ABSTRACT
Introduction: The ongoing development of genomic medicine and the use of molecular and imaging markers in personalized medicine (PM) has arguably challenged the field of health economic modeling (HEM). This study aims to provide detailed insights into the current status of HEM in PM, in order to identify if and how modeling methods are used to address the challenges described in literature.
Areas covered: A review was performed on studies that simulate health economic outcomes for personalized clinical pathways. Decision tree modeling and Markov modeling were the most observed methods. Not all identified challenges were frequently found, challenges regarding companion diagnostics, diagnostic performance, and evidence gaps were most often found. However, the extent to which challenges were addressed varied considerably between studies.
Expert commentary: Challenges for HEM in PM are not yet routinely addressed which may indicate that either (1) their impact is less severe than expected, (2) they are hard to address and therefore not managed appropriately, or (3) HEM in PM is still in an early stage. As evidence on the impact of these challenges is still lacking, we believe that more concrete examples are needed to illustrate the identified challenges and to demonstrate methods to handle them.
1. Introduction
With the advent of personalized medicine (PM), the delivery of health care is shifting towards selecting and monitoring the best available treatment for each individual patient based on patient characteristics and diagnostic information. More specifically, ‘Personalized medicine seeks to improve stratification and timing of health care by utilizing biological information and biomarkers on the level of molecular disease pathways, genetics, proteomics as well as metabolomics’ [Citation1]. Personalized clinical processes typically involve multiple diagnostic tests and treatments over time, and the sequences of tests and treatments may differ between individual patients. Furthermore, treatment decisions are becoming increasingly preference sensitive [Citation2], due to the fact that there is no longer a ‘one-size-fits-all’ approach and the need to appraise the combined information from multiple sources, such as numerous test results, patients’ characteristics, and medical histories. Examples of personalized clinical processes include treatment targeting based on risk-stratification by patient characteristics and response monitoring using biomarkers [Citation3,Citation4]. The shift towards more interactive and dynamic, and therefore more complex, clinical treatment processes is associated with challenges not only regarding the delivery of health care, but also regarding the health economic evaluation of medical technologies [Citation5].
For instance, the use of randomized controlled trials (RCTs) for collecting evidence, and to inform health economic evaluations, is increasingly being questioned in a PM context [Citation5–Citation9]. RCTs are designed to draw conclusions on a population-level, while PM focusses on patient-level outcomes. The resulting data gaps characterize the increasingly common work setting in which evaluations of health care interventions need to be performed, initiating the need for accumulating other types of data, e.g. observational (big) data, or expert elicitation techniques [Citation10,Citation11]. Furthermore, new challenges occur with respect to analyzing trial data at the individual level and identifying and handling multiple subgroups [Citation11]. Therefore, health economic evaluation in the context of PM increasingly relies on modeling approaches and new methods, such as dynamic simulation modeling and the use of machine learning or other statistical approaches [Citation12].
However, PM also challenges the field of health economic modeling (HEM), as there is a need for models to accurately capture the interactions and dynamics present in personalized clinical processes [Citation13,Citation14]. Annemans et al. [Citation13] report on 10 methodological challenges that need to be considered when ‘designing and conducting robust model-based economic evaluations in the context of personalized medicine’. In general, these challenges raised by Annemans et al. focus towards the need to appropriately represent the dynamics of personalized treatment decisions with a wealth of diagnostics and surrounding uncertainty. More specifically, these challenges can mostly be translated into appropriate handling of the diagnostic performance of tests, combinations of tests, greater uncertainty due to more complex analysis, and data gaps. Phillips et al. [Citation15] complement these challenges by taking into account patients’ and physicians’ preferences, patients’ characteristics (such as age, gender, comorbidities, and medical history), and considering the impact of drug therapies and companion diagnostics simultaneously. Other challenges relate to the absence of implemented guidelines, criteria, and standards for the evaluation of new technologies in PM [Citation16,Citation17].
Given these methodological challenges, the appropriateness of the commonly used modeling method for HEM, i.e. Markov modeling, is being questioned [Citation14,Citation18], as this modeling approach may not be able to fully capture the complex treatment processes associated with PM [Citation19–Citation22]. Consequently, the use of more advanced modeling methods, such as discrete event simulation, agent based modeling, and system dynamics, might be more appropriate in this personalized context [Citation11,Citation23–Citation25].
