ABSTRACT
Background
This study aims to report on the design of a model to determine the cost-effectiveness of prevention and treatment of early psychosis (PsyMod) and to estimate ten-year cost-effectiveness and budget impact of interventions targeting individuals with ultra-high risk (UHR) of developing psychosis or with first episode psychosis (FEP).
Methods
PsyMod was built in parallel with the development of a new standard of care for treatment of early psychosis in the Netherlands. PsyMod is a state-transition cohort simulation model and considers six health states, namely ultra-high risk of psychosis (UHR), FEP, post-FEP, no-UHR, recovery/remission, and death. Results are expressed as total healthcare costs, QALYs, incremental cost-effectiveness ratio (ICER), and budget impact.
Results
PsyMod was used to extrapolate budget impact and cost-effectiveness of cognitive behavioural therapy for preventing FEP for individuals at UHR of psychosis (CBTuhr) compared to care as usual. CBTuhr resulted in a per-patient increase of 0.06 QALYs and a per patient cost reduction of €654 (dominant ICER) with a reduction in 5-year healthcare costs of €1,002,166.
Conclusions
PsyMod can be used to examine cost-effectiveness and budget impact of interventions targeting prevention and treatment of FEP and is freely available for academic purposes upon request by the authors.
1. Introduction
Schizophrenia is among the world’s leading causes of disability affecting the lives of patients (and their families) during the critical years of adolescence and early adulthood [Citation1–Citation5]. Besides having a negative impact on social life and work [Citation6,Citation7], schizophrenia is associated with an increase in substance abuse, depression, violence, and suicide attempts [Citation7]. Consequently, health-related and economic costs to patients, their families and society are substantial [Citation8,Citation9] and go beyond direct medical costs [Citation10]. While the main driver of indirect costs seems to be long-term unemployment [Citation11], hospitalization alone may account for up to 77% of the direct cost [Citation12].
The high burden of schizophrenia spectrum and other psychotic disorders (as it is formally defined in the Diagnostic and Statistical Manual of Mental Disorders fifth edition [Citation13]) underscores the importance of early detection and proactive intervention in patients at risk of a first psychosis [Citation14,Citation15]. Likewise, delayed detection and treatment, resulting in longer duration of untreated psychosis (DUP), is associated with poor outcomes [Citation16–Citation18]. The two main goals of early intervention programmes are hence (1) to reduce the DUP, and (2) to provide consistent and comprehensive care throughout the most critical first years of the disorder [Citation19]. In recent years these programmes became more and more available with evidence suggesting their effectiveness and cost-effectiveness [Citation7,Citation10,Citation12,Citation20–Citation26].
Also regarding other elements of care for psychosis or schizophrenia, a range of cost-effectiveness studies have been performed. Several of these used health economic decision models, using various modelling techniques [Citation27]. Most model-based economic evaluations focused on pharmacological interventions in stages later than ‘ultra-high risk of psychosis’ (UHR) or ‘first episode psychosis’ (FEP) [Citation28–Citation37]. Moreover, there is currently no cost-effectiveness model available combining pathways for patients with UHR and FEP in one model which is useful to evaluate the simultaneous impact of various interventions targeting different stages of (the development of) schizophrenia.
Therefore, we developed a new model, which was based on McGorry et al.’s clinical staging model [Citation38]. With the introduction of a clinical staging model of psychiatric disorders by McGorry and colleagues [Citation38], the potential to improve and individualise treatment and timing of interventions increased. Policy-makers are in need of reliable information on both costs and health effects of current practices as well as alternatives to enable well informed decisions [Citation39]. McGorry’s model includes, among others, the stages 1) ultra-high risk of psychosis; 2) first episode of psychotic disorder; 3a) incomplete remission from first episode; 3b) recurrence or relapse of psychotic disorder; 3c) multiple relapses; and 4) severe, persistent or unremitting illness. Patients with UHR demonstrate certain levels of intensity, severity and/or pathognomonic symptoms (e.g. a Global Assessment of Functioning-score <70) [Citation40]. The development of this staging model in which the importance of identification and treating patients at UHR for psychosis was emphasized, boosted an interest in preventive psychiatry and it has been demonstrated that early detection and intervention in people at ultra-high risk of developing psychosis can be successful to prevent or delay a first psychosis [Citation40–Citation42].
The goal of the current manuscript is therefore to present a health-economic model to determine the cost-effectiveness of prevention and treatment of early psychosis (named PsyMod). PsyMod was developed in parallel with the development of a new Standard of Care for psychosis in the Netherlands. The model aims to estimate the long-term cost-effectiveness of interventions targeting individuals at ultra-high risk (UHR) of developing a first psychosis or those with a current first episode psychosis (FEP). Here, we describe (1) the process of developing a conceptual model, (2) the final structure of the model, and (3) the parameters used to populate the model. Finally, PsyMod will be illustrated by evaluating the cost-effectiveness and budget impact of cognitive behaviour therapy to prevent FEP in individuals at UHR.
