ABSTRACT
Background: Drug-resistant tuberculosis (DR-TB) continues to be a major public health challenge with suboptimal treatment outcomes including well-documented treatment-related toxicities. We compared the cost-effectiveness of bedaquiline (BDQ) containing regimens with injectable containing regimens (short-course regimen [SCR] and long-course regimen [LCR]) in India, Russia, and South Africa.
Methods: The analysis evaluated the direct costs of DR-TB treatment which included drugs, hospitalization, injectable-related adverse event costs, and other costs. Scenarios altered regimen costs, SCR/LCR ratio, and substitution rate between regimens (whether BDQ or injectable containing).
Results: BDQ containing regimens are more cost effective based on cost per treatment success compared with injectable containing regimens, reducing these in SCR by 18–20% and in LCR by 49–54%. Average cost effectiveness ratios (ACERs) of BDQ containing regimens are lower. The incremental cost effectiveness ratio (ICER) is negative. Exclusive use of BDQ containing regimens results in approximately 61,000 more patients treated successfully over 5 years.
Conclusions: Across all countries, BDQ containing regimens are dominant compared to injectable containing regimens, entailing lower treatment costs to achieve better clinical outcomes. This analysis can provide insight and support to local and global decision-makers and public health organizations to allocate efficiently resources improving patient and public health outcomes.
1. Introduction
Tuberculosis (TB) is the leading global cause of death due to an infectious agent [Citation1]. In 2016, of the 490,000 people estimated to have developed multidrug-resistant (MDR) TB and extremely drug-resistant (XDR) TB, collectively referred to as drug-resistant (DR) TB in this study, only 22% started on treatment, reporting low treatment success (54%) [Citation1–Citation3].
To address this, the World Health Organization (WHO) endTB Strategy specifically calls out the need for optimal use of current and new tools, including new drugs and new treatment regimens [Citation4]. In 2016, the WHO published new guidelines on the treatment of drug-resistant tuberculosis (DR-TB), conditionally recommending a short-course (SCR) regimen lasting 9–11 months, in addition to the existing long-course (LCR) regimen that lasts 20–24 months [Citation5,Citation6]. Both regimens require the use of second-line injectable drugs that have well-documented severe toxicities (hearing loss and kidney failure) [Citation7–Citation13].
Increasingly more countries are developing ambitious implementation plans to achieve the set endTB target of optimal use of current and new tools, specifically looking at replacing second-line injectables with innovative drugs within DR-TB treatment regimens in programmatic settings. Such efforts require a parallel comprehensive determination of comparative regimen-specific outcomes and associated costs.
Bedaquiline (BDQ) was approved in 2012 as the first ever drug to treat DR-TB, the first new TB drug in over 40 years [Citation14,Citation15] and is currently indicated for the treatment of pulmonary MDR-TB [Citation16] . As of June 2018, 105 countries (including 29 of the 30 WHO designated high MDR-TB burden countries) have included BDQ into their DR-TB treatment [Citation17]. From a policy perspective, governments have increasingly recognized the utility of BDQ in the treatment of DR-TB in programmatic settings [Citation18–Citation22]. Both clinical trials and recent real-world evidence reviews and studies have reported BDQ’s clinical effectiveness in the treatment of DR-TB with consistently high sputum culture conversion rates and improved outcomes across a broad range of patient populations [Citation23–Citation30].
Importantly, the South African government has recently taken a decision to make BDQ available to all eligible DR-TB patients while shifting at the same time to injection-free DR-TB regimens overall [Citation31].
Unique to this study, it was of interest to investigate pragmatically the cost per treatment success of different DR-TB treatment regimens to build a contextual understanding of the cost-effectiveness profile of BDQ-containing regimens. This approach should support governments, local and global decision-makers, and public health organizations in efficiently allocating resources to improve patient and public health outcomes.
The analysis includes defining direct medical costs associated with BDQ-containing regimens compared to existing DR-TB standard-of-care injectable-containing regimens for SCR and LCR in select WHO designated high-burden countries: India, Russia, and South Africa. These countries were chosen because they embody diverse healthcare settings with unique DR-TB epidemiology profiles, are more broadly representative of the region they are part of, and account for 52% of the global DR-TB burden [Citation1].
2. Methods
2.1. Analysis development
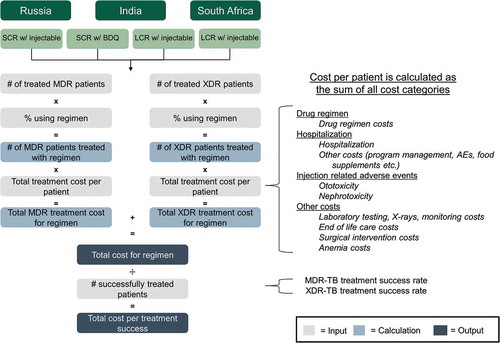
An analysis was developed to compare the cost-effectiveness of BDQ-containing SCRs and LCRs with that of injectable-containing SCR and LCR from a cost per treatment success perspective (calculation overview in ). In the costing, we only considered direct costs. A health provider annual cost perspective as well as a 5-year time horizon perspective were employed, looking at a base case and three scenarios.