It is unknown how current models address these challenges reported by experienced modelers and which modeling methods are being used to do so. Therefore, we assess the level of support for the methodological challenges described in literature by (1) identifying if and how the methodological challenges regarding modeling in the context of PM are being addressed, (2) exploring the different modeling approaches in PM in use to date, and (3) determining which alternative modeling methods may be appropriate to handle the specific issues in PM. Although several reviews have been published on modeling in PM [Citation26–Citation29], these focus on patient stratification using pharmacogenetics. We contribute to this literature by researching the challenges for HEM in PM in general, including patient stratification by other means than pharmacogenetics, e.g. the use of imaging technologies or risk stratification by patient characteristics.
2. Literature review
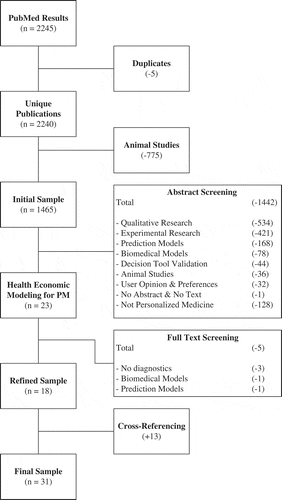
We performed a search in PubMed, employing primary search terms on Personalized Medicine and Precision Medicine combined with additional search terms on modeling and simulation, both in the title or the abstract of the publication. The search strategy was further specified by adding well-known key words used in health economics. The exact search algorithm can be found in Supplementary materials 1. The narrow primary search terms were required to include all sorts of patient stratification, e.g. pharmacogenetics, imaging technologies, and stratification by patient characteristics. No specific start date was applied and the search was updated until the 27 November 2015. The final sample was enriched by cross-referencing to include as many relevant publications as possible [Citation30].
To maintain the broad perspective of this review, and therefore prevent erroneous excluding of publications, only duplicates and animal studies were removed from the initial search results. The unique sample was first assessed based on title and abstract by one reviewer (KD). Next, screening the full text of the remaining publications resulted in inclusion into, or exclusion from, the final sample. Only publications relating to HEM studies in PM were included for full text screening. Publications were considered to meet the PM criterion when tests or prediction models were used to stratify patients into subgroups, for screening or targeting purposes, or when tests were used to monitor treatment effectiveness and thereby support patient-level treatment decisions. The refined sample was enriched by cross-referencing. Cross-references were included based on full text screening and until theoretical saturation was reached when inclusion of additional publications did not result in additional insights in a specific disease area, for example the screening for breast cancer. A second reviewer was consulted if it was unclear whether a publication should be included or excluded. The final decision to include or exclude a publication was made based on consensus between authors KD and HK. Reasons for exclusion were categorized into not mutually exclusive categories, as specified in Supplementary materials 2.
3. Scoring checklist representing the main challenges
In order to extract the data from the final sample, the general study characteristics and information on the used modeling method were summarized first. This includes the target disease, the description on how the treatment process was personalized, the used modeling method, the model structure, and the performed analyses. The treatment processes were characterized by the purpose of the stratification (screening, targeting, or monitoring) and whether the stratification was prognostic or predictive [Citation31]. Screening is referred to as the process of diagnosing a patient with a specific disease, whereas treatment targeting relates to selecting a treatment, from a set of treatment options that is expected to be most beneficial to a specific patient, based on patient-specific characteristics or diagnostic information. Treatment monitoring concerns the process of repeatedly assessing a patient’s response to the current treatment, in order to stop this treatment or switch to another treatment, if the patient is not benefiting from the current treatment.
Next, a checklist presenting the main methodological challenges for HEM in the context of PM was developed using the literature, where the publications by Annemans et al. [Citation13] and Phillips et al. [Citation15] served as the reference. After reviewing and classifying relevant papers, 10 different items in the checklist were used for the analysis of the final sample, based on the challenges derived from literature [Citation13,Citation14,Citation17,Citation20] (). The checklist was used to highlight the challenges in the final sample. For the first seven items in the checklist a positive result indicates that the authors addressed the challenge in the model, whereas for the remaining items eight to ten a positive result indicates that the authors identified that specific challenge and reported this in the publication. When a challenge was addressed or identified by the authors, this was scored as ‘+’, when challenges were not addressed or identified this was scored as ‘–’. If applicable, additional information on the extent to which challenges were addressed or mentioned was recorded.
Table 1. Checklist presenting the main challenges described in literature and used for analysis of the final sample. A positive score on challenges 1–7 indicates that the corresponding challenge is addressed in the model presented in the publication. A positive score in challenges 8–10 indicates that the authors identified the corresponding challenge and reported on the challenge in the publication. Negative scores indicate that the corresponding challenge is not addressed or identified and reported by the authors.
