?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Objective
To estimate the cost-effectiveness of second-line pharmacological treatments in patients with acromegaly resistant to first-generation somatostatin analogues (FG SSA) from the Spanish National Health System (NHS) perspective.
Methods
A Markov model was developed to analyze the cost-effectiveness of pegvisomant and pasireotide in FG SSA-resistant acromegaly, simulating a cohort of patients from the treatment beginning to death. Treatment with pegvisomant or pasireotide was compared to FG SSA retreatment. Efficacy data were obtained from clinical trials and utilities from the literature. Direct health costs were obtained from Spanish sources (€2018).
Results
The Incremental Cost Effectiveness Ratio (ICER) of pegvisomant vs. FG SSA was €85,869/Quality-adjusted life years (QALY). The ICER of pasireotide vs. FG SSA was €551,405/QALY. The ICER was mainly driven by the incremental efficacy (4.41 QALY for pegvisomant vs. FG SSA and 0.71 QALY for pasireotide vs. FG SSA), with a slightly lower increase in costs with pegvisomant (€378,597 vs. FG SSA) than with pasireotide (€393,151 vs. FG SSA).
Conclusion
The ICER of pasireotide compared to FG SSA was six times higher than the ICER of pegvisomant vs. FG SSA. Pegvisomant is a more cost-effective alternative for the treatment of acromegaly in FG SSA-resistant patients in the Spanish NHS.
1. Introduction
Acromegaly is a disease caused by an excessive secretion of growth hormone (GH), generally caused by the presence of a pituitary adenoma. It is included in the group of rare diseases due to its low prevalence. European epidemiological data show an incidence rate of 0.2–1.1 cases per 100,000 and a prevalence rate of 2.8–13.7 cases per 100,000 inhabitants [Citation1]. According to the Spanish Acromegaly Registry, the estimated prevalence rate of acromegaly in Spain is 36 cases per million, ranging from 15.7 to 75.8 depending on the geographic area [Citation2] with an increased prevalence in women (60.8%) [Citation3].
The disease develops insidiously, causing an important delay in diagnosis. The mean age at diagnosis is 45.5 years, with a mean time of 6 years from the onset of the first symptoms of acromegaly to diagnosis [Citation3]. The clinical manifestations of acromegaly depend on growth hormone (GH) and insulin-like growth factor 1 (IGF-1) levels, the patient’s age, the size of the tumor and the delay in diagnosis. Facial changes (frontal prominence and prognathism), acral growth, hypertension, symptoms of hyperglycemia, cardiomyopathy, heart failure, and sleep apnoea are generally observed [Citation4].
The objectives of acromegaly treatment are: to diminish the tumor mass; normalize IGF-1 and GH levels; control symptoms and comorbidities; improve quality of life; and prevent premature mortality due to the disease [Citation4].
There are three therapeutic approaches for the treatment of acromegaly: surgery, pharmacologic therapy and radiotherapy [Citation4,Citation5]. Surgery is the main first-line treatment. Pharmacological treatment is used as a primary treatment (in patients for whom surgery is not suitable or is not effective or in the interim period until radiotherapy is completely effective) or if surgery fails. First-line pharmacological treatment is based on first-line somatostatin analogues (FG SSA, octreotide, and lanreotide) [Citation2,Citation4,Citation6]. Pegvisomant (GH receptor antagonist) [Citation7] or pasireotide (second generation somatostatin analogue) [Citation8] is used when the patient does not respond adequately to or does not tolerate FG SSA (second-line pharmacological treatment) [Citation9]. Although there are not comparative head-to-head data available, results of their respective clinical trials show that the efficacy (both in terms of biochemical and tumor control) and safety profile of pegvisomant and pasireotide differs considerably [Citation10,Citation11].
The objective of this analysis was to estimate the cost-effectiveness of second-line pharmacological treatments (pegvisomant or pasireotide) in patients with acromegaly resistant to first-generation somatostatin analogues (FG SSA) from the perspective of the Spanish National Health System (NHS). Therefore, it aims to provide information to support the decision-making process regarding the selection of second-line acromegaly treatments from the perspective of the Spanish National Health System (NHS).
2. Methods
2.1. Summary of the economic model
A Markov model was used to simulate the lifetime disease progression of a cohort of 1,000 patients with acromegaly requiring second-line pharmacological treatment. Three mutually exclusive health states were included: controlled patient, uncontrolled patient, and death. Patients transition in six-month cycles based on the different transition probabilities calculated using published clinical trials (see below).
The alternative treatments evaluated were FG SSA (octreotide LAR or lanreotide ATG), pasireotide, and pegvisomant. It was not possible to switch treatment from pasireotide to pegvisomant or vice versa in the absence of available data to support this possibility. Combination treatment (off-label use) was not considered in the model.