Broadly, the model is structured as a sum of direct costs related to treatment of DR-TB drugs, hospitalization, injection-related AEs, and ‘other’ costs. For each cost category, average cost per treated patient for SCR and LCR, with either BDQ or injectable-containing regimens, was obtained from the published literature and multiplied by number of treated patients. The total cost per successfully treated patient was determined by dividing total cost by the number of successfully treated patients. This calculation was performed for each of the four regimens, for each of the three countries. Total treatment cost was obtained by summing the total costs across all cost categories.
2.2. Analysis inputs for base case
Numbers of DR-TB treated patients and costs of treatment were obtained from published literature and reports including WHO reports, Global Drug Facility (GDF) reports, and National TB Program reports [Citation1,Citation5,Citation19,Citation22,Citation30,Citation32–Citation44]. These data were used to derive analysis inputs based on the assumptions listed in . Definitions used in developing the analysis are listed in Supplementary Table 1.
Table 1. Model assumptions used to derive analysis inputs.
Direct costs captured included costs associated with drugs, hospitalization (including the actual hospitalization costs and costs associated with program management, adverse events (AEs), and nutrition supplements related to hospitalization), injection-related AEs (ototoxicity and nephrotoxicity), and ‘other’ costs (laboratory testing, X-rays, and monitoring, anemia management, surgical intervention, and end-of-life care).
A conservative approach was undertaken in defining regimen composition (Supplementary Table 2) and treatment length based on international and national guidelines. Drugs included in LCR assumed a standard mix for conventional MDR-TB treatment regimen composition as opposed to a fully active XDR-TB treatment regimen composition [Citation45]. Treatment length utilized in calculations linked to SCRs was 9–10 months, and only 18 months for LCRs. Notably, although Russia has already included BDQ in LCRs [Citation33], we excluded it from the LCR to compare differences in cost-effectiveness between BDQ-containing LCRs and injectable-containing LCRs.
Costs of treating XDR-TB were derived by scaling-up cost of treating MDR-TB costs as listed in . Pre-XDR-TB costs were not called out separately but modeled in a consistent manner to LCR values. Previous analyses have estimated that XDR-TB is approximately three to four times costlier to treat than MDR-TB [Citation32,Citation39]. Given the difference in time, country, and costs between these studies, it is notable that the relative cost ratio of XDR-TB to MDR-TB is consistent. Conservatively, we assumed a threefold scale-up factor for associated XDR-TB costs, relative to MDR-TB.
Table 2. Determination of costs of treatment of XDR-TB.
Furthermore, in the base case, we modeled an even split between injectable-containing regimens and BDQ-containing regimens across both SCR and LCR to more accurately compare their relative cost-effectiveness.
All costs were either adjusted for inflation to 2018 prices or adjusted for different countries based on gross domestic product based on purchasing power parity (PPP) using conversion rates published by the Organization for Economic Co-operation and Development (OECD) [Citation46]. All currency values reported are in United States dollars (USD).
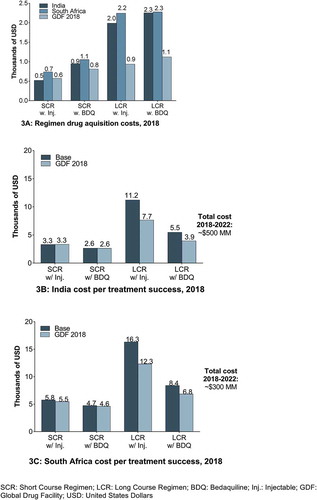
Drug regimen costs were determined from publicly available sources [Citation34,Citation35,Citation37,Citation38]. GDF prices were obtained from listed GDF pricing and local country pricing was obtained from publicly available local tender pricing data (). In South Africa, base case regimen pricing used was from 2017 GDF pricing as there is no public domain reference of local tender pricing [Citation34]. Based on local expert opinion input, South African local tender pricing is in line with GDF pricing and evolution trends. In India, based on local expert opinion input, the base case scenario assumes that there is an equal 50/50 split of regimens procured via GDF and via local tenders. The background consideration to this being drug quality assurance concerns [Citation47,Citation48]. As part of the drug regimen cost inputs, a BDQ drug acquisition cost assumption, proportionally matching the recently announced GDF regimen savings [Citation49] with a non-tiered $600 BDQ price, was included in calculations for India and South Africa. Of note, this is different from the 2017 BDQ tiered pricing of $900 and $3,000 available via GDF.
Hospitalization cost per patient, obtained from publicly available peer-reviewed literature [Citation19,Citation22,Citation39], is calculated by dividing total hospitalization costs by total treated patients, agnostic of number of patients hospitalized (). Therefore, our regimen-specific hospitalization costs already include differences in time to sputum culture conversion, treatment success rate, and hospitalization rate, which are the cause of lower hospitalization costs for BDQ-containing regimens compared to injectable-containing regimens. BDQ-containing LCR hospitalization cost per patient is 33% lower than injectable-containing LCRs in Russia, 34% lower in India, and 25% lower in South Africa. Similar cost reduction due to BDQ, despite differences in treatment paradigm and healthcare infrastructure in these countries, indicates BDQ has a relatively consistent effect on hospitalization cost. In addition, given that most hospitalization data in the literature evaluates LCRs, we scaled down LCR hospitalization costs based on treatment duration to derive SCR hospitalization costs, keeping BDQ effect proportional.