4. Results
The search strategy yielded 2245 publications on PubMed (). From this initial sample, five duplicates and 775 animal studies were excluded. The abstracts of all remaining 1465 publications were read and finally resulted in the exclusion of 1442 publications. These excluded publications were predominantly publications of a qualitative nature (n = 534, 37%) and experimental nature (n = 421, 29%). Several other excluded publications were publications on mathematical or statistical models developed to support medical decision-making (n = 168, 12%). A total of 128 publications were excluded from the final sample because they covered adjacent topics, such as personalized speaking tools or personalized computer systems (9%). The detailed reasons for exclusion are presented in Supplementary materials 2. After exclusion based on title and abstract, 23 publications remained. From these publications the full text was screened, resulting in five more exclusions from the sample for different reasons (i.e. papers presenting a biomedical model [Citation32], a prediction model [Citation33], or that did not include diagnostics or personalized risk estimations [Citation34–Citation36]). Analyzing the full text of the included articles resulted in an enrichment of the sample with 13 additional publications by cross-referencing. Many candidate cross-reference articles were not included due to the absence of diagnostics or personalized risk estimations [Citation37–Citation46]. Finally, a total of 31 publications were available for analysis [Citation47–Citation77].
The final sample concerned studies in various disease areas, including oncology (n = 17), cardiovascular disease (n = 5), human immunodeficiency virus (n = 2), hepatitis C virus (n = 2), Alzheimer’s disease (n = 1), neonatal disease (n = 1), rheumatoid arthritis (n = 1), depressive disorder (n = 1), and type 2 diabetes (n = 1) (). Most studies in the sample stratify patients for targeted therapy (n = 19, 61%), of which four studies combine treatment targeting with response monitoring and one article combines treatment targeting with screening. In total 13 studies stratify patients for screening purposes (42%). No articles used tests for response monitoring only. This indicates that patient stratification is used only for predictive purposes in 45% of the included publications (n = 14), for prognostic purposes in 39% of the included publications (n = 12), and for both predictive and prognostic purposes in 16% of the included publications (n = 5).
Table 2. Summary of the study characteristics of the final sample of publications.
The most frequently used modeling methods are decision tree modeling (n = 15, 48%) and Markov modeling (n = 12, 39%), which are often combined (n = 6, 19%). Other frequently used modeling methods are microsimulation modeling (n = 4, 13%), and mathematical modeling by equations (n = 3, 10%). Other observed modeling methods include partially observable Markov decision process (POMDP) modeling, deterministic dynamic compartment modeling (DDCM), and discrete event simulation (DES). Whereas decision trees, Markov models, and DDCM [Citation78] simulate cohorts of patients, POMDP modeling [Citation79], microsimulation modeling [Citation80], and DES [Citation81] are used for patient-level simulations. As presents, there is not only an increase in publications over time, but also an increase in the use of modeling methods other than decision tree analysis and Markov modeling. Regarding the motivation of the used modeling methods, one article mentions the straightforward interpretation of a decision tree as reason for its use [Citation49], whereas another article, using a Markov model, suggests that it may not be appropriate to model a screening process as a homogenous process [Citation59].
Table 3. Summary of the distribution of the publications and the use of cohort modeling3 or alternative modeling methods over time.
shows that not all methodological challenges as included in the checklist were systematically addressed or identified and reported in the final sample. The challenges regarding physicians’ preferences, disease-specific outcome measures, greater uncertainty, and absence of guidelines are addressed or identified and reported in at most five of the included publications. Yet, the challenges regarding the patients’ preferences, diagnostic performance, multiple tests, companion diagnostics, and the lack of evidence are more frequently addressed or identified and reported (in at least 10 publications). However, the latter result needs to be perceived in relative terms, as the extent to which challenges are addressed varies considerably between studies. For example, several studies modeled multiple tests, but assumed a fixed sequence of these tests. Although in practice, test sequences might be dynamic and thereby influence the diagnostic performance of the process as a whole. Furthermore, of the 19 publications in which a lack of evidence is identified, these data gaps are not purely caused by stratification in 37% of the cases (n = 7).
Table 4. Results of the analysis on whether the challenges for HEM in PM are addressed or identified and reported in the final sample of publications.
5. Conclusion
From the articles that were included, it can be concluded that patient stratification, using diagnostics or risk models, is mostly performed for treatment targeting or screening purposes. Only in few of the included publications diagnostics are used for treatment monitoring. Overall, the most frequently observed modeling methods are decision tree analysis and Markov modeling, which is in line with literature [Citation14,Citation18]. However, an increase in the use of more advanced modeling methods is observed. Finally, the results show that the methodological challenges for HEM in the context of PM described in literature are not (yet) frequently addressed or identified and reported.
