The new direct-acting antivirals (DAAs) in hepatitis C treatment has led to the redefinition of the funding model of these extremely expensive drugs [Citation1,Citation2].
France is known to have a compulsory universal welfare system that allows access to medicines for all patients according to their needs, regardless of their financial means. In France, the hepatitis C prevalence would correspond to 367,000 seropositive patients (0.84% of the general population), of which about 20% are undiagnosed for chronic hepatitis [Citation3].
Despite the therapeutic improvement they brought, DAAs were endangering the sustainability of the French health-care system [Citation4]. This innovation prompted legislators to regulate their use, in order to ensure equity for patients with hepatitis C.
The French government decided to streamline treatment expenses by implementing a specific financing mechanism and by restricting access to drugs for several patient profiles. Indeed, prices charged by pharmaceutical companies and the number of patients expecting treatment triggered a significant risk on health insurance expenditures: the total estimated cost of these treatments in France is around 5 € billion per year (i.e. 20% of total drug spending [Citation5]).
Because of the high cost of DAAs, an existing financing mechanism to cap the expenses incurred by health insurance under the treatment of hepatitis C was changed by the legislator and adapted to meet the specific problems caused by their cost. This progressive contribution from the pharmaceutical companies commercializing these treatments is triggered when the following two conditions are met: the sum of sales (excluding tax) resulting from the use of drugs for the treatment of the disease is greater than a threshold amount set at €450 million in 2014 and at €700 million in 2015 (called W rate); the growth rate of the turnover is higher than 10% (rate set in 2014 that may change in the future). In practice, the contribution depends on the difference found with the W rate fixed by the French Parliament each year. In addition to this, there is another principle: each company is liable for a share of the contribution determined in proportion of its turnover, subject to a ceiling of 15% of its turnover.
As a result, the savings generated by this mechanism amounted to 76.5 million for the French health system in 2014 through the signing of an agreement between the firms commercializing DAAS and the French Economic Committee of the Health Products (excluding the tax on sales paid by companies that did not sign). It is essential to note that the face values of these drugs do not correspond to their real price and that prices of DAAs in France are currently the lowest in Europe [Citation6].
In parallel, the French Health Ministry decided to closely frame the prescriptions of DAAs and limit their financial impact through several measures. In fact, in Europe, these treatments obtained a EU marketing authorization for all patients with hepatitis C, regardless of the patient’s profile and the French ministerial directives decided to restrict their use by limiting health insurance’s reimbursement through the selection of patients depending on their genotypes, disease stages and comorbidities [Citation7]. For now, the only therapeutic indications eligible for the care by health insurance are chronic hepatitis C in adults with stage of F3, F4 or “severe F2” liver fibrosis or simultaneously infected with HIV; with mixed cryoglobulinemia (II and III) and systemic symptoms or with B lymphoma associated with HCV regardless of the stage of fibrosis. In addition, it was decided that Multi-Disciplinary Team (MDT) meetings in hepatitis C reference center (HCRC) would be implemented for treatment initiations. A list of HCRC (35 in France) was established by the Health Ministry and the French law now financially penalizes the health insurance in case of non-compliance to these regulatory and organizational constraints. After these legislative developments, French hospitals had to get organized and apply the measures sought by the authorities in the field.
The largest university public hospital group in Europe (the Assistance-Publique Hôpitaux de Paris (AP-HP) including 37 French public hospitals supporting 7 million patients each year) tackled the challenge of organizing the patient’s access to DAAs from both medical and financial points of view.
The AP-HP accounts for 20% of total French expenses for DAAs, which exceeded 300 million Euros (almost 30% of AP-HP drugs’ budget) in 2014. In accordance with the legislation, AP-HP managers set up a working group to define the new organization of patient’s access to these treatments in the Paris area where every HCRC are 7 AP-HP hospitals. This group defined a practical organization and developed recommendations for the management of hepatitis C patients in AP-HP that should help minimize financial risks and are intended to harmonize practices in different AP-HP hospitals.