Costs associated with management of ototoxicity were calculated as the sum of per patient management costs and cost of switching to a BDQ -containing regimen (). Switching to a BDQ-containing regimen is expected to be a significant portion of ototoxicity cost associated with injectable-containing regimens, as patients will no longer be able to continue the injectable-containing regimen [Citation40]. To calculate this cost of switching, we added the incremental cost of initiating a new BDQ-containing regimen, while subtracting the cost of the injectable-containing regimen for the duration not utilized (e.g. number of months not utilized of the full regimen treatment length, assuming injectable-related AEs on average would lead to need for discontinuation at the 2-month mark from initiation).
References used for treatment success rate inputs into the analysis were as follows: for injectable-containing SCRs, the recently released STREAM Stage 1 preliminary results of ~80% favorable outcomes [Citation50] and for injectable-containing LCRs, the WHO Global TB Report 2017 overall treatment success rate of 54% [Citation1]. For BDQ-containing LCRs, the treatment success rate calculated in the 2017 WHO meta-analysis (n = 537) of 79.7% sputum culture conversion rate at 6-months (95% confidence interval 75.2% to 83.5%) [Citation51] was used as the closest indicator to results in programmatic settings. For BDQ-containing SCRs, the assumption of a 5% increment on current STREAM SCR treatment success rate [Citation50] was derived based on predictive modeling. Achievement of treatment success rates higher than this is likely to be stifled by mortality and loss to follow-up, the sum of the two being assumed to top 15% under the best of circumstances in the settings being considered.
2.3. Analysis outputs
Cost-effectiveness was assessed by deriving comparative costs per treatment success in 2018 for BDQ-containing regimens and injectable-containing regimens, with the costs being split into four categories (drug regimen, hospitalization, injection-related AEs, and other costs). Cost per treatment success without ototoxicity was also calculated as a subset of the overall cost per treatment success (which was irrespective of ototoxicity). Total DR-TB treatment cost across all regimens (2018–2022) was calculated for each country, and a final analysis was run to quantity comparative the average cost-effectiveness ratios (ACERs).
2.4. Scenarios
Due to inherent variability around some factors which affect DR-TB treatment costs and therefore BDQ-containing regimens’ potential cost-effectiveness, scenarios were built that changed the following inputs: regimen price, choice of injectable used in the DR-TB regimen, SCR/LCR ratios, and rate at which injectable-containing regimens are substituted for BDQ-containing regimens.
Three scenarios () were analyzed in addition to the base case, in order to quantify BDQ-containing regimen impact and further understand economic ramifications: (i) use of lower GDF pricing announced in June 2018 in India and South Africa [Citation34]; (ii) shifting to National Strategic Plan (NSP) prevalence with local procurement and capreomycin in India [Citation35,Citation52]; and (iii) total conversion from injectable-containing regimens to BDQ-containing regimens in all countries.
Table 3. Scenarios included in the analysis.
3. Results
BDQ-containing SCRs are more cost-effective based on cost per treatment success in 2018 compared with injectable-containing SCRs, lowering costs by $659 in India and by $1,022 in South Africa ()). BDQ-containing LCRs reduce the costs per treatment success in 2018 by $106,480 in Russia, by $5,751 in India, and by $7,915 in South Africa, when compared with injectable-containing LCRs ()).
Figure 2. (A) Short-course (SCR) regimen cost per treatment success, 2018. (B) Long-course (LCR) regimen cost per treatment success, 2018. (C) ACER of BDQ-containing regimens relative to ACER of injectable-containing regimens, 2018.
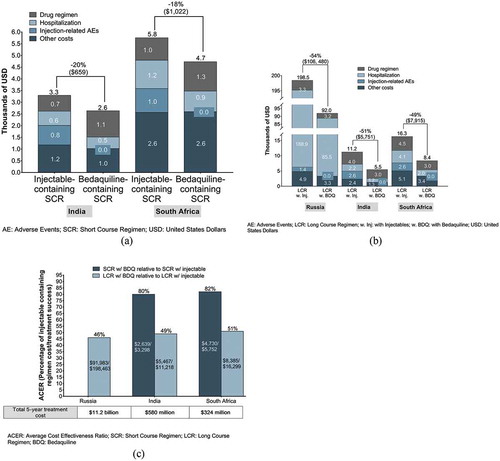
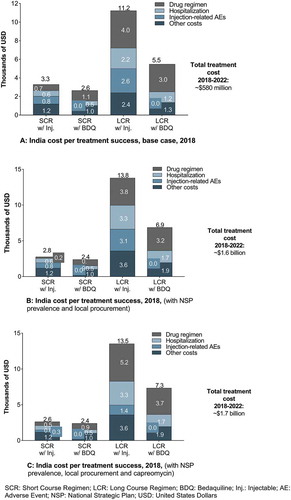
Critically, from a health provider perspective, in BDQ-containing SCRs, the cost per treatment success without ototoxicity improves by $3,147 and $5,361 for India and South Africa, respectively, representing savings of more than 50% (). In addition, in BDQ-containing LCRs, cost per treatment success without ototoxicity provides savings of $147,129, $20,211, and $14,214 for Russia, South Africa, and India, respectively, representing savings between 62% and 72% ().