6. Expert commentary
The findings from the literature study can have different implications. For instance, they may indicate that the impact of the challenges for HEM in PM is less severe than expected, that the challenges are hard to address and there is a lack of methods to overcome the challenges, or that we are still in an early stage of personalization and that the complexity of Personalized Medicine, therefore, is not yet a major issue. The observation that the most frequently used modeling methods are still cohort models may also indicate that we are still in a premature stage. Currently, it seems to be sufficient to stratify patients into relatively large subgroups, as there is also no regulatory incentive to further personalize these models.
However, treatment decisions are becoming increasingly complex, involving multiple biomarkers or panels of markers from next-generation sequencing to feed a sequence of clinical decisions. In this context, patient-level models are likely to become standard, as cohort models can no longer reflect the dynamic treatment processes in heterogeneous subgroups of individuals and may lead to biased estimates of the impact of new technologies. Representing the variation in patients’ clinical pathways is particularly problematic for cohort models, as this would either require numerous separate cohort models, representing all plausible sequences, or one very large model including all these sequences. In both cases, however, models will become substantially complex to manage and models’ cognitive ease will decrease dramatically. Conversely, more advanced modeling methods can represent the dynamics of individual pathways in a straightforward and more natural manner.
Policymakers will need to incentivize the use of appropriate modeling approaches to accurately represent clinical practice and accept that this might result in more complex health economic models, possibly at the expense of these models’ cognitive ease. It is necessary to accept this increase in complexity, as health economic models are likely to become biased and may lose their value in supporting decision-making when they are not matched with the dynamics and complexity of current and future clinical processes.
That the challenges are present, but may be hard to address using current approaches, is illustrated by the fact that many authors do recognize the challenges described in literature, but do not actually address them in the corresponding models. For example, the relevance of shared decision-making is highlighted in several publications, as authors argue the need for physicians to provide patients with personalized information on expected treatment outcomes and to involve these patients in decision-making [Citation36,Citation52,Citation70,Citation72,Citation75]. The observation that this interactive and complex decision-making process is not yet integrated into the corresponding models, however, illustrates the challenge to further personalize these models. Another example can be found in breast cancer screening, as individualization of the screening process is considered very important [Citation37,Citation82], whereas all included models are still cohort-based.
Although patient-level simulation seems appropriate, the present review insufficiently can conclude the usefulness of modeling techniques beyond cohort models to adequately represent personalized clinical processes. Yet, it seems obvious that modeling patients on an individual level is desirable and advanced methods, such as microsimulation modeling and discrete event simulation, may be essential. However, to utilize the full potential of these advanced modeling methods, more evidence, for example on subgroup-specific event rates, costs, and quality of life, may be required compared to less advanced methods. Since the results show that evidence gaps are a frequently experienced challenge, the use of advanced methods may therefore not always be feasible to address the challenges associated with PM.
The results of this study provide insights into the level of support in modeling practice for the methodological challenges described in literature. These findings can be used to focus development of a framework for HEM in this context, as they indicate which challenges require additional guidance the most. Additional further research is recommended to investigate the impact of the challenges for HEM in PM when truly personalized clinical processes are being modeled and whether this will affect decisions made by policy makers. Furthermore, whether the use of advanced modeling methods indeed can solve at least some of these challenges, needs further investigation while being weighed against the increased complexity of these models.
Our study has certain limitations. First of all, it is interesting to note that the number of papers that were finally selected from the initial search results is relatively low, whereas PM is a growing field of research and the health economic issues related to various targeted drugs receive global recognition. This might be partly caused by the strict inclusion criteria required for the research objectives and by the fact that we only consulted one database. However, we minimalized the effect of this limitation by cross-referencing and found that the results for the publications obtained by cross-referencing to be in line with the publications in the initial sample. Furthermore, underreporting may have resulted in the absence of mentioned challenges for HEM in PM and the lack of motivation for the used modeling methods. This also relates to the limited space that is available in peer-reviewed journals for reporting methods and results.
7. Five-year view
The continuing personalization of clinical pathways highlights the need for using more advanced modeling methods to accurately represent the complex context of clinical practice and, therefore, to be meaningful for supporting decision-making. If not already, regulatory agencies will need to critically review the modeling methods that are being used to translate clinical practice into health economic models, initiating the need for using appropriate modeling methods. Furthermore, efforts to illustrate and guide the use of more advanced modeling methods, such as discrete event simulation and system dynamics modeling, will strengthen and spread the knowledge base of these methods, increasing their use for the evaluation of health care interventions, also by clinical experts. Finally, the pharmacoeconomic and clinical communities will realize that using more advanced modeling methods does not necessarily result in more complex models. On the contrary, using more advanced modeling methods will enable models to continue being valuable for what they are needed for, which is supporting decisions in an increasingly complex environment.