2. Development of the model
2.1. Model design and structure
Using the clinical staging model from McGorry et al. [Citation38], the model was built in parallel with the development of a multidisciplinary standard of care for treatment of early psychosis in the Netherlands [Citation43]. During a period of two years, a group of healthcare providers (n = 10), patient (family) representatives (n = 2), and researchers (n = 6) (hereafter: guideline group, GG) came together at approximately six-week intervals to develop a new guideline and to reflect on the model. An iterative process was started to simplify the conceptual model such that it could be supported with epidemiological evidence, while still maintaining a satisfying level of face validity according to the experts. Epidemiological evidence was gathered to guide this process by means of a comprehensive quasi-systematic literature review in PsycINFO® (see appendix 1).
After arriving at a simplified conceptual model, the next step was to use available evidence from the literature either to populate the model with regard to the epidemiology of the onset and course of psychosis (e.g. transition parameters from one health state to another), or to calibrate modelled epidemiological outcomes (e.g. the ratio in prevalence between two stages; see below). Lastly, a draft version of the model was presented at a health economic study group conference (lolaHESG 2015), in order to receive comments from independent peers.
A flow-diagram regarding the involvement of expert opinion during model development is found in appendix 2.
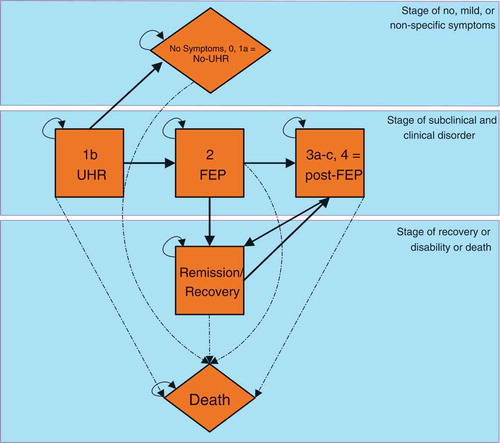
The disease progression model as defined by McGorry et al [Citation38], including the states 1b) ultra-high risk of psychosis; 2) first episode of psychotic disorder; 3a) incomplete remission from first episode; 3b) recurrence or relapse of psychotic disorder; 3c) multiple relapses; and 4) severe, persistent or unremitting illness, was simplified by combining the states 3a, 3b, 3c and 4 into a single post-FEP state. This simplification was made as experts deemed the epidemiological evidence-base to be insufficient for providing reliable input on the transitions between these states. Moreover, our aim was limited to developing a model able to assess the cost-effectiveness of services targeting individuals at ultra-high risk of developing psychosis and with a first episode of psychosis. In addition, we added a no-UHR state to capture individuals who no longer have a risk-status of developing a psychosis, a state representing recovery/remission, and a death state (see ).
In PsyMod, simulated patients will start in the UHR stage. In the UHR stage, individuals can transition to either FEP, no-UHR, or death. Once a transition to no-UHR has occurred, patients remain in this state until the end of the time horizon (or when they die). Similarly, once patients transfer to FEP, a transition back to UHR is not possible. From FEP, patients can only move to the post-FEP state or to remission/recovery. A transition to death is always possible and death is an absorbing end-state. In principle, only at one instance can individuals transition back and forth between two health states in PsyMod. This is the case for the states of post-FEP and remission/recovery.
Given the chronic character of the condition and as epidemiological evidence regarding the incidence and prevalence often reports time intervals of one year, it was chosen to set the cycle length of the model accordingly. A time-horizon of ten years was deemed long enough by the experts to capture the costs and effects of interventions targeting either individuals at ultra-high risk of developing psychosis and interventions targeting individuals with a first episode of psychosis. For example, it has been demonstrated that almost all transition from UHR to FEP occur within 10 years of entry to the clinic [Citation44]. The simulated cohorts starts at an age of 25 years (in line with the mean age in Ising et al. [Citation45]). The model was constructed such that both a five-year and ten-year time horizon can be applied.
A health care system perspective was assumed due to the paucity on evidence regarding societal costs. Future outcomes were discounted at 4% (costs) and 1.5% (effects), in accordance with the pertinent Dutch guideline for pharmacoeconomic research [Citation46]. Half-cycle correction was applied [Citation47]. All costs are expressed in 2018 Euros, by indexing unit cost prices as reported in the Dutch guideline for economic evaluations [Citation46] using the consumer price index.