Table 4. SCR and LCR cost per treatment success without ototoxicity 2018.
Total 5-year costs for health providers are ~$11.2 billion, ~$580 million, and ~$324 million for Russia, India, and South Africa, respectively ()). This is calculated as cost per treatment success multiplied by the total number of treated patients.
We compared the ACERs, defined as the cost per treatment success, of BDQ-containing SCRs and LCRs in the three countries for 2018. The ACERs of BDQ-containing regimens were calculated as a percentage of the ACERs of injectable-containing regimens in the respective countries ()).
BDQ-containing SCRs provide lower ACERs (80% and 82% in India and South Africa, respectively, of injectable-containing SCRs). In addition, BDQ-containing LCRs also provide lower ACERs (46%, 49%, and 51% in Russia, India, and South Africa, respectively, of injectable-containing LCRs).
Notably, despite differences in healthcare infrastructure and standards of care, the cost-effectiveness of BDQ-containing regimens as a percentage of the cost-effectiveness of injectable-containing regimens remains similar across countries.
3.1. Scenario 1: reduction in GDF pricing in India and South Africa
The GDF announced new lower prices in June 2018 for injectable-containing SCRs ($571) and injectable-containing LCRs ($812) ()).
In India ()), the new GDF pricing lowers the cost per treatment success for BDQ-containing LCRs to $3,950 (28% reduction) and for injectable-containing LCRs to $7,657 (32% reduction). For SCR, cost per treatment success for BDQ-containing regimens is $2,627 and $3,329 for injectable-containing regimens. Overall 5-year treatment cost is reduced from ~$580M to ~$500M, driven by a reduction in LCR costs.
In South Africa ()), the cost per treatment success is $12,309 with injectable-containing LCRs (24% reduction) and $6,833 with BDQ-containing LCRs (18% reduction). For SCR, the cost per treatment success moves to $5,454 for injectable-containing SCRs and to $4,588 for BDQ-containing regimens (5% and 3% reductions, respectively). Overall 5-year treatment cost is reduced from ~$324M to ~$300M, driven by a reduction in LCR costs.
Overall, BDQ-containing SCR and LCRs remain cost-effective in a scenario of lower GDF regimen prices, with LCR savings for cost per treatment success in 2018 (compared with injectable-containing LCRs) of $3,707 (48% savings) in India and $5,476 (44% savings) in South Africa. BDQ-containing SCRs offer savings for cost per treatment success in 2018 (compared with injectable-containing SCRs) of $702 (21% savings) in India and $866 (16% savings) in South Africa.
3.2. Scenario 2: shifting to National Strategic Plan (NSP) prevalence with local procurement and capreomycin in India
The NSP prevalence data estimates that the number of XDR patients will be significantly higher (~ninefold higher), which drives higher hospitalization costs with LCRs and increases by 23%–25% their cost per treatment success. When combined with a shift from mixed procurement (local and GDF) to 100% local procurement (with greater than 50% reduction in injectable-containing SCR pricing), this results in 15% decrease in cost per treatment success for injectable-containing SCRs and a 7% decrease in BDQ-containing SCRs (,b)). Overall, there is an increase in the 5-year (2018–2022) total treatment costs to ~$1.6B from $580M in the base case.
A shift to capreomycin in India does not affect expressively cost per treatment success (~5%) since the observed increased drug acquisition cost and nephrotoxicity-related costs are offset by lower ototoxicity costs ()).
Cost savings provided by BDQ-containing regimens relative to injectable-containing regimens, on a cost per treatment success basis, are decreased in the SCR and increased in LCR.
3.3. Scenario 3: total conversion to bedaquiline-containing regimens in Russia, India, and South Africa
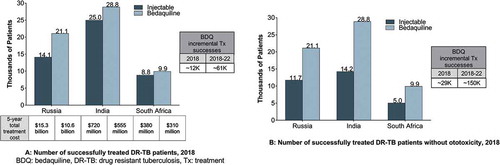
Exclusive use of BDQ-containing regimens over injectable-containing regimens results in approximately 12,000 more patients treated successfully across the three countries in 2018 and approximately 61,000 more patients treated successfully over a 5-year period, from 2018 to 2022 ()). Considering treatment of DR-TB patients without ototoxicity further increases the total incremental treatment successes to approximately 29,000 in 2018 and 150,000 from 2018 to 2022 ()).