Key issues
Health economic models in personalized medicine are to a large extend based on cohorts of patients instead of individual patients.
Some of the specifics of Health Economic Modeling in Personalized Medicine, such as patient-level models and the representation of the dynamics and sequence of preference-sensitive clinical decisions, are still not addressed appropriately.
This may indicate that the impact of these challenges is less severe than expected, that the challenges are hard to address and there is a lack of methods to overcome the challenges, or that we are still in an early stage of personalization and that the complexity of Personalized Medicine, therefore, is not yet a major issue.
Further research should focus on identifying the extent of these challenges when truly personalized processes are being modeled and the added value of advanced modeling methods in this context.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Contribution statement
The research design, including the search strategy and inclusion criteria, was developed as a combined effort of all authors. The search and primary analysis was performed by KD in close cooperation with HK and under the supervision of MIJ. The initial manuscript was drafted by KD and revised by HK and MIJ. The overall guarantor of this study is MIJ.
Supplementary_Materials.zip
Download Zip (20.6 KB)Supplemental Material
Supplemental data for this article can be accessed here.
References
- Schleidgen S, Klingler C, Bertram T, et al. What is personalized medicine: sharpening a vague term based on a systematic literature review. BMC Med Ethics. 2013;14:1–12.
- Van Til JA, IJzerman MJ. Why should regulators consider using patient preferences in benefit-risk assessment?. Pharmacoeconomics. 2014;32:1–4.
- Gheita TA, Gheita HA, Kenawy SA. The potential of genetically guided treatment in Behçet’s disease. Pharmacogenomics. 2016;17:1165–1174.
- Liu Y, Hegde P, Zhang F, et al. Prostate cancer – a biomarker perspective. Front Endocrinol (Lausanne). 2012;3:72.
- Towse A, Garrison LP Jr. Economic incentives for evidence generation: promoting an efficient path to personalized medicine. Value in Health: the Journal of the International Society for Pharmacoeconomics and Outcomes Research. 2013;16:S39–43.
- Booth CM, Tannock IF. Randomised controlled trials and population-based observational research: partners in the evolution of medical evidence. Br J Cancer. 2014;110:551–555.
- Ferrante Di Ruffano L, Davenport C, Eisinga A, et al. A capture-recapture analysis demonstrated that randomized controlled trials evaluating the impact of diagnostic tests on patient outcomes are rare. J Clin Epidemiol. 2012;65:282–287.
- Horgan D, Lawler M, Brand A. Getting Personal: accelerating Personalised and Precision Medicine Integration into Clinical Cancer Research and Care in Clinical Trials. Public Health Genomics. 2015;18:325–328.
- Lawler M, Sullivan R. Personalised and Precision Medicine in Cancer Clinical Trials: panacea for Progress or Pandora’s Box?. Public Health Genomics. 2015;18:329–337.
- Crown WH. Potential application of machine learning in health outcomes research and some statistical cautions. Value in Health. 2015;18:137–140.
- IJzerman MJ, Manca A, Keizer J, et al. Implementation of comparative effectiveness research in personalized medicine applications in oncology: current and future perspectives. Comp Effectiveness Res. 2015;5:65.
- Marshall DA, Burgos-Liz L, Pasupathy KS, et al. Transforming Healthcare Delivery: integrating Dynamic Simulation Modelling and Big Data in Health Economics and Outcomes Research. PharmacoEconomics. 2015;34:115–126.
- Annemans L, Redekop K, Payne K. Current methodological issues in the economic assessment of personalized medicine. Value in Health: the Journal of the International Society for Pharmacoeconomics and Outcomes Research. 2013;16:S20–6.
- Miller JD, Foley KA, Russell MW. Current Challenges in Health Economic Modeling of Cancer Therapies: A Research Inquiry. American Health Drug Benefits. 2014;7:153–162.
- Phillips KA, Liang S-Y, Van Bebber S, et al. To the translation of genomic information into clinical practice and health policy: utilization, preferences, and economic value. Curr Opin Mol Ther. 2008;10:260–266.
- Ginsburg GS, Kuderer NM. Comparative effectiveness research, genomics-enabled personalized medicine, and rapid learning health care: a common bond. J Clinical Oncology: Official Journal Am Soc Clin Oncol. 2012;30:4233–4242.
- Postma MJ, Boersma C, Vandijck D, et al. Health technology assessments in personalized medicine: illustrations for cost–effectiveness analysis. Expert Rev Pharmacoecon Outcomes Res. 2011;11:367–369.