2.2. Model parameters
Data concerning transition rates between health states were extracted from existing literature. Costs and quality of life associated with each stage were based on the resource use and utilities as observed in a recent Dutch UHR trial with a four-year follow-up [Citation45]. The 1-year incidence of psychosis in the Netherlands was taken from estimates reported in the Dutch Handbook of Early Psychosis [Citation48].
Efforts made to validate the model were based on the Assessment of the Validation Status of Health-Economic decision models (AdViSHe) questionnaire [Citation49]. The conceptual model was validated on face validity (by experts) and cross validity testing (e.g. to other staging and health economic models). Input data was validated based on face validity only as model fit testing (e.g. using R2 statistics) was deemed not applicable (i.e. no parameters of the model were based on regression models). Validation of the results and assumptions of the model was done by external expert review (i.e. the Lowlands Health Economic Study Group), and in addition via extreme value testing (e.g. to see whether any errors occurred), and by checking whether the relative number of patients in each cycle and state is consistent with empirical evidence. Operational validity was assessed by face validity (e.g. by asking experts whether model outcomes were within a likely (or expected) range) and cross validation testing of model outcomes (e.g. check whether internal epidemiology was consistent with previous research).
2.2.1. Transition probabilities
Transitions to death were based on general mortality statistics in the Netherlands as reported by Statistics Netherlands [Citation50] (based on the age specific mean mortality rate of people in the Netherlands) and excess mortality in individuals with psychosis as reported by Saha et al. [Citation3].
The expert panel deemed the meta-analysis of Fusar-Poli et al. (2012) to be the most complete source of input on the transitions up to FEP [Citation51]. Fusar-Poli et al. (2012) conducted a meta-analysis of transition rates in individuals at UHR. A total of twenty-seven included studies comprised 2,502 patients with a mean age of 19.9 years. Transition risks were reported for the time points after six months, after one year, and after two and three years.
Whereas the estimate of the one-year transition by Fusar-poli et al. (2012) from UHR to FEP could be directly used as a transition parameter in the Markov model, the transitions between the model states before FEP (UHR to UHR; UHR to no-UHR and no-UHR to no-UHR) had to be calibrated, such that the two-year and three-year transitions reported by Fusar-Poli et al. (2012) were approximated by the model, see and for the parameter values and appendix 3 for additional details.
Table 1. State dependent yearly transition probabilities, as the mortality rate is age dependent it is not presented in this table.
Table 2. justification of epidemiology parameters.
Austin et al. (2013) was used as our main source of epidemiological input from the states starting at FEP [Citation52]. Austin et al. (2013) reported on the disease progress of FEP patients over a ten-year time-horizon in the Danish OPUS trial. OPUS originally enrolled 496 individuals with first episode of psychosis, and 304 (61%) participated in the 10-year follow-up. The authors present prevalence rates of ‘recovery’, ‘symptom remission’, ‘positive and/or negative symptoms’, and ‘institutionalisation’ at two, five, and ten-year follow-up. Experts considered this study to be appropriate for epidemiological input, as the long-term follow-up, together with the multi-year, multi-state report on prevalence rates, resulted in a higher internal consistency of the modelled epidemiology than the use of multiple, different studies. The OPUS categories of ‘recovery’ and ‘symptom remission’ were considered to represent our model state remission/recovery, whereas the OPUS categories ‘positive and/or negative symptoms’ and ‘institutionalisation’ were interpreted as representing our model state post-FEP. and and appendix 3 provide more details on the model transitions derived from Austin et al. (2013) [Citation52].
The resulting estimates were also validated using a more recent meta-analysis of Fusar-poli et al. 2016 [Citation53]. This meta-analysis was used for validation purposes only as it does not provide information regarding health states other than FEP and the prevalence of FEP for various points in time. In this meta-analysis, the risk of a recurrence after FEP was estimated to be 0.42 (95%CI: 0.30–0.54), 0.78 (95%CI: 0.58–0.93), and 0.84 (95%CI: 0.70–0.94) after one, two, and three years’ post-FEP respectively. This was in line with the estimates calculated in PsyMod (0.50, 0.74, 0.86).