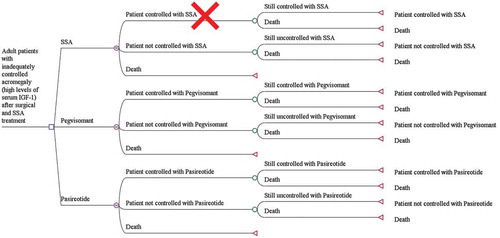
The model was built using TreeAge Pro 2018, TreeAge Software, Williamstown, MA, USA. shows the decision tree used in the model for the three states and alternatives considered.
Figure 1. Decision tree model in patients with acromegaly requiring second-line pharmacological treatment.
Live years gained (LYG), quality-adjusted life years (QALYs), and the associated costs for each alternative were calculated. Incremental cost-effectiveness ratios (ICER) of the comparisons were calculated using the formula:
2.2. Analysis perspective and discount
The analysis was performed from the perspective of the Spanish NHS. Only direct health-care costs (cost of medication, management of adverse events, follow-up and management of comorbidities) were considered.
For the base case, a 3% discount was applied to both costs and benefits (Life Years Gained [LYG] and QALYs) [Citation12].
2.3. Time horizon
The model was developed to cover the lifetime progression of the disease. Taking into account that the mean diagnosis age of acromegaly in Spain is 45 years [Citation2] and adjusting for life expectancy according to age and gender [Citation13], time horizon was set to 42 years for the base case analysis.
2.4. Patient populations and treatments
The cohort of patients was defined based on baseline characteristics of the clinical trials analyzed [Citation10,Citation11]. These studies included adult patients with inadequately controlled acromegaly (high levels of serum GH and/or IGF-1) after surgical and FG SSA treatment ().
Table 1. Description of the studies, mean dose, efficacy, and safety results considered in the model.
The mortality rate derived from each state of health was obtained based on the general mortality data adjusted by age in the Spanish population [Citation13]. From this data, and as described in the literature [Citation14], we assumed a death relative risk ratio of 1.1 and 2.5 for the controlled and uncontrolled acromegaly patients, respectively.
The mean dose, efficacy, and clinically significant adverse events of each treatment were extracted from clinical trials for pegvisomant [Citation11], pasireotide [Citation10] and FG SSA [Citation10] (). In the base case, the efficacy rate obtained using the mean study dose was used. The efficacy of FG SSA was 0% since the baseline population considered in the model is resistant to this medication, and in line with the efficacy results of the comparator arm (FG SSA) in the pasireotide clinical trial used in the model [Citation10] (). Efficacy (i.e. controlled patients) was defined as a mean GH concentration < 2.5 µg/L and/or normalized IGF-1 concentration (between the upper and the lower limits of normality) for FG SSA and pasireotide [Citation15]. Pegvisomant efficacy was defined in terms of normalized IGF-1 concentration only, since, according to its mechanism of action, it does not reduce GH levels [Citation11,Citation16].
Although the maximum dose of pegvisomant is 30 mg/day according to its label [Citation7], the model assumed the efficacy range shown in the clinical trial (maximum dose studied: 20 mg/day) (). The mean dose considered in the model (15 mg/day) is in line with the mean dose used in Spanish patients according to the real-world study ACROSTUDY (15.5 mg/day) [Citation15]. It was assumed that the efficacy shown in the pegvisomant clinical trial at 12 weeks is maintained at 24 weeks, in order to allow comparison with the pasireotide clinical trial [Citation10]. This assumption is plausible considering the long-term treatment outcomes observed in ACROSTUDY [Citation15].
2.5. Estimation of utilities
QALYs were calculated taking into account the quality of life (“utility“) and the LYG in each state of health. The reduction in the quality of life (”disutility”) produced by the treatment-related adverse events was also considered. The utility and disutility values applied in the model were obtained from the literature [Citation17–Citation20] ().
Table 2. Utility values (quality of life) used in the model.
2.6. Estimation of resources and costs
The estimation of costs considered in the model was made using published Spanish official rates (Table 1 in Appendix). Diagnostic-related Groups (DRGs) method was used for the allocation of resources or processes.
Medication costs:
Medication costs include both pharmacological costs and drug administration-related costs, if applicable. Ex-factory prices (EFP) were used to calculate the cost per mg, and applicable official discounts were applied (−4% for pasireotide, −7.5% for pegvisomant 25 and 30 mg and −15% for lanreotide and pegvisomant 10, 15, and 20 mg). Medication cost per cycle was calculated with the cost per mg and the mean dose () for each treatment alternative ().
Table 3. Costs per cycle (6 months) used in the model (€2018) [Citation5,Citation13,Citation16,Citation20,Citation24–Citation28]. Percentage of the unit cost imputed to each item is shown in column ‘%’. Inthe case of pharmacological costs, the percentages reflect the proportion of drug used in the studies considered in the model (see ), and unit costs are calculated considering the mean dose and the dosing schedule in the label (see ).