- Sailer AM, Van Zwam WH, Wildberger JE, et al. Cost-effectiveness modelling in diagnostic imaging: a stepwise approach. Eur Radiol. 2015;25:3629–3637.
- Ferrusi IL, Leighl NB, Kulin NA. Marshall DA. Do economic evaluations of targeted therapy provide support for decision makers? . J Oncol Practice.. 2011;7:36s–45s.
- Golubnitschaja O, Yeghiazaryan K, Costigliola V, et al. Risk assessment, disease prevention and personalised treatments in breast cancer: is clinically qualified integrative approach in the horizon?. Epma J. 2013;4:6.
- Hulme C, Browne C, Mansfield J, et al. Determining cost-effectiveness of advanced cancer care: a systematic review of economic models. BMJ Support Palliat Care. 2011;1:A21–A2.
- Mullins CD, Montgomery R, Tunis S. Uncertainty in assessing value of oncology treatments. Oncologist. 2010;15(Suppl 1):58–64.
- Lee KA, Dziadkowiec O, Meek P. A systems science approach to fatigue management in research and health care. Nurs Outlook. 2014;62:313–321.
- Marshall DA, Burgos-Liz L, IJzerman MJ, et al. Applying dynamic simulation modeling methods in health care delivery research—the SIMULATE checklist: report of the ISPOR simulation modeling emerging good practices task force. Value in Health. 2015;18:5–16.
- Marshall DA, Burgos-Liz L, IJzerman MJ, et al. Selecting a dynamic simulation modeling method for health care delivery research-part 2: report of the ISPOR dynamic simulation modeling emerging good practices task force. Value in Health: the Journal of the International Society for Pharmacoeconomics and Outcomes Research. 2015;18:147–160.
- Beaulieu M, De Denus S, Lachaine J. Systematic review of pharmacoeconomic studies of pharmacogenomic tests. Pharmacogenomics. 2010;11:1573–1590. Epub 2010/12/03
- Phillips KA, Van Bebber SL. A systematic review of cost-effectiveness analyses of pharmacogenomic interventions. Pharmacogenomics. 2004;5:1139–1149. Epub 2004/12/09
- Plumpton CO, Roberts D, Pirmohamed M, et al. A systematic review of economic evaluations of pharmacogenetic testing for prevention of adverse drug reactions. PharmacoEconomics. 2016;34:1–23.
- Verhoef TI, Redekop WK, Darba J, et al. A systematic review of cost-effectiveness analyses of pharmacogenetic-guided dosing in treatment with coumarin derivatives. Pharmacogenomics. 2010;11:989–1002. Epub 2010/07/07
- Wolfswinkel JF, Furtmueller E, Wilderom CPM. Using grounded theory as a method for rigorously reviewing literature. Eur J Inf Syst. 2013;22:45–55.
- Nalejska E, Mączyńska E, Lewandowska MA. Prognostic and predictive biomarkers: tools in personalized oncology. Mol Diagn Ther. 2014;18:273–284.
- Peer X, An G. Agent-based model of fecal microbial transplant effect on bile acid metabolism on suppressing Clostridium difficile infection: an example of agent-based modeling of intestinal bacterial infection. J Pharmacokinet Pharmacodyn. 2014;41:493–507.
- Schubert KO, Clark SR, Baune BT. The use of clinical and biological characteristics to predict outcome following First Episode Psychosis. Aust N Z J Psychiatry. 2015;49:24–35.
- Guerriero C, Cairns J, Roberts I, et al. The cost-effectiveness of smoking cessation support delivered by mobile phone text messaging: txt2stop. Eur J Health Econ. 2013;14:789–797.
- Lee T, Biddle AK, Lionaki S, et al. Personalized prophylactic anticoagulation decision analysis in patients with membranous nephropathy. Kidney Int. 2014;85:1412–1420.
- Soeteman DI, Stout NK, Ozanne EM, et al. Modeling the effectiveness of initial management strategies for ductal carcinoma in situ. J Natl Cancer Inst. 2013;105:774–781.
- Al-Badriyeh D, Slavin M, Liew D, et al. Pharmacoeconomic evaluation of voriconazole versus posaconazole for antifungal prophylaxis in acute myeloid leukaemia. J Antimicrob Chemother. 2010;65:1052–1061.
- Cutler CS, Lee SJ, Greenberg P, et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood. 2004;104:579–585.
- Desai K, Sansom SL, Ackers ML, et al. Modeling the impact of HIV chemoprophylaxis strategies among men who have sex with men in the United States: HIV infections prevented and cost-effectiveness. Aids. 2008;22:1829–1839.