Moreover, the total costs of treatment over 5 years are lower with BDQ-containing regimens across the three countries when compared to injectable-containing regimens. Total conversion to BDQ from injectable yields 5-year cost savings of ~$4.7B ($15.3B to $10.6B) in Russia, ~$165M in India ($720M to $550M), and $70M in South Africa ($380M to $310M) ()).
The ICER for BDQ-containing regimens () is negative (a reassuring indicator for health providers). Total conversion to the use of BDQ-containing regimens yields ICER decreases ICER of $122,878, $7,721, and $10,341 in Russia, India, and South Africa, respectively. Also, when hospitalization is excluded from ICER calculation, total conversion to the use of BDQ-containing regimens yields ICER decreases of $9,068, $6,397 and $7,775 in Russia, India and South Africa, respectively.
Table 5. ICER calculation.
4. Discussion
Together, the cost-effectiveness analysis in the base case and the three scenarios explored further quantifies the value that BDQ-containing regimens bring to health systems. BDQ-containing DR-TB regimens provide improved cost-effectiveness and have a significant cost per treatment success advantage over injectable-containing regimens in both SCR and LCR across India, South Africa, and Russia while lowering total health provider treatment expenditure.
The cost savings with BDQ-containing regimens are obtained at a modeled drug acquisition cost for BDQ comparable to other second-line anti-DR-TB generic drugs in resource-limited settings, which is singular for innovative drugs in this space and linked to a public health focus.
In the LCR, BDQ-containing regimens decrease the drug regimen cost, also providing substantial savings in hospitalization and averting injectable-related AE costs. In the SCR, BDQ-containing regimens provide significant savings over injectable-containing regimens through lowering hospitalization, lowering other costs, and averting injectable-related AE costs.
More broadly, these findings indicate that BDQ-containing regimens can be included in the standard of care for DR-TB in both the LCRs and SCRs from a cost-effectiveness perspective.
Russia, India, and South Africa contain diverse healthcare settings and standards of care for MDR-TB [Citation53–Citation57], and BDQ-containing regimens achieve improved cost per treatment success in all cases modeled.
These results are consistent with other BDQ-specific cost-effectiveness studies, concluding that BDQ is highly likely to be cost-effective in most environments [Citation22], that inclusion of BDQ to background regimens provides reductions in healthcare costs in countries with high DR-TB burdens [Citation19], and that there is higher projected net monetary benefit and improved cost-efficiency with greater access to BDQ -containing regimens in India [Citation21]. Most recently, South Africa-specific publications showed that providing BDQ to all patients with DR-TB could significantly increase treatment success rate at a cost over 10 years, which represents only 1% of the annual National Tuberculosis Program (NTP) budget [Citation18] and that when comparing a BDQ -containing regimen to an injectable-containing regimen, the resulting ICER for the BDQ-containing regimen was both cost saving and more effective after adjusting for adverse drug reactions [Citation58].
It is important to note that the clinical advantage of BDQ-containing regimens lies in their accelerated time to sputum conversion and higher rates of treatment success, translating into higher avoidance of treatment failures, one of the primary drivers of cost-effectiveness. While removal of injectables from treatment regimens also reduces AEs such as ototoxicity and nephrotoxicity, reduction in hospitalization costs and other costs associated with treatment failure is primarily due to improved efficacy of BDQ-containing regimens. There is a trend toward implementing injectable-free regimens for the treatment of DR-TB in a programmatic setting, outside of clinical trials or observational research, led by the South African government through its milestone decision to recommend BDQ for the first time anywhere in the world for all DR-TB patients [Citation31].
4.1. Study limitations
There are several limitations to our study. The current analysis does not explore the economic impact of TB transmission dynamics. Each untreated or failing patient continues to transmit the disease and expands the reservoir of potential future XDR-TB cases [Citation59,Citation60]. An assessment of the economic impact of stemming transmission of DR-TB should be undertaken to provide a broader understanding of the cost-effectiveness discussion in the public health context. Kunkel’s mathematical decision model found that if BDQ safety and efficacy are assumed to be sufficiently high, the optimal strategy would be to provide BDQ to all patients with MDR-TB. Such a liberal use of BDQ would lower transmission and could potentially impact the trajectory of an epidemic [Citation61].
Another limitation is that the study does not make individual drug acquisition cost comparisons or cost per patient treated comparisons but seeks to report overall cost per patient successfully treated. This approach primarily addresses national level objectives in the context of achieving global goals to ending TB that require focus on optimal use of current and new tools. With this approach, cost optimization takes precedent to cost minimization [Citation4].
In selecting parameters for comparative treatment success rates, which represent analysis inputs for the calculation of cost per treatment success output, we used the most broadly applicable and robustly collected data sources. Apart from the new anti-MDR-TB drugs, which have undergone randomized controlled trials to support their use, there are almost no data to help quantify the contribution of individual drugs to the effectiveness of DR-TB regimens. This limitation is not likely to impact the generalizability of the study results.