In cases where the cost per mg of the drug was not linear (lanreotide), a conservative approach was applied and the presentation with the lowest price per milligram was used. In the case of pasireotide, since the clinical trial comprises doses of 40 mg and 60 mg, both costs were used. Cost of FG SSA treatment was calculated considering the proportion of use of octreotide and lanreotide in the clinical trial [Citation10] ().
The cost of a nursing visit was used as an estimation of the cost of intramuscular administration ().
Follow-up costs:
Only differential follow-up costs derived from each treatment alternative were taken into account as shown in . Differential follow-up costs identified were the cost of liver ultrasound and resonance imaging, according to international guidelines [Citation5] and expert’s opinion.
Adverse events management costs:
Adverse events management costs were calculated adopting a microcosting approach [Citation21]. Unit costs are the mean unit costs of the official rates in the Spanish regional health systems (Table 1 in Appendix).
The cost for hyperglycemia (grade ≥3) was calculated from the mean cost of grade 3 or 4 hyperglycaemic events due to pharmacological treatment obtained from the literature [Citation22] and the Spanish Ministry of Health statistics [Citation23] (). It was assumed that hyperglycaemic adverse events take place at the beginning of treatment. Therefore, its associated costs and disutility values were allocated in the first cycle only.
Comorbidity management costs:
The comorbidities considered in the model were the most prevalent in patients with acromegaly [Citation24]: hypertension, arrhythmia, glucose-homeostasis alterations, diastolic/systolic dysfunction, and sleep apnoea. For the prevalence of cardiac comorbidities in patients with controlled/uncontrolled acromegaly, we used a study that addresses this in the same population of patients and considered the prevalence in controlled patients to be the same as in the control group in that study [Citation25] (Table 2 in Appendix). In patients with uncontrolled acromegaly, a higher prevalence was considered according to the literature [Citation25,Citation26] (Table 2 in Appendix). Comorbidity management costs were obtained from the literature [Citation27–Citation31] ().
2.7. Sensitivity analyses
The following univariate sensitivity analyses (SA) were performed in order to analyze the influence of the efficacy and costs variables in the results obtained in the model:
SA 1: A dose titration was considered instead of using clinical trial mean doses. Patients started treatment with the lowest dose (10 mg/day pegvisomant; 40 mg/month pasireotide) and increased the dose in case of lack of efficacy (in increments of 5 mg pegvisomant; 20 mg pasireotide) up to the maximum dose (30 mg/day pegvisomant; 60 mg/month pasireotide).
SA 2: 100% patients in the somatostatin analogue alternative were treated with octreotide.
SA 3: 100% patients in the somatostatin analogue alternative were treated with lanreotide.
SA 4: Pasireotide efficacy was defined in terms of normalization of IGF-1 levels rather than normalization of IGF-1 and GH levels.
SA 5: A 10% increase in the efficacy of pegvisomant was assumed to verify the influence of this variable.
SA 6: The specific disutility value of diabetes in women (−0.23) obtained from the literature [Citation18], was used to verify the influence of this variable.
SA 7: The efficacy and safety of pegvisomant in a real-world study was used [Citation32]. Since there are not real-world efficacy data for pasireotide, at least in a study with sufficient methodological quality, the efficacy of pasireotide in clinical trial was considered ().
SA 8: An analysis with no discount was performed, in accordance with the recommendations of the Spanish economic evaluation guideline [Citation12].
SA 9: An analysis with a 5% discount was performed, in accordance with the recommendations of the Spanish economic evaluation guideline [Citation12].
SA 10–12: A time horizon of 5, 10, and 15 years was considered to verify the effect of this variable.
In addition to these univariate analyses, a probabilistic sensitivity analysis (PSA) was performed using a Monte Carlo methodology [Citation33]. One thousand simulations were carried out, taking into account the different distributions of the variables. Costs were described by gamma distributions and efficacies by beta distributions. The α and λ parameters of the gamma distributions and the α and β of the beta distributions were obtained from the means and standard deviations of each variable using the moments method (Table 3 in Appendix).
3. Results
3.1. Base case and deterministic sensitivity analyses
shows the results of costs and QALYs for each treatment alternative in the base case scenario. Pegvisomant treatment versus FG SSA treatment had an incremental benefit of 5.15 life years gained (LYG) and 4.41 QALYs with an incremental cost of €378,597. Therefore, the ICER of pegvisomant versus FG SSA was €73,514/LYG and €85,869/QALY ().
Table 4. Results of the cost-effectiveness model, base case. Time horizon: Lifetime.
According to the deterministic sensitivity analyses, a titration dosing of pegvisomant (SA 1) increased its ICER to €88,490/QALY (). Conversely, when compared to lanreotide (SA 3), pegvisomant ICER decreased to €71,344/QALY (). Taking into account the efficacy and safety data in real clinical practice (SA 7), pegvisomant ICER increased to €93,696/QALY ().
Table 5. Results of the cost-effectiveness model, univariate sensitivity analyses.