- Herman WH, Hoerger TJ, Brandle M, et al. for the Diabetes Prevention Program Research G. The cost-effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance.. Ann Intern Med. 2005;142:323–332.
- Hertenstein B, Heil G, Heimpel H. Allogenic bone marrow transplantation of chemotherapy for patients with acute myeloid leukemia in first complete remission: a decision analysis approach. Ann Hematol. 1996;72:223–230.
- Kasuya M, Meguro K. Health economic effect of donepezil treatment for CDR 0.5 converters to Alzheimer’s disease as shown by the Markov model. Arch Gerontol Geriatr. 2010;50:295–299.
- Kurosawa S, Yamaguchi T, Miyawaki S, et al. A Markov decision analysis of allogeneic hematopoietic cell transplantation versus chemotherapy in patients with acute myeloid leukemia in first remission. Blood. 2011;117:2113–2120.
- Lee SJ, Kuntz KM, Horowitz MM, et al. Unrelated donor bone marrow transplantation for chronic myelogenous leukemia: a decision analysis. Ann Intern Med. 1997;127:1080–1088.
- Paltiel AD, Freedberg KA, Scott CA, et al. HIV preexposure prophylaxis in the United States: impact on lifetime infection risk, clinical outcomes, and cost-effectiveness. Clin Infect Dis. 2009;48:806–815.
- Rein DB, Smith BD, Wittenborn JS, et al. The cost-effectiveness of birth-cohort screening for hepatitis C antibody in U.S. primary care settings. Ann Intern Med. 2012;156:263–270.
- Ayer T, Alagoz O, Stout NK. OR Forum—A POMDP approach to personalize mammography screening decisions. Oper Res. 2012;60:1019–1034.
- Carles M, Vilaprinyo E, Cots F, et al. Cost-effectiveness of early detection of breast cancer in Catalonia (Spain). BMC Cancer. 2011;11:192.
- Chen A, Dowdy DW, Garcia-Lerma JG. Clinical effectiveness and cost-effectiveness of HIV pre-exposure prophylaxis in men who have sex with men: risk calculators for real-world decision-making. Plos One. 2014;9:e108742.
- Djalalov S, Yong J, Beca J, et al. Genetic testing in combination with preventive donepezil treatment for patients with amnestic mild cognitive impairment: an exploratory economic evaluation of personalized medicine. Mol Diagn Ther. 2012;16:389–399.
- Eckman MH, Rosand J, Greenberg SM, et al. Cost-effectiveness of using pharmacogenetic information in warfarin dosing for patients with nonvalvular atrial fibrillation. Ann Intern Med. 2009;150:73–83.
- Ferket BS, Van Kempen BJH, Heeringa J, et al. Personalized prediction of lifetime benefits with statin therapy for asymptomatic individuals: a modeling study. Plos Med. 2012;9:e1001361.
- Greeley SAW, John PM, Winn AN, et al. The cost-effectiveness of personalized genetic medicine: the case of genetic testing in neonatal diabetes. Diabetes Care. 2011;34:622–627.
- Guzauskas GF, Hughes DA, Bradley SM, et al. A risk-benefit assessment of prasugrel, clopidogrel, and genotype-guided therapy in patients undergoing percutaneous coronary intervention. Clin Pharmacol Ther. 2012;91:829–837.
- Handorf EA, McElligott S, Vachani A, et al. Cost effectiveness of personalized therapy for first-line treatment of stage IV and recurrent incurable adenocarcinoma of the lung. J Oncol Pract. 2012;8:267–274.
- Hochheiser LI, Juusola JL, Monane M, et al. Economic utility of a blood-based genomic test for the assessment of patients with symptoms suggestive of obstructive coronary artery disease. Popul Health Manag. 2014;17:287–296.
- Juusola JL, Brandeau ML, Owens DK, et al. The cost-effectiveness of preexposure prophylaxis for HIV prevention in the United States in men who have sex with men. Ann Intern Med. 2012;156:541–550.
- Kievit W, De Bruin JH, Adang EM, et al. Cost effectiveness of a new strategy to identify HNPCC patients. Gut. 2005;54:97–102.
- Kobayashi T, Goto R, Ito K, et al. Prostate cancer screening strategies with re-screening interval determined by individual baseline prostate-specific antigen values are cost-effective. Eur J Surg Oncol. 2007;33:783–789.
- Krieckaert CL, Nair SC, Nurmohamed MT, et al. Personalised treatment using serum drug levels of adalimumab in patients with rheumatoid arthritis: an evaluation of costs and effects. Ann Rheum Dis. 2015;74:361–368.
- Ladabaum U, Wang G, Terdiman J, et al. Strategies to identify the Lynch syndrome among patients with colorectal cancer: a cost-effectiveness analysis. Ann Intern Med. 2011;155:69–79.