For overall treatment of DR-TB in LCRs, we used outcome data published in WHO reports. The consistency of the treatment success outcome (48–54%) over several successive reporting periods underscores this robustness; the overall treatment success of 54% was demonstrated in a 9,154 individual patient data MDR-TB meta-analysis [Citation62]. For BDQ -containing regimens, we used data from a five study meta-analysis conducted by WHO in 2016 [Citation51]. This was chosen in lieu of clinical trial data; clinical trials tend to have a much more narrowly defined patient population [Citation26,Citation27]. The overall population in the WHO meta-analysis covered nine countries from around the world and used both fixed-effects and random-effects models to account for heterogeneity among the studies, thus representing the best available evidence of treatment success of BDQ-containing regimens under programmatic settings. For SCRs, the paucity of data on treatment success rates is more pronounced, as there have not been publications on ‘real-world’ use. Moreover, BDQ is currently not part of the WHO SCR recommendations, thus precluding the availability in the public domain of programmatic outcome data including treatment success rate for BDQ-containing SCRs.
Changes to DR-TB treatment at a local level, especially in standards of ancillary and supportive care, may affect relative cost savings brought about by broad use of BDQ-containing regimens. Specifically, the long-term societal and individual related economic impact brought about by more effective regimens has not been evaluated. This relates to both development costs incurred at country level and catastrophic costs incurred at household level, which should be a focus for future studies.
5. Conclusions
Across all three countries, BDQ-containing regimens are dominant compared to injectable-containing regimens since they entail lower treatment costs to achieve better clinical outcomes.
Therefore, BDQ-containing regimens reduce the costs per treatment success by 18–20% in SCRs and 49–50% in LCRs, also resulting in approximately 61,000 more patients treated successfully over a 5-year period and 150,000 additional patients treated successfully without ototoxicity over the same period (when compared to standard of care injectable-containing regimens).
This analysis can provide insight and support to both local and global decision-makers and public health organizations to allocate resources efficiently to improve patient and public health outcomes.
Key issues
Using a health economics analysis, we have demonstrated that substituting BDQ for second-line injectable agents in DR-TB regimens would lead to more DR-TB patients achieving treatment success in India, South Africa, and Russia at a lower cost to the health system.
BDQ-containing SCRs are more cost-effective based on cost per treatment success than injectable-containing SCRs by 20% ($659), and 18% ($1,022) in 2018 in India and South Africa, respectively.
BDQ-containing LCRs reduce the cost per treatment success in 2018 by 54% ($106,480) in Russia, 51% ($5,751) in India, and 49% ($7,915) in South Africa (when compared to injectable-containing LCRs).
Cost savings realized by complete transition to BDQ-containing injectable-free regimens would result in an additional 61,000 patients treated successfully in the three countries (India, Russia, and South Africa) over a 5-year period, assuming transition occurred at the beginning of 2018, and distinctly, an additional 150,000 patients treated successfully without ototoxicity.
These findings indicate that BDQ-containing regimens can be included in the standard of care for DR-TB in both LCRs and SCRs from a cost-effectiveness perspective.
Author Contributions
AMI, AMA and SW were involved in collecting data and adapting as well as running the model which formed the basis of this analysis. AMI, CK, LM, AW, VS, and AT were involved in providing major advice on direction of the analysis, as well as on the inputs and outputs. All authors were involved in the preparation of the manuscript, are responsible for all content and editorial decisions, and jointly made the decision to submit the manuscript for publication.
Declaration of interest
A Thomas, AM Ionescu, AM Agnarson, C Kambili, L Metz, A Williams, V Singh were all employees of Johnson & Johnson at the time of conduct of this analysis. J Kfoury and S Wang were employees of L. E. K. consulting at the time of conduct of this analysis. L. E. K. consulting received funding from Johnson and Johnson to conduct the analysis. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download Zip (38.7 KB)Acknowledgments
Prasad Kulkarni of SmartAnalyst provided assistance with the preparation of the manuscript.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- World Health Organization. Global Tuberculosis Report 2017. 2017, World Health Organization: Geneva, Switzerland.
- World Health Organization. Multidrug-Resistant Tuberculosis (MDR-TB) 2017 Fact Sheet Update. 2017.
- World Health Organization. TB drug resistance types. [cited 2018 June]. Available from: http://www.who.int/tb/areas-of-work/drug-resistant-tb/types/en/.
- World Health Organization. The End TB Strategy. 2015: Geneva, Switzerland.
- World Health Organization. Guidelines for treatment of drug-resistant tuberculosis. 2016: Geneva, Switzerland.
- World Health Organization. The shorter MDR-TB regimen FactSheet. (May 2016).
- Reuter A, Tisile P, Von Delft D, et al. The devil we know: is the use of injectable agents for the treatment of MDR-TB justified? Int J Tuberc Lung Dis. 2017;21(11):1114–1126. .
- Arnold A, Cooke GS, Kon OM, et al. Adverse effects and choice between the injectable agents amikacin and capreomycin in multidrug-resistant tuberculosis. Antimicrob Agents Chemother. 2017;61(9).
- Kuaban C, Noeske J, Rieder HL, et al. High effectiveness of a 12-month regimen for MDR-TB patients in Cameroon. Int J Tuberc Lung Dis. 2015;19(5):517–524.