Pasireotide treatment versus FG SSA treatment showed an incremental benefit of 1.20 life years gained (LYG) and 0.71 QALYs with an incremental cost of €393,151 (). Therefore, the ICER of pasireotide versus FG SSA was €327,626/LYG and €551,405/QALY ().
According to the sensitivity analyses, applying a diabetes disutility value of −0.23 (SA 6), increased pasireotide ICER to €1,051,411/QALY (). In contrast, when control of IGF-1 levels was used to assess the efficacy of pasireotide (SA 4), its ICER decreased to €354,068/QALY ().
In both comparisons, increasing the time horizon (SA 10–12) resulted in a decrease in ICERs ().
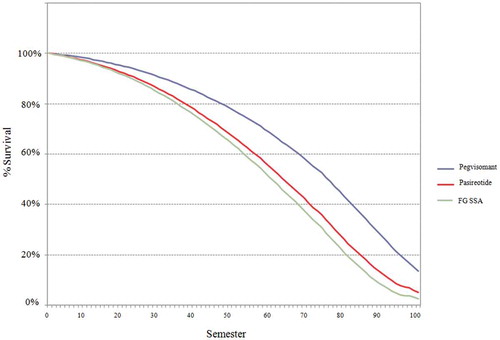
3.2. Survival curves
shows the survival curves with the evolution of the simulated cohort according to the treatment alternatives analyzed.
3.3. Probabilistic sensitivity analyses
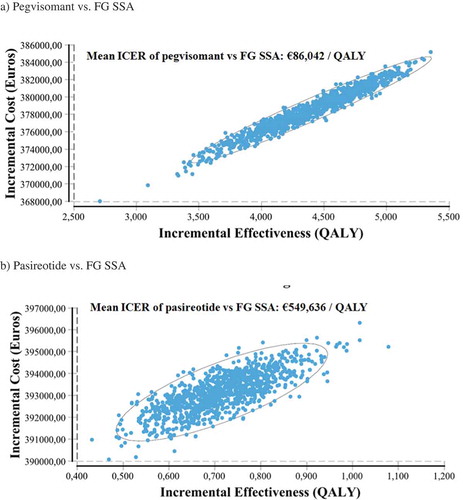
The probabilistic sensitivity analyses showed a mean ICER of €86,042/QALY (95% CI [€75,206-€104,634/QALY]) for pegvisomant versus FG SSA and a mean ICER of €549,636/QALY (95% CI [€435,303-€730,321/QALY]) for pasireotide versus FG SSA (). These results are consistent with the abovementioned results.
Table 6. Results of the probabilistic sensitivity analyses.
shows the incremental cost-effectiveness plane of the 1,000 simulations performed for each comparison.
Figure 3. Incremental cost-effectiveness plane and confidence ellipses of the 1,000 simulations performed in the probabilistic sensitivity analyses of pegvisomant vs. FG SSA (a) and pasireotide vs. FG SSA (b). ICER: Incremental cost-effectiveness ratio. QALY: quality-adjusted life years. FG SSA: first-generation somatostatin analogues (octreotide, lanreotide).
4. Discussion
Transsphenoidal surgery is the first-line treatment for most patients with acromegaly. In patients with persistent disease after surgery, FG SSA are indicated as first-line pharmacological treatment [Citation4,Citation9]. If there is an inadequate response, contraindication or lack of tolerance to FG SSA, treatment with pegvisomant [Citation4,Citation7,Citation9], or with the recently approved pasireotide, is recommended (second-line pharmacological treatment) [Citation8,Citation9].
In patients not fully controlled with FG SSA, it is important to take into account not only the efficacy and safety data from pivotal clinical trials, but also to the cost-effectiveness evidence to support the decision-making process in the selection of treatment alternatives. For this purpose, we evaluated the cost-effectiveness of pegvisomant or pasireotide treatment compared to FG SSA (octreotide and lanreotide) treatment from the perspective of the Spanish NHS.
The results of this model showed that the use of pasireotide versus FG SSA had an ICER six times greater than the use of pegvisomant versus FG SSA (€85,869/QALY vs. € 551,405/QALY) (). This difference in ICERs was mainly driven by the incremental effectiveness (4.41 QALYs for pegvisomant vs. FG SSA and 0.71 QALYs for pasireotide vs. FG SSA), with a slightly lower increase in costs with pegvisomant (€378,597 vs. FG SSA) than with pasireotide (€393,151 vs. FG SSA) (). This difference is consistent with a higher survival and diseases control rates with pegvisomant treatment ( and ).
The results of the sensitivity analyses confirmed the robustness of the model (). In general terms, medication costs had the greatest influence on the results. Thus, the increase in pegvisomant or pasireotide doses by dose titration considerably increased both ICERs, due to the increase in medication costs (). Similarly, when only octreotide was considered as FG SSA, both ICERs increased, since octreotide is less expensive than lanreotide ().