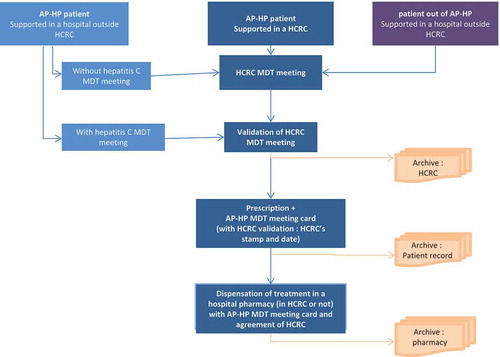
In December 2014, on the initiative of the central purchasing agency of AP-HP, the group of experts implemented an organizational chart of MDT meetings practical arrangements for all patients: patients supported in a HCRC; in a hospital outside a reference center or in a hospital outside AP-HP (). MDT meetings hepatitis C in a hospital outside a reference center are allowed but the final validation always comes back to one of the HCRC. An AP-HP hepatitis C MDT meeting is valid if at least three of the following five disciplines are represented: hepatology, infectious diseases, internal medicine, virology, pharmacy. A hospital outside a reference center can ask any HCRC for their patient records to be validated. If HCRC accepts the proposal, this agreement must be mention on the prescription (with HCRC’s stamp) on the day of the meeting.
The validation request of a reference center is performed on a standardized AP-HP MDT meetings card, also developed by the group of experts. It contains the patient’s confidential clinical data on the front; the proof of study in MDT meeting and the agreement of HCRC on the back side. It is available on the AP-HP website. The same card is used for MDT meetings in one HCRC or in one hospital outside a reference center.
These treatments shall be given in any hospital pharmacy (in HCRC or not) provided that the agreement and the proof of the HCRC MDT meetings is mentioned on this card which allows to respect the confidentiality of clinical patient data.
The decisions of the group of experts apply to the entire AP-HP and are transmitted to local referents. The implementation of MDT meetings, as offered by the AP-HP group of experts, supplies a framework for the requirements with a control by pharmacists and is an effective self-regulatory mechanism. It allows controlling prescriptions and the reimbursement of these expensive treatments. This organization implemented in the AP-HP and the use of the standardized AP-HP MDT meetings cards were extended throughout the region. It was taken over by the French Health Ministry on a national level in order to apply such process to other French hospitals. This kind of organization could be a structural model for other institutions or hospitals worldwide if they are faced with the question of fairness for patients with hepatitis C.
Because patients requiring treatment are now cured on a short time frame, it is expected that consumptions and expenditures related to DAAs will decrease. It may be possible to reopen the debate whether patients should receive treatment for chronic HCV infection [Citation8]. For the French hepatologists, access to universal treatment is a short-term goal [Citation7]: they would like to extend the reimbursed indications to support a larger number of patients (adults infected simultaneously with HBV; with nephropathy related to HCV; with chronic fatigue; with a high risk of transmitting HCV). Could it be possible to expand the population of patients treated to all patients? We can wonder about the sustainability of the system and the need to maintain the same organization on the long term. The results should be scrupulously analyzed.
Declaration of interest
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Acknowledgment
The authors would like to thank all the AP-HP’s members of the hepatitis C group of experts.
Additional information
Funding
References
- Traynor K. Effectiveness, costs weigh on HCV treatment decisions. Am J Health Syst Pharm. 2014 July 15;71:1156–1157.
- Only just the beginning of the end of hepatitis C [editorial]. Lancet. 2014;383(9914):281.
- Brouard C, Le Strat Y, Larsen C, et al. Estimation du nombre de personnes non diagnostiquées pour une hépatite C chronique en France en 2014. Implications pour des recommandations de dépistage élargi. Bull Epidémiol Hebd. 2015;(19–20):329–339 in French. [cited 2015 Oct 05]. Available from http://www.invs. sante.fr/beh/2015/19-20/2015_19-20_1.html
- Brennan T, Shrank W. New expensive treatments for hepatitis C infection. JAMA. 2014;312(6):593–594.
- Inspection Générale des Affaires Sociales (IGAS). Evaluation médico-économique en santé, 2014 Dec. [in French]. [cited 2015 Oct 07]. Available from; http://www.ladocumentationfrancaise.fr/var/storage/rapports-publics/154000078.pdf
- Comité Economique des Produits de Santé (CEPS). Rapport d’activité 2014/2015, 2015 Sep [in French]. [cited 2015 Oct 07]. Available from: http://www.sante.gouv.fr/IMG/pdf/RA_2014_FINAL_V2_01102015.pdf
- Association Française pour l’Etude du Foie (AFEF). Recommandations AFEF sur la prise en charge des hépatites virales C, 2015 Jun [in French]. [cited 2015 Aug 27]. Available from: http://www.afef.asso.fr/rc/org/afef/nws/News/2015/20150527-184857-777/src/nws_fullText/fr/Recommandations%20AFEF%20H%C3%A9patite%20C%20Juin%202015.pdf
- Ghany MG. The ongoing debate of who to treat for chronic hepatitis C virus. JAMA Intern Med. 2015;175(2):169–170.