- Sagwa EL, Ruswa N, Mavhunga F, et al. Comparing amikacin and kanamycin-induced hearing loss in multidrug-resistant tuberculosis treatment under programmatic conditions in a Namibian retrospective cohort. BMC Pharmacol Toxicol. 2015;16:36.
- Melchionda V, Wyatt H, Capocci S, et al. Amikacin treatment for multidrug resistant tuberculosis: how much monitoring is required? Eur Respir J. 2013;42(4):1148–1150.
- Seddon JA, Godfrey-Faussett P, Jacobs K, et al. Hearing loss in patients on treatment for drug-resistant tuberculosis. Eur Respir J. 2012;40(5):1277–1286.
- Duggal P, Sarkar M. Audiologic monitoring of multi-drug resistant tuberculosis patients on aminoglycoside treatment with long term follow-up. BMC Ear Nose Throat Disord. 2007;7:5.
- Mahajan R. Bedaquiline: first FDA-approved tuberculosis drug in 40 years. Int J Appl Basic Med Res. 2013;3(1):1–2.
- Brigden G, Hewison C, Varaine F. New developments in the treatment of drug-resistant tuberculosis: clinical utility of bedaquiline and delamanid. Infect Drug Resist. 2015;8:367–378.
- FDA. Janssen Products. SIRTURO (bedaquiline) Prescribing Information 2018.
- DR-TB Scale-Up Treatment Action team (DR-TB STAT). Global Snapshot. [Cited 2018 Jun 28, 2018]; Available from: http://drtb-stat.org/global-snapshot/.
- Schnippel K, Firnhaber C, Conradie F, et al. Incremental cost effectiveness of bedaquiline for the treatment of rifampicin-resistant tuberculosis in South Africa: model-based analysis. Appl Health Econ Health Policy. 2018;16(1):43–54. .
- Lu X, Smare C, Kambili C, et al. Health outcomes of bedaquiline in the treatment of multidrug-resistant tuberculosis in selected high burden countries. BMC Health Serv Res. 2017;17(1):87. .
- Van Deun A, Decroo T, Piubello A, et al. Principles for constructing a tuberculosis treatment regimen: the role and definition of core and companion drugs. Int J Tuberc Lung Dis. 2018;22(3):239–245.
- Mehra M, Kambili C, Potluri R, et al. Modeling the impact of bedaquiline treatment strategies on the multidrug-resistant tuberculosis burden in India. Int J Tuberc Lung Dis. 2017;21(8):902–909.
- Vassall AW. Cost effectiveness of introducing bedaquiline in MDR-TB regimens an exploratory analysis. 2013.
- Olayanju O, Limberis J, Esmail A, et al. Long-term bedaquiline-related treatment outcomes in patients with extensively drug-resistant tuberculosis from South Africa. Eur Respir J. 2018;51(5):1800544.
- Borisov SE, Dheda K, Enwerem M, et al. Effectiveness and safety of bedaquiline-containing regimens in the treatment of MDR- and XDR-TB: a multicentre study. Eur Respir J. 2017;49(5):1700387.
- Skrahina A. Bedaquiline in MDR-TB treatment: Belarus experience. IJM. 2016;5:S62–S63.
- Pym A, Diacon AH, Tang S-J, et al. Bedaquiline in the treatment of MDR and XDR-TB. ERJ. 2016;47(2):564–574.
- Diacon AH, Pym A, Grobusch MP, et al. MDR-TB and culture conversion with bedaquiline. NEJM. 2014;371:723–732.
- World Health Organization. The use of bedaquiline in the treatment of multidrug resistant tuberculosis. 2013.
- World Health Organization. Report of the Guideline Development Group Meeting on the use of bedaquiline in the treatment of multidrug-resistant tuberculosis: A review of available evidence (2016) 2017.
- Schnippel K, Ndjeka N, Maartens G, et al. Effect of bedaquiline on mortality in South African patients with drug-resistant tuberculosis: a retrospective cohort study. Lancet Respir Med, 2018.
- Government of South Africa. Health releases data on reduction in TB mortality cases. 2018; [cited 2018 June]. Available from: https://www.gov.za/speeches/health-releases-data-reduction-tb-mortality-case-19-jun-2018-0000.
- Marks SM, Flood J, Seaworth B, et al. Treatment practices, outcomes, and costs of multidrug-resistant and extensively drug-resistant tuberculosis, United States, 2005–2007. Emerg Infect Dis. 2014;20(5):812–821.
- The Russian Society of Phthisiatricians. Federal Clinical Recommendations for diagnosis and treatment tuberculosis of the respiratory system with multiple and broad medicinal resistance of the pathogen. 2015.
- The Stop TB Partnership. GDF products list. 2016 Jun 13, 2018]; Available from: http://www.stoptb.org/gdf/drugsupply/drugs_available.asp.
- India National Pharmaceutical Pricing Authority. Price fixing order. 2018 Jun 13, 2018]; Available from: http://www.nppaindia.nic.in/ceiling/press02April18/Formulation_Prices(841).pdf.