It should be noted that besides the higher cost of pegvisomant relatively to pasireotide, the total cost of pasireotide is higher than the cost of pegvisomant. This is mainly driven by the cost of comorbidities (due to lower efficacy) and adverse event management (especially hyperglycaemic adverse event) with pasireotide. A recent study has also suggested this possibility [Citation34]. Indeed, both treatments have differential effects in glucose homeostasis: pasireotide has been found to cause, compared to FG SSA, increased fasting plasma glucose (FPG) and glycated hemoglobin (HbA1c), thereby causing a higher incidence of drug-related hyperglycaemic adverse events [Citation10]. In contrast, pegvisomant has favorable effects on glucose metabolism, since it improves insulin sensitivity, decreases FPG, improves glucose tolerance and decreases HbA1c levels [Citation35].
The results from our model show that treatment with pegvisomant or pasireotide would not be cost-effective compared to FG SSA, since the accepted efficiency threshold set at €25,000/QALY would be exceeded [Citation36]. However, the use of the conventional efficiency threshold for the evaluation of orphan medicinal products is questionable and under debate [Citation37–Citation39]. Their higher acquisition costs and the difficulties in assessing their long-term efficacy mean that orphan drugs are not really comparable to other medicinal products using cost-effectiveness thresholds which are usually employed.
It is important to note the changes in the treatment of acromegaly in recent years. According to data from the French registry [Citation40], which evaluates the evolution of the management of acromegaly over the last three decades, the number of cured patients after surgery has barely changed while the percentage of patients with a controlled disease by medical treatment has increased. Therefore, nowadays the majority of acromegaly patients can achieve an adequate control of the disease through progressive treatment. However, therapeutic choices may determine the time needed to reach control and the cost-effectiveness of patient management. Second-line treatments are more expensive than FG SSA (); however, their use is needed and recommended in guidelines to control the disease and reduce mortality and comorbidities. Besides, maintaining a treatment in resistant patients has an important associated cost and little benefit. This analysis shows that maintaining FG SSA treatment in patients resistant to this therapy has a cost of €250,650, with an effectiveness of 10.1 QALYs ().
Several studies have been conducted to assess the cost of acromegaly treatment. Studies on the analysis of preoperative treatment to improve the results of surgery have suggested that this strategy is cost-effective [Citation41]. In 2004, Didoni et al. conducted a study in 134 Italian patients over a period of 7 years [Citation42]. The costs of hospitalization, specialist visits, and drugs for comorbidities were €7,968 per year for controlled patients and €12,533 per year for uncontrolled patients [Citation42]. A Spanish study evaluated the cost in 11 consecutive patients with invasive macroadenoma in 2007 and established it to be between €7,072 and €9,874 per patient per year [Citation43]. In other study, the mean cost per patient per year in 53 Canadian patients with acromegaly during a mean follow-up of 49 months was $8,111 [Citation44]. Valentim et al. [Citation45] conducted a cost-effectiveness study of octreotide LAR and lanreotide SR in Brazil, and found that octreotide LAR was more cost-effective than lanreotide SR. In Spain, Roset et al. [Citation46] analyzed treatment costs in 74 patients with acromegaly between 2005 and 2007: the mean annual cost per patient was €9,668. The cost of patients treated with surgery alone was €2,501 per year compared to €9,745 per year for patients receiving medical treatment alone [Citation46]. In cases where both treatments were required, the annual cost varied from €10,866 and €12,364 [Citation46]. Recently, two systematic reviews have been published on the cost-effectiveness of pharmacological treatments for patients with acromegaly that includes the abovementioned studies and conclude that cost-effectiveness analyses of second-line treatments were controversial and had important limitations [Citation47,Citation48].
In all the studies considered, pharmacological treatment was the variable with the deepest impact in total direct costs. It was estimated that pharmacological treatment could account for 71% of the direct costs of the disease [Citation46]. It should be noted that comorbidity costs are always higher in uncontrolled patients than in controlled patients [Citation42,Citation46]. Most of the studies do not perform an exhaustive comparison between the different treatments, so there may be relevant aspects of the direct and indirect costs that are not considered [Citation49].
Since this model is based on efficacy data from separate clinical trials (with differences in the inclusion criteria, follow-up periods or in the control groups) the results should be confirmed by randomized clinical trials in which both alternatives are compared directly, and also in clinical practice (i.e. real-world data). Among the limitation of the study, the crossover of the treatment with pasireotide to pegvisomant or vice versa has not been analyzed because there are no data from clinical trials to support the modeling of this possibility. In addition, the cost estimate of this model only considers the direct costs of the disease due to the lack of data on indirect costs. Actual costs may be significantly higher if indirect costs are taken into account. No study in real-world evidence for pasireotide was included in the analysis, since only a retrospective study of very small sample size was available [Citation50].
Currently, radiotherapy is considered an adjuvant treatment for patients with recurrent or persistent tumors after surgery and for patients resistant or intolerant to medical treatments [Citation5,Citation9] and, therefore, was not included in this analysis.