2.2.2. Modelled epidemiology
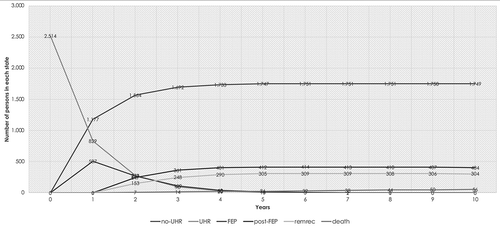
Next, we estimated the required size of the simulated population to be in line with Dutch epidemiology in order to estimate the cost-effectiveness and total budget impact. In a given year, approximately 3,000 people in the Netherlands develop a FEP [Citation48]. Furthermore, Fusar-poli et al. (2012) estimated the transition probability from UHR to FEP to be 35.8% [Citation51]. This would mean that in the Netherlands, there are approximately 3,000/0.358 = 8,330 new UHR patients annually (assuming every person with FEP would first be UHR). However, interventions targeting UHR can only be offered to people already in the mental health system (because otherwise they could not be screened for UHR). It is estimated that approximately 30% of the UHR patients are currently in the mental health system [Citation54]. Hence, the estimated size of the simulated cohort was 8,330 * 0.3 = 2,514. The absolute number of individuals from one yearly cohort of people with UHR, combined with the transition matrix presented in , results in the modelled disease progression for this cohort over a time-horizon of ten years as depicted in .
provides an overview of the input and output regarding the epidemiology, divided in parameters that were taken straight from the literature (direct input), parameters that were used to calibrate the epidemiology (calibrated parameters) and output from the model that could be compared with outputs observed in literature (outcomes used for calibration). The use of these three types of parameters was necessary 1) as not all parameters could be directly observed in literature; and 2) to make sure that model outcomes are in line with existing literature. The process of calibration is explained in more detail in appendix 3.
2.3. Measurement and valuation of costs and health effects
The multicentre trial conducted in the Netherlands by Ising et al. [Citation12,Citation45] was selected by experts to provide input on the cost and quality of life estimates of the different states in the model. Ising et al. [Citation12,Citation45] provide input on the four-year costs and quality of life of people at ultra-high risk of developing psychosis receiving usual care. As a part of the trial-population developed a first episode of psychosis, these four-year cost and quality of life data could be used to estimate the costs and quality of life associated with the different states in the model.
In order to estimate health care costs and utilities for each health state, the trial data was categorized according to the following assumptions:
For UHR: mean costs and utilities of patient in the first two years of the trial who did not develop FEP.
For no-UHR: mean costs and utilities of patients in the third and fourth year of the trial who did not develop FEP. One should keep in mind that these are still people with minor to moderate mental problems.
For FEP: mean costs and utilities of patients in the first two years after FEP.
For Remission/recovery: assumed to be equal to no-UHR + €1,000 (before indexing to 2018; based on clinical opinion). This assumption was made as it was considered that patients in the remission/recovery state were considered to use slightly more health care sources compared to no-UHR.
Post-FEP: mean costs and utilities of patients in the third and fourth year after FEP, excluding cost and utilities for those in remission/recovery.
presents cost and utility estimates per health state in care as usual based on Ising et al. (2016) The associated resource use costs can be found in the online supplement of Ising et al. (2016) [Citation45], and have been adapted as mentioned above to fit the health states of the model. The costs presented below also include costs of treatments included in care as usual which were defined in accordance with the Dutch clinical guidelines (see below).
Table 3. costs and utilities associated with each state.
2.4. Interventions included in the base case (care as usual)
Care as usual was defined based on Dutch clinical guidelines. Experts estimated that care as usual required three treatments for patients with FEP: 1) medication (to 100% of the patients); 2) CBT focusing on patients with FEP (to 50% of the patients); and 3) family intervention (to 5% of the patients). Moreover, in care as usual, no interventions are offered to UHR patients (except for treatments they are receiving for other than UHR).
2.5. Model output
Results in PsyMod are expressed as total healthcare costs, QALYs, life years per scenario (i.e. base case or alternative scenario), and budget impact. The incremental cost-effectiveness ratio (ICER) is then calculated as follows: (Costsalternative – Costsbase case)/(QALYsalternative – QALYsbase case). This way, the ICER represents the incremental costs per QALY gained.
2.5.1. Probabilistic sensitivity analysis
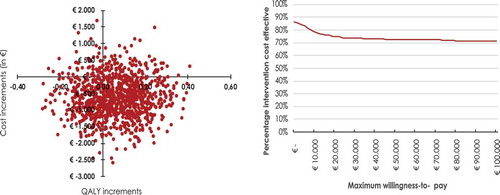
Parameter uncertainty is examined in the model through probabilistic sensitivity analysis. We characterise uncertain parameters (i.e. all input parameters in the model) by using probability distributions (e.g. normal distribution or beta distribution). As utility values are bound between 0 to 1, the beta distribution was used for these parameters. Transition probabilities are also bound between 0 and 1, but transitions coming from one health state should sum to 1 (e.g. otherwise you would lose/gain ‘patients’ moving from these health states). Hence, the Dirichlet distribution was used, which is basically a multivariate beta distribution. To capture uncertainty around the costs associated with each health state, the gamma distribution was used. After assigning distributions to all parameters, the model was re-run 1,000 times in which each time a random draw from the parameter distributions for every parameter was taken, leading to 1,000 ICERs. Next, all 1,000 simulations were plotted on a cost-effectiveness plane (CE-plane) to capture the uncertainty in the ICER estimate. Finally, a cost-effectiveness acceptability curve is constructed for the costs per QALY gained in which the likelihood that the alternative scenario is cost-effective is presented given different willingness-to-pay thresholds.