- Ladapo JA, Jaffer FA, Hoffmann U, et al. Clinical outcomes and cost-effectiveness of coronary computed tomography angiography in the evaluation of patients with chest pain. J Am Coll Cardiol. 2009;54:2409–2422.
- Leunis A, Redekop WK, Van Montfort KA, et al. The development and validation of a decision-analytic model representing the full disease course of acute myeloid leukemia. Pharmacoeconomics. 2013;31:605–621.
- Lieberthal RD, Dudash K, Axelrod R, et al. An economic model to value companion diagnostics in non-small-cell lung cancer. Per Med. 2013;10:139–147.
- De Lima Lopes G Jr., Segel JE, Tan DS, et al. Cost-effectiveness of epidermal growth factor receptor mutation testing and first-line treatment with gefitinib for patients with advanced adenocarcinoma of the lung. Cancer. 2012;118:1032–1039.
- Liu S, Schwarzinger M, Carrat F, et al. Cost effectiveness of fibrosis assessment prior to treatment for chronic hepatitis C patients. Plos One. 2011;6:e26783.
- Mvundura M, Grosse SD, Hampel H, et al. The cost-effectiveness of genetic testing strategies for Lynch syndrome among newly diagnosed patients with colorectal cancer. Genet Med. 2010;12:93–104.
- O’Donoghue C, Eklund M, Ozanne EM, et al. Aggregate cost of mammography screening in the United States: comparison of current practice and advocated guidelines. Ann Intern Med. 2014;160:145.
- Perlis RH, Patrick A, Smoller JW, et al. When is pharmacogenetic testing for antidepressant response ready for the clinic? A cost-effectiveness analysis based on data from the STAR*D study. Neuropsychopharmacology. 2009;34:2227–2236.
- Petta S, Cabibbo G, Enea M, et al. Group WEFs. Personalized cost-effectiveness of boceprevir-based triple therapy for untreated patients with genotype 1 chronic hepatitis C. Dig Liver Dis. 2014;46:936–942.
- Ramsey SD, Burke W, Clarke L. An economic viewpoint on alternative strategies for identifying persons with hereditary nonpolyposis colorectal cancer. Genet Med. 2003;5:353–363.
- Van Ravesteyn NT, Miglioretti DL, Stout NK, et al. What level of risk tips the balance of benefits and harms to favor screening mammography starting at age 40?. Ann Intern Med. 2012;156:609–617.
- Reyes CM, Allen BA, Terdiman JP, et al. Comparison of selection strategies for genetic testing of patients with hereditary nonpolyposis colorectal carcinoma: effectiveness and cost-effectiveness. Cancer. 2002;95:1848–1856.
- Schousboe JT, Kerlikowske K, Loh A, et al. Personalizing mammography by breast density and other risk factors for breast cancer: analysis of health benefits and cost-effectiveness. Ann Intern Med. 2011;155:10–20.
- Sullivan SD, Garrison LP Jr., Rinde H, et al. Cost-effectiveness of risk stratification for preventing type 2 diabetes using a multi-marker diabetes risk score. J Med Econ. 2011;14:609–616.
- Sung L, Buckstein R, Doyle JJ, et al. Treatment options for patients with acute myeloid leukemia with a matched sibling donor: a decision analysis. Cancer. 2003;97:592–600.
- Vilaprinyo E, Forné C, Carles M, et al. Interval Cancer Study G. Cost-effectiveness and harm-benefit analyses of risk-based screening strategies for breast cancer. Plos One. 2014;9:e86858.
- Kermack WO, McKendrick AGA. Contribution to the mathematical theory of epidemics. Proceedings of the Royal Society of London A: Mathematical, Physical and Engineering Sciences; 1927;115:700–721.
- Kaelbling LP, Littman ML, Cassandra AR. Planning and acting in partially observable stochastic domains. Artif Intell. 1998;101:99–134.
- Zucchelli E, Jones AM, TRice N The evaluation of health policies through microsimulation methods. Health, Econometrics and Data Group (HEDG) Working Papers., 2010.
- Karnon J, Stahl J, Brennan A, et al. Force I-SMGRPT. Modeling using discrete event simulation: a report of the ISPOR-SMDM modeling good research practices task force–4.. Value in Health: the Journal of the International Society for Pharmacoeconomics and Outcomes Research. 2012;15:821–827.
- Gail M, Rimer B. Risk-based recommendations for mammographic screening for women in their forties. J Clinical Oncology: Official Journal Am Soc Clin Oncol. 1998;16:3105–3114. Epub 1998/09/17