Combination therapy (i.e. pegvisomant + FG SSA, off label use), although prescribed in clinical practice and recommended in guidelines and consensus [Citation5,Citation9], was outside the scope of this analysis. However, it would be interesting to study their cost-effectiveness in future analyses [Citation51].
Here, we present the first exhaustive analysis studying the cost-effectiveness of second-line treatment in patients resistant to FG SSA.
5. Conclusions
In conclusion, the proposed model shows that the ICER of pasireotide compared to FG SSA was six times higher than the ICER of pegvisomant vs. FG SSA. According to this analysis, pegvisomant is the most cost-effective alternative in the treatment of acromegaly in FG SSA-resistant patients for the Spanish NHS.
Article highlights
In patients not fully controlled with first-generation somatostatin analogues (FG SSA), it is important to take into account not only the efficacy and safety data from pivotal clinical trials, but also to the cost-effectiveness evidence to support the decision-making process in the selection of treatment alternatives.
The Incremental Cost Effectiveness Ratio (ICER) of pegvisomant vs. FG SSA was €85,869/Quality-adjusted life years (QALY).
The ICER of pasireotide vs. FG SSA was €551,405/QALY, six times higher than the ICER of pegvisomant vs. FG SSA.
The difference in both ICERs was mainly driven by the incremental efficacy (4.41 QALY for pegvisomant vs. FG SSA and 0.71 QALY for pasireotide vs. FG SSA), with a slightly lower increase in costs with pegvisomant (€378,597 vs. FG SSA) than with pasireotide (€393,151 vs. FG SSA).
Pegvisomant is a more cost-effective monotherapy alternative for the treatment of acromegaly in FG SSA-resistant patients in the Spanish NHS.
Author contribution statement
CR-T and DR-R developed the economic model. FC, VG, NM, LS, and CP contributed to analysis conceptualization, design, and revision of the model. All authors had access to data, contributed to analysis conceptualization, methodology development, and manuscript preparation. All authors listed made substantial contributions to the analysis conceptualization and/or design, analysis and/or interpretation of data and manuscript preparation and/or review. All authors read, edited and approved the final manuscript. All authors agree to be accountable for all aspects of the work. CP is the guarantor of the overall content of this manuscript.
Conflict of interest
This analysis was sponsored by Pfizer (Spain). CR-T and DR-R are employees of Health Value who received an honorarium from Pfizer (Spain) in connection with the development of this manuscript. Medical writing support was provided by CR-T and DR-R (Health Value) and was funded by Pfizer. CP, LS-C, and NM are employees of Pfizer (Spain). FC has received speaker honoraria from Pfizer (Spain) and Novartis. VG declared no conflict of interest.
Declaration of interest
C Peral, N Mir, and L Sánchez-Cenizo are all employees of Pfizer SLU, Madrid, Spain. F Cordido has received speaker honoraria from Pfizer (Spain). D Rubio-Rodríguez and C Rubio-Terrés are employees of Health Value who received an honorarium from Pfizer (Spain) in connection with the development of this manuscript. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures
A reviewer on this manuscript has disclosed they have received a speaker fee from Novartis in the past. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Supplemental Material
Download MS Word (69 KB)Acknowledgments
We thank Javier Parrondo at JParrondo Health for his collaboration in the development of the preliminary economic model.
Supplementary material
Supplementary data can be accessed here.
Additional information
Funding
References
- Lavrentaki A, Paluzzi A, Wass JA, et al. Epidemiology of acromegaly: review of population studies. Pituitary. 2017;20(1):4–9.
- Mestron A, Webb SM, Astorga R, et al. Epidemiology, clinical characteristics, outcome, morbidity and mortality in acromegaly based on the Spanish Acromegaly Registry (Registro Espanol de Acromegalia, REA). Eur J Endocrinol. 2004;151(4):439–446.
- Sesmilo G, Gaztambide S, Venegas E, et al. REA investigators. Changes in acromegaly treatment over four decades in Spain: analysis of the Spanish Acromegaly Registry (REA). Pituitary. 2013;16(1):115–121.
- Cordido F, García Arnés JA, Marazuela Aspiroz M, et al. grupo de Neuroendocrinología de la Sociedad Española de Endocrinología y Nutrición. [Practical guidelines for diagnosis and treatment of acromegaly. Grupo de Neuroendocrinología de la Sociedad Española de Endocrinología y Nutrición]. Endocrinol Nutr. 2013;60(8):457.e1–457.e15.
- Katznelson L, Laws ER Jr, Melmed S, et al. Endocrine society. Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(11):3933–3951.
- Sesmilo G, Webb SM. Neuroendocrinology group of the spanish society of endocrinology and nutrition. Twelve years of the Spanish acromegaly registry: a historical view of acromegaly management in Spain. Endocrinol Nutr. 2010;57(2):39–42.