- Sharma A, Hill A, Kurbatova E, et al. Estimating the future burden of multidrug-resistant and extensively drug-resistant tuberculosis in India, the Philippines, Russia, and South Africa: a mathematical modelling study. Lancet Infect Dis. 2017;17(7):707–715.
- Russia State Authority. National Register of selling prices. 2018. [cited 2018 June]. Available from: www.grls.rosminzdrav.ru.
- Russian Government. Unified information system in the sphere of procurement. [cited 2018 June]. Available from www.zakupki.gov.ru.
- Pooran A, Pieterson E, Davids M, et al. What is the cost of diagnosis and management of drug resistant tuberculosis in South Africa? PLoS One. 2013;8(1):e54587.
- Schnippel K, Firnhaber C, Berhanu R, et al. Direct costs of managing adverse drug reactions during rifampicin-resistant tuberculosis treatment in South Africa. Int J Tuberc Lung Dis. 2018;22(4):393–398.
- Falzon D, Schünemann HJ, Harausz E, et al. World Health Organization treatment guidelines for drug-resistant tuberculosis, 2016 update. Eur Respir J. 2017;49(3):1602308.
- Government of India, M.o.H.F.W. Central Tuberculosis Division, Guideline for PMDT in India. 2017.
- Government of South Africa, Introduction of new drugs and drug regimens for the management of drug resistant tuberculosis in South Africa: policy framework. 2015.
- World Health Organization. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. Geneva, Switzerland: World Health Organization; 2014.
- Centers for Disease Control and Prevention. Revised definition of extensively drug-resistant tuberculosis. Morbidity and Mortality Weekly Report. 2006;55:1176.
- Organisation for Economic Co-operation and Development (OECD). Purchasing power parities (PPP) (indicator). 2018. [Cited 2018 Jun 11]; Available from: https://data.oecd.org/conversion/purchasing-power-parities-ppp.htm.
- Laserson KF, Kenyon AS, Kenyon TA, et al. Substandard tuberculosis drugs on the global market and their simple detection. Int J Tuberc Lung Dis. 2001;5(5):448–454.
- Bate R, Jensen P, Hess K, et al. Substandard and falsified anti-tuberculosis drugs: a preliminary field analysis. Int J Tuberc Lung Dis. 2013;17(3):308–311.
- The StopTB Partnership 14 June 2018, USD 31 Million Saved by Stop TB Partnership’s Global Drug Facility Through Newly-Reduced Prices for TB Medicines. 2018.
- World Health Organization (WHO). Position statement on the continued use of the shorter MDR-TB regimen following an expedited review of the STREAM Stage 1 preliminary results. Geneva, Switzerland: World Health Organization; 2018.
- World Health Organization, Review of available evidence on the use of bedaquiline for the treatment of multidrug-resistant tuberculosis: Data analysis report. 2016.
- Government of India. National Strategic Plan for tuberculosis elimination 2017–2025. 2017; [cited 2018 June]. Available from: https://tbcindia.gov.in/WriteReadData/NSP%20Draft%2020.02.2017%201.pdf.
- Floyd K, Hutubessy R, Samyshkin Y, et al. Health-systems efficiency in the Russian Federation: tuberculosis control. Bull World Health Organ. 2006;84(1):43–51.
- Satyanarayana S, Subbaraman R, Shete P, et al. Quality of tuberculosis care in India: a systematic review. Int J Tuberc Lung Dis. 2015;19(7):751–763.
- Zinatsa F, Osborne EL, Assefa MT, et al. Voices from the frontline: barriers and strategies to improve tuberculosis infection control in primary health care facilities in South Africa. BMC Health Serv Res. 2018;18(1):269.
- Loveday M, Wallengren K, Brust J, et al. Community-based care vs. centralised hospitalisation for MDR-TB patients, KwaZulu-Natal, South Africa. Int J Tuberc Lung Dis. 2015;19(2):163–171.
- John D, Chatterjee P, Murthy S, et al. Cost effectiveness of decentralised care model for managing MDR-TB in India. Indian Journal of Tuberculosis; 2017;65(3):208–217.
- Schnippel K, Firnhaber C, Page-Shipp L, et al. Impact of adverse drug reactions on the incremental cost-effectiveness of bedaquiline for drug-resistant tuberculosis. Int J Tuberc Lung Dis. 2018;22(8):918–925.
- Kodama C, Wedzicha JA, Iversen M, et al. Mycobacterium tuberculosis transmission from patients with drug-resistant compared to drug-susceptible TB: a systematic review and meta-analysis. Eur Respir J. 2017;50(4):1701044.
- Mandal S, Arinaminpathy N. Transmission modeling and health systems: the case of TB in India. Int Health. 2015;7(2):114–120.
- Kunkel A, Cobelens FG, Cohen T, et al. Tradeoffs in introduction policies for the anti-tuberculosis drug bedaquiline: a model-based analysis. PLoS Med. 2016;13(10):e1002142.
- Ahuja SD, Ashkin D, Avendano M, et al. Correction: multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLoS Med. 2012;9(9).