3. Results of model testing
To apply PsyMod to a real-world example, we extrapolated the results of a trial-based economic evaluation of Ising et al. (2016) in which the cost-effectiveness of cognitive behaviour therapy for preventing first-episode psychosis for individuals at UHR of psychosis (CBTuhr) [Citation45]. This study demonstrated that CBTuhr resulted in a significantly lower proportion of transitions to psychosis compared to care as usual (with a risk difference of 0.122). Moreover, the intervention cost of CBTuhr was estimated to be €1,924 per patient. In the here presented application of PsyMod, CBTuhr (and the corresponding cost and effect parameters) was added to the model as a ‘new’ intervention at an arbitrary coverage rate of 25% and compared to care as usual. In addition, the ten-year cost-effectiveness and five-year budget impact of adding CBTuht to care as usual was examined using PsyMod (in accordance with the Dutch guidelines for budged impact and economic evaluations) [Citation46].
Running the model with these scenarios resulted in the cost-effectiveness plane and CEAC as presented in & . On average, adding CBTuhr to care as usual resulted in a per-patient increase of 0.06 QALYs and a per-patient cost reduction of €654 over a ten-year time horizon resulting in a dominant ICER. On a national level, looking at the budget impact, the implementation of CBTuhr would reduce total five-year (undiscounted) healthcare costs by €1,002,166 indicating that the required intervention costs of € 1.2 million are more than offset. For the first five years, yearly savings are: €161,154; €208,556; €214,245; €213,400; and €204,812 respectively (undiscounted, in accordance with Dutch guidelines [Citation46]).
4. Discussion
PsyMod is the first model developed with the potential to assess the cost-effectiveness of competing interventions targeting UHR and FEP. Models are a useful tool to simulate real-world situations and can provide valuable insights for policymakers on how to optimally allocate available resources. However, all simulation approaches are based on assumptions. In our paper, we aimed to be transparent about the assumptions underlying PsyMod, such that users can form a good judgement on the validity and usefulness of its results. Results of PsyMod were compared to clinical studies and calibrated in such a way that they correspond with observed epidemiology. Moreover, PsyMod was used to extrapolate findings of the cost-effectiveness of CBTuhr and showed that CBTuhr is likely to be a promising intervention on the long-term in terms of cost-effectiveness. One should keep in mind, however, that in this analysis the costs of identifying persons at UHR were not considered.
Albeit similar models do not exist, health economic modelling has been used in the past to examine treatment effects for schizophrenia or psychosis specifically. For example, Valmaggia et al. [Citation36] employed a simple decision tree model (i.e. only looking at ‘transition to psychosis’ or ‘no transition’) to evaluate the effects of Outreach and Support in South London, an early intervention in people at high risk of psychosis. They concluded that services that permit early detection of people at high risk of psychosis may be cost saving. Next, McCrone et al. [Citation32] used a Markov model to examine the effects of early intervention services on service costs for people with first‐episode psychosis. Instead of using health states, the model was constructed to map care pathways. Again, early intervention was demonstrated to be cost saving.
Most of the currently available health economic models focus solely on antipsychotics with a variety of health states ranging from symptomatic health states (e.g. remission/no remission [Citation55–Citation57];) to adverse events only [Citation58,Citation59]. For example, a study of Perlis et al. [Citation57] examined the cost-effectiveness of a genetic test that may identify individuals with greater likelihood of responding to clozapine treatment for schizophrenia and focused on ‘recovered from psychosis’, or ‘psychosis’ [Citation57]. Palmer et al. [Citation55,Citation56] examined the cost-effectiveness of three antipsychotic treatments for people with schizophrenia using a Markov model. Albeit being technically slightly different to more recent Markov models, this model focused on symptomatic health states, i.e. ‘positive & negative syndromes’, ‘negative syndromes only’, ‘positive syndromes only’, ‘no positive & no negative syndromes’, ‘suicide’, and ‘death’.