- EMA. Ficha Técnica Somavert (Pegvisomant). 2016. [Cited 2018 Jun]. [ Available from: http://www.ema.europa.eu/docs/es_ES/document_library/EPAR_-_Product_Information/human/000409/WC500054629.pdf
- EMA. Ficha Técnica Signifor Pasireotida). 2016. [Cited 2018 Jun]. Available from: http://www.ema.europa.eu/docs/es_ES/document_library/EPAR_-_Product_Information/human/002052/WC500128056.pdf.
- Melmed S, Bronstein MD, Chanson P, et al. A consensus statement on acromegaly therapeutic outcomes. Nat Rev Endocrinol. 2018;14(9):552–561.
- Gadelha MR, Bronstein MD, Brue T, et al. Pasireotide C2402 study group. Pasireotide versus continued treatment with octreotide or lanreotide in patients with inadequately controlled acromegaly (PAOLA): a randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2(11):875–884. .
- Trainer PJ, Drake WM, Katznelson L, et al. Treatment of acromegaly with the growth hormone-receptor antagonist pegvisomant. N Engl J Med. 2000;342(16):1171–1177.
- López Bastida J, Oliva J, Antoñanzas F, et al. [A proposed guideline for economic evaluation of health technologies]. Gac Sanit. 2010;24(2):154–170.
- INE. Defunciones por edad, mes y sexo. Datos provisionales Año 2015 2016. 2015. [Cited 2018 Jun]. Available from: http://www.ine.es.
- Holdaway IM, Bolland MJ, Gamble GD. A meta-analysis of the effect of lowering serum levels of GH and IGF-I on mortality in acromegaly. Eur J Endocrinol. 2008;159(2):89–95.
- Bernabeu I, Pico A, Venegas E, et al. Spanish ACROSTUDY group. Safety of long-term treatment with Pegvisomant: analysis of Spanish patients included in global ACROSTUDY. Pituitary. 2016;19(2):127–137. .
- van der Lely AJ, Hutson RK, Trainer PJ, et al. Long-term treatment of acromegaly with pegvisomant, a growth hormone receptor antagonist. Lancet. 2001;358(9295):1754–1759.
- Brazzelli M, Cruickshank M, Kilonzo M, et al. Clinical effectiveness and cost-effectiveness of cholecystectomy compared with observation/conservative management for preventing recurrent symptoms and complications in adults presenting with uncomplicated symptomatic gallstones or cholecystitis: a systematic review and economic evaluation. Health Technol Assess. 2014;18(55):1–101.
- García-Soidán FJ, Villoro R, Merino M, et al. Estado de salud, calidad de vida y utilización de recursos sanitarios de los pacientes con diabetes mellitus en España. Medic Fam. 2017;43(6):416–424.
- Rowles SV, Prieto L, Badia X, et al. Quality of life (QOL) in patients with acromegaly is severely impaired: use of a novel measure of QOL: acromegaly quality of life questionnaire. J Clin Endocrinol Metab. 2005;90(6):3337–3341.
- Salomon JA, Vos T, Hogan DR, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the global burden of disease study 2010. Lancet. 2012;380(9859):2129–2143.
- Ferreira CNRC, Santana CF Acromegaly patients with inadequate response to maximum dose octreotide-lar who progress to treatment with pegvisomant: economic evaluation and incremental budget impact analysis from the public perspective to são paulo state. ISPOR 5th Latin America conference; September 2015; Santiago de Chile: ISPOR; 2015.
- Llibre-Codina JM, Casado-Gómez MA, Sánchez-de la Rosa R, et al. [Cost of nucleoside analogue reverse transcriptase inhibitor-related toxicity in HIV-1-infected patients]. Enferm Infecc Microbiol Clin. 2007;25(2):98–107.
- Registro de altas. CIE9 MC CMBD 2013 [Internet]. 2013. [Cited 2018 Nov 04]. Available from: http://pestadistico.inteligenciadegestion.msssi.es
- Colao A, Ferone D, Marzullo P, et al. Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr Rev. 2004;25(1):102–152.
- Colao A, Pivonello R, Grasso LF, et al. Determinants of cardiac disease in newly diagnosed patients with acromegaly: results of a 10 year survey study. Eur J Endocrinol. 2011;165(5):713–721.
- Davi‘ MV, Dalle Carbonare L, Giustina A, et al. Sleep apnoea syndrome is highly prevalent in acromegaly and only partially reversible after biochemical control of the disease. Eur J Endocrinol. 2008;159(5):533–540.
- Andreu AL, Chiner E, Sancho-Chust JN, et al. Effect of an ambulatory diagnostic and treatment programme in patients with sleep apnoea. Eur Respir J. 2012;39(2):305–312.
- Ballesta M, Carral F, Olveira G, et al. Economic cost associated with type II diabetes in Spanish patients. Eur J Health Econ. 2006;7(4):270–275.
- Delgado JF, Oliva J, Llano M, et al. Health care and nonhealth care costs in the treatment of patients with symptomatic chronic heart failure in Spain. Rev Esp Cardiol. 2014;67(8):643–650.
