing during checkpoint blockade immunotherapy in non-small cell lung cancer. Adv Sci. 2020;7(9):1903410. doi: 10.1002/advs.201903410
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE collaborative group. J Clin Oncol. 2008;26(21):3552–3559. doi: 10.1200/JCO.2007.13.9030
- Xia L, Mei J, Kang R, et al. Perioperative ctDNA-Based molecular residual disease detection for non-small cell lung cancer: a prospective multicenter cohort study (LUNGCA-1). Clin Cancer Res. 2022;28(15):3308–3317. doi: 10.1158/1078-0432.CCR-21-3044
- Chae YK, Oh MS. Detection of minimal residual disease using ctDNA in lung cancer: current evidence and future directions. J Thorac Oncol. 2019;14(1):16–24. doi: 10.1016/j.jtho.2018.09.022
- Barlesi F, Mazieres J, Merlio JP, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet. 2016;387(10026):1415–1426. doi: 10.1016/S0140-6736(16)00004-0
- Wu YL, Tsuboi M, He J, et al. Osimertinib in resected EGFR-Mutated non-small-cell lung cancer. N Engl J Med. 2020;383(18):1711–1723. doi: 10.1056/NEJMoa2027071
- Mok T, Wu YL, Lee JS, et al. Detection and dynamic changes of EGFR mutations from circulating tumor DNA as a predictor of survival outcomes in NSCLC patients treated with first-line intercalated erlotinib and chemotherapy. Clin Cancer Res. 2015;21(14):3196–3203. doi: 10.1158/1078-0432.CCR-14-2594
- Herbst RS, Giaccone G, Marinis F, et al. Atezolizumab for first-line treatment of PD-L1-Selected patients with NSCLC. N Engl J Med. 2020;383(14):1328–1339. doi: 10.1056/NEJMoa1917346
- Paz-Ares L, Spira A, Raben D, et al. Outcomes with durvalumab by tumour PD-L1 expression in unresectable, stage III non-small-cell lung cancer in the PACIFIC trial. Ann Oncol. 2020;31(6):798–806. doi: 10.1016/j.annonc.2020.03.287
- Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy–inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res. 2012;18(24):6580–6587. doi: 10.1158/1078-0432.CCR-12-1362
- Guo N, Lou F, Ma Y, et al. Circulating tumor DNA detection in lung cancer patients before and after surgery. Sci Rep. 2016;6(1):33519. doi: 10.1038/srep33519
- Waldeck S, Mitschke J, Wiesemann S, et al. Early assessment of circulating tumor DNA after curative-intent resection predicts tumor recurrence in early-stage and locally advanced non-small-cell lung cancer. Mol Oncol. 2022;16(2):527–537. doi: 10.1002/1878-0261.13116
- Pellini B, Chaudhuri AA. Circulating tumor DNA minimal residual disease detection of non-small-cell lung cancer treated with curative Intent. J Clin Oncol. 2022;40(6):567–575. doi: 10.1200/JCO.21.01929
- Pollock PM, Harper UL, Hansen KS, et al. High frequency of BRAF mutations in nevi. Nat Genet. 2003;33(1):19–20. doi: 10.1038/ng1054
- Assaf ZJF, Zou W, Fine AD, et al. A longitudinal circulating tumor DNA-based model associated with survival in metastatic non-small-cell lung cancer. Nat Med. 2023;29(4):859–868. doi: 10.1038/s41591-023-02226-6