Several strengths and limitations apply to our modelling approach. By closely working together with a group of experts in the field of early psychosis, we increased the clinical relevance of our model. Likewise, by presenting a draft version of the model at a health economic study group conference (lolaHESG 2015), we were able to integrate expert opinion on our assumptions from an early state on and from an interdisciplinary perspective. PsyMod incorporates evidence from a range of different input sources. Epidemiological evidence on transition rates was complemented with clinical expert opinion. Furthermore, costs and health utilities were derived from an RCT with long follow-up [Citation12]. At first glance, a Dutch study looking at costs of antipsychotic medication, psychiatric and somatic health care for patients estimated vastly higher annual costs compared to the estimates used in PsyMod [Citation60]. However, this study was conducted in substantially older patients, based on claims data, and did not follow a cohort approach (only a snapshot in time and hence a relatively high number of patients with long-term care). Finally, PsyMod was partly validated using the AdviSHE tool (i.e. not all items from the AdviSHE tool could be covered). Several other limitations need to be acknowledged. Empirical evidence in our model is based on a limited number of sources. Available evidence on UHR and FEP regarding prevalence and long-term implications is scarce. In addition, a number of original studies that could have been useful to inform our model were either conducted with small sample sizes (i.e. smaller than 100 participants), or did not cover the stages of interest in our model [Citation61,Citation62]. Therefore, we chose to base our assumptions on a limited number of relevant publications, complemented with expert opinion. Further, PsyMod was constructed from a Dutch perspective. No direct conclusions can be drawn about its applicability to other countries. Moreover, given the lack of accurate data on societal costs, PsyMod incorporates health care costs only and thus solely provides a health care perspective. The choice for a cohort Markov model automatically implies that we did not model individual patient characteristics (in contrast to taking a micro-simulation approach) and that transition probabilities between states were assumed to be stable over time. However, a more individual patient-oriented simulation, e.g., a micro-simulation, requires substantially more information to construct the model which was not available. The stages severe, persistent or unremitting illness as outlined in McGorry et al. [Citation38], were simplified by combining these into a single post-FEP state. This simplification was justified by the fact that experts deemed the epidemiological evidence-base to be insufficient for providing reliable input on the transitions between these states, but might bias our result (e.g. by resulting in less extreme cases by merging states). Lastly, all simulated patients start in the UHR state, which implicitly assumes that every individual developing FEP qualifies for UHR first. In reality, it is likely that several phenotypic pathways leading to FEP exists outside of the UHR paradigm (e.g. in patients in which early psychotic symptoms may be absent) [Citation40,Citation63]. Furthermore, if a proportion of the UHR patients is not detected this would mean a reduction in the demonstrated benefits. When extrapolating the results for CBTuhr using PsyMod, we have accounted for this by assuming a non-optimal coverage rate (i.e. 25%).
4.1. Conclusion
PsyMod is the result of a comprehensive modelling exercise and can be used to examine the cost-effectiveness and budget impact of interventions targeting prevention and treatment of first episode of psychosis. PsyMod can be used for various interventions (e.g. medication, cognitive behaviour therapy, et cetera) in a relatively easy and accessible way. Moreover, PsyMod is freely available for academic purposes upon request from the authors. PsyMod may stimulate the generation and uptake of cost-effectiveness evidence and thereby help to increase efficiency of mental health care, particularly in the Netherlands. Further research should examine the transferability of the results provided by the model to other countries and to determine what changes may be necessary to make the model applicable to other settings.
Article Highlights
PsyMod is a methodologically sound model that can be used to examine the cost-effectiveness and budget impact of interventions targeting prevention and treatment of first episode of psychosis.
PsyMod can be used for various interventions (e.g. medication, cognitive behaviour therapy, et cetera) in a relatively easy and accessible way.
A real-world application for healthcare in the Netherlands of the PsyMod in which cognitive behaviour therapy for preventing FEP for individuals at UHR of psychosis (CBTuhr) was compared to care as usual (CAU) demonstrated that CBTuhr dominated CAU while reducing total five-year (undiscounted) healthcare costs by €1,002,166.
PsyMod is freely available for academic purposes upon request by the authors.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Authors contributions
Overall project coordination: JL, FS, SMAAE, MH. Writing of manuscript: BW, FWT. Critical appraisal of manuscript: SK, TF, MVDG, WV, LDH, HI, FS. All authors have read and approved the manuscript.
Additional material
Appendix 1: ‘Appendix 1 – Literature search’
Appendix 2: ‘Appendix 2 - Involvement of expert opinion in the model development adapted from Ramos et al.’
Appendix 3: ‘Appendix 3 – transition parameters’
Availability of data and materials
The model (PsyMod) is freely available upon request at the authors.
Supplemental Material
Download MS Word (119.5 KB)Acknowledgments
We would like to thank everyone participating in the development of the multidisciplinary guideline for early psychosis in the Netherlands and provided us with the necessary expert input. In addition, we would like to thank all people participating in the Lowlands Health Economic Study Group 2015 session which was focused around PsyMod.
Supplementary Material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Organization WH. Schizophrenia. 2015 [updated 2015; cited 2015 Sept 2]. Available from: http://www.who.int/mental_health/management/schizophrenia/en/
- Lauber C, Keller C, Eichenberger A, et al. Family burden during exacerbation of schizophrenia: quantification and determinants of additional costs. Int J Social Psychiatry. 2005;51(3):259–264.
- Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123–1131.
- Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380(9859):2197–2223.
- Neil AL, Carr VJ, Mihalopoulos C, et al. Costs of psychosis in 2010: findings from the second Australian National Survey of Psychosis. Aust N Z J Psychiatry. 2014;48(2):169–182.
- Melau M, Jeppesen P, Thorup A, et al. The effect of five years versus two years of specialised assertive intervention for first episode psychosis-OPUS II: study protocol for a randomized controlled trial. Trials. 2011;12(1):72.
- Hastrup LH, Kronborg C, Bertelsen M, et al. Cost-effectiveness of early intervention in first-episode psychosis: economic evaluation of a randomised controlled trial (the OPUS study). Br J Psychiatry. 2013;202(1):35–41.
- Stant AD, TenVergert EM, Wunderink L, et al. Economic consequences of alternative medication strategies in first episode non-affective psychosis. Eur Psychiatry. 2007;22(6):347–353.
- Mangalore R, Knapp M. Cost of schizophrenia in England. J Ment Health Policy Econ. 2007;10(1):23.
- Angelo C, Vittorio M, Anna M, et al. Cost-effectiveness of treating first-episode psychosis: five-year follow-up results from an Italian early intervention programme. Early Interv Psychiatry. 2011;5(3):203–211.
- Carr VJ, Neil AL, Halpin SA, et al. Costs of schizophrenia and other psychoses in urban Australia: findings from the Low Prevalence (Psychotic) Disorders Study. Australas Psychiatry. 2003;37(1):31–40.
- Ising HK, Smit F, Veling W, et al. Cost-effectiveness of preventing first-episode psychosis in ultra-high-risk subjects: multi-centre randomized controlled trial. Psychol Med. 2014;2014:1–12.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®). Philadelphia, USA: American Psychiatric Pub; 2013.
- Edwards J, McGorry PD. Implementing early intervention in psychosis: A guide to establishing psychosis services. London: Taylor & Francis; 2002.
- Larsen TK, Friis S, Haahr U, et al. Early detection and intervention in first-episode schizophrenia: a critical review. Acta Psychiatr Scand. 2001;103(5):323–334.
- Birchwood M, Todd P, Jackson C. Early intervention in psychosis. The Critical Period Hypothesis. Br J Psychiatry Suppl. 1998;1998(172):53–59.
- Jeppesen P, Petersen L, Thorup A, et al. The association between pre-morbid adjustment, duration of untreated psychosis and outcome in first-episode psychosis. Psychol Med. 2008;38(08):1157–1166.
- Marshall M, Lewis S, Lockwood A, et al. Association between duration of untreated psychosis and outcome in cohorts of first-episode patients: a systematic review. Arch Gen Psychiatry. 2005;62(9):975–983.
- McGorry PD, Killackey E, Yung AR. Early intervention in psychotic disorders: detection and treatment of the first episode and the critical early stages. Med J Aust. 2007;187(7 Suppl):S8–10.
- McCrone P, Singh SP, Knapp M, et al. The economic impact of early intervention in psychosis services for children and adolescents. Early Interv Psychiatry. 2013;7(4):368–373.
- Cullberg J, Mattsson M, Levander S, et al. Treatment costs and clinical outcome for first episode schizophrenia patients: a 3-year follow-up of the Swedish “Parachute Project” and Two Comparison Groups. Acta Psychiatr Scand. 2006;114(4):274–281.
- Breitborde NJ, Woods SW, Srihari VH. Multifamily psychoeducation for first-episode psychosis: A cost-effectiveness analysis. Psychiatric Serv. 2009;60(11):1477–1483.
- McCrone P, Craig TK, Power P, et al. Cost-effectiveness of an early intervention service for people with psychosis. Br J Psychiatry. 2010;196(5):377–382.
- Mihalopoulos C, McGorry PD, Carter RC. Is phase-specific, community-oriented treatment of early psychosis - an economically viable method of improving outcome? Acta Psychiatr Scand. 1999;100(1):47–55.
- Mihalopoulos C, Harris M, Henry L, et al. Is early intervention in psychosis cost-effective over the long term? Schizophr Bull. 2009;35(5):909–918.
- Correll CU, Galling B, Pawar A, et al. Comparison of Early Intervention Services vs Treatment as Usual for Early-Phase Psychosis: A Systematic Review, Meta-analysis, and Meta-regression. JAMA Psychiatry. 2018 Jun 1;75(6):555–565. PubMed PMID: 29800949; PubMed Central PMCID: PMCPMC6137532. eng.