- Montes-Santiago J, Rodil V, Formiga F, et al. Working group on heart failure and atrial fibrillation of the Spanish Society of Internal Medicine (SEMI). Features and costs of patients admitted for cardiac arrhythmias in Spain. Rev Clin Esp. 2013;213(5):235–239.
- Sicras-Mainar A, Navarro-Artieda R. [Cost of arterial hypertension according to levels of morbidity in primary care setting]. Med Clin. 2009;133(8):290–295.
- Freda PU, Gordon MB, Kelepouris N, et al. Long-term treatment with pegvisomant as monotherapy in patients with acromegaly: experience from ACROSTUDY. Endocr Pract. 2015;21(3):264–274. .
- Eckhardt R, Ulam S, von Neumann J, and the Monte Carlo method. Los Alamos Sci. 1987;15(6):131-146.
- Muhammad A, van der Lely AJ, Delhanty PJD, et al. Efficacy and safety of switching to pasireotide in patients with acromegaly controlled with pegvisomant and first-generation somatostatin analogues (PAPE study). J Clin Endocrinol Metab. 2018;103(2):586–595.
- Feola T, Cozzolino A, Simonelli I, et al. Pegvisomant improves glucose metabolism in acromegaly: a meta-analysis of prospective interventional studies. J Clin Endocrinol Metab. 2019. DOI:10.1210/jc.2018-02281
- Vallejo-Torres L, García-Lorenzo B, Castilla I, et al. Valor Monetario de un Año de Vida Ajustado por Calidad: estimación empírica del coste de oportunidad en el Sistema Nacional de Salud.: ministerio de Sanidad, Servicios Sociales e Igualdad. Servicio de Evaluacio´n del Servicio Canario de la Salud. 2015.
- Drummond MF, Wilson DA, Kanavos P, et al. Assessing the economic challenges posed by orphan drugs. Int J Technol Assess Health Care. 2007;23(1):36–42.
- Hughes DA, Tunnage B, Yeo ST. Drugs for exceptionally rare diseases: do they deserve special status for funding? Qjm. 2005;98(11):829–836.
- Picavet E, Dooms M, Cassiman D, et al. Drugs for rare diseases: influence of orphan designation status on price. Appl Health Econ Health Policy. 2011;9(4):275–279.
- Maione L, Brue T, Beckers A, et al. French acromegaly registry group. Changes in the management and comorbidities of acromegaly over three decades: the French Acromegaly Registry. Eur J Endocrinol. 2017;176(5):645–655.
- Margusino-Framiñán L, Pertega-Diaz S, Pena-Bello L, et al. Cost-effectiveness analysis of preoperative treatment of acromegaly with somatostatin analogue on surgical outcome. Eur J Intern Med. 2015;26(9):736–741.
- Didoni G, Grottol S, Gasco V, et al. Cost-of-illness study in acromegalic patients in Italy. J Endocrinol Invest. 2004;27(11):1034–1039.
- Luque-Ramírez M, Paramo C. Varela da Costa C, García-Mayor RV. Cost of management of invasive growth hormone-secreting macroadenoma. J Endocrinol Invest. 2007;30(7):541–545.
- Wilson LS, Shin JL, Ezzat S. Longitudinal assessment of economic burden and clinical outcomes in acromegaly. Endocr Pract. 2001;7(3):170–180.
- Valentim J, Passos V, Mataveli F, et al. Cost-effectiveness analysis of somatostatin analogues in the treatment of acromegaly in Brazil. Arq Bras Endocrinol Metabol. 2008;52(9):1452–1460.
- Roset M, Merino-Montero S, Luque-Ramírez M, et al. Spanish group of the OASIS study. Cost of clinical management of acromegaly in Spain. Clin Drug Investig. 2012;32(4):235–245.
- Leonart LP, Borba HHL, Ferreira VL, et al. Cost-effectiveness of acromegaly treatments: a systematic review. Pituitary. 2018;21(6):642–652.
- Orlewska E, Stępień R, Orlewska K. Cost-effectiveness of somatostatin analogues in the treatment of acromegaly. Expert Rev Pharmacoecon Outcomes Res. 2019;19(1):15–25.
- Biermasz NR, Roelfsema F, Pereira AM, et al. Cost-effectiveness of lanreotide Autogel in treatment algorithms of acromegaly. Expert Rev Pharmacoecon Outcomes Res. 2009;9(3):223–234.
- Shimon I, Adnan Z, Gorshtein A, et al. Efficacy and safety of long-acting pasireotide in patients with somatostatin-resistant acromegaly: a multicenter study. Endocrine. 2018 Nov;62(2):448–455.
- Duan L, Huang M, Yan H, et al. Cost-effectiveness analysis of two therapeutic schemes in the treatment of acromegaly: a retrospective study of 168 cases. J Endocrinol Invest. 2015;38(7):717–723.