- Kruse G, Wong BJ, Duh MS, et al. Systematic Literature Review of the Methods Used to Compare Newer Second-Generation Agents for the Management of Schizophrenia: A focus on Health Technology Assessment. PharmacoEconomics. 2015;33(10):1049–1067.
- Bobes J, Cañas F, Rejas J, et al. Economic consequences of the adverse reactions related with antipsychotics: an economic model comparing tolerability of ziprasidone, olanzapine, risperidone, and haloperidol in Spain. Prog Neuro Psychopharmacol Biol Psychiatry. 2004;28(8):1287–1297.
- Druais S, Doutriaux A, Cognet M, et al. Cost effectiveness of paliperidone long-acting injectable versus other antipsychotics for the maintenance treatment of schizophrenia in France. PharmacoEconomics. 2016;34(4):363–391.
- Drukker M, Joore M, van Os J, et al. The use of a Cumulative Needs for Care Monitor for individual treatment v. care as usual for patients diagnosed with severe mental illness, a cost-effectiveness analysis from the health care perspective. Epidemiol Psychiatr Sci. 2012;21(04):381–392.
- Lin I, Muser E, Munsell M, et al. Economic impact of psychiatric relapse and recidivism among adults with schizophrenia recently released from incarceration: a Markov model analysis. J Med Econ. 2015;18(3):219–229.
- McCrone P, Knapp M, Dhanasiri S. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123–1131.
- O’Day K, Rajagopalan K, Meyer K, et al. Long-term cost-effectiveness of atypical antipsychotics in the treatment of adults with schizophrenia in the US. Clinicoecon Outcomes Res. 2013;5:459–470.
- Phanthunane P, Vos T, Whiteford H, et al. Cost-effectiveness of pharmacological and psychosocial interventions for schizophrenia. Cost Eff Resour Allocation. 2011;9(1):6.
- Tempest M, Sapin C, Beillat M, et al. Cost-effectiveness Analysis of Aripiprazole Once-Monthly for the Treatment of Schizophrenia in the UK. J Ment Health Policy Econ. 2015;18(4):185–200.
- Valmaggia L, McCrone P, Knapp M, et al. Economic impact of early intervention in people at high risk of psychosis. Psychol Med. 2009;39(10):1617–1626.
- Zeidler J, Mahlich J, Greiner W, et al. Cost effectiveness of paliperidone palmitate for the treatment of schizophrenia in Germany. Appl Health Econ Health Policy. 2013;11(5):509–521.
- McGorry PD, Hickie IB, Yung AR, et al. Clinical staging of psychiatric disorders: a heuristic framework for choosing earlier, safer and more effective interventions. Aust N Z J Psychiatry. 2006;40(8):616–622.
- Vos, Theo, University of Queensland. School of Population Health. Centre for Burden of Disease and Cost Effectiveness and Deakin University. Deakin Population Health Strategic Research Centre Assessing cost-effectiveness in prevention: (ACE-Prevention): final report. Centre for Burden of Disease and Cost Effectiveness, School of Population Health, University of Queensland, Brisbane, 2010.
- McGorry PD, Mei C. Ultra-high-risk paradigm: lessons learnt and new directions. Evid Based Ment Health. 2018;21(4):131–133.
- van der Gaag M, Smit F, Bechdolf A, et al. Preventing a first episode of psychosis: meta-analysis of randomized controlled prevention trials of 12 month and longer-term follow-ups. Schizophr Res. 2013;149(1–3):56–62.
- Fusar-Poli P, Yung A, McGorry P, et al. Lessons learned from the psychosis high-risk state: towards a general staging model of prodromal intervention. Psychol Med. 2014;44(1):17–24.
- Zorginstituut Nederland. Zorgstandaard Psychose 2017. [cited 2019 Jan 14]. Available from: https://www.zorginzicht.nl/bibliotheek/schizofrenie-inclusief-module-vroege-psychose/Paginas/Home.aspx
- Nelson B, Yuen HP, Wood SJ, et al. Long-term Follow-up of a Group at Ultra High Risk (“Prodromal”) for Psychosis: the PACE 400 StudyFollow-up of Prodromal PsychosisFollow-up of Prodromal Psychosis. JAMA Psychiatry. 2013;70(8):793–802.
- Ising HK, Lokkerbol J, Rietdijk J, et al. Four-Year Cost-effectiveness of Cognitive Behavior Therapy for Preventing First-episode Psychosis: the Dutch Early Detection Intervention Evaluation (EDIE-NL) Trial. Schizophr Bull. 2017;43.2:365-374.
- Zorginstituut Nederland. Richtlijn voor het uitvoeren van economische evaluaties in de gezondheidszorg. Diemen: Zorginstituut Nederland; 2015.
- Naimark DM, Bott M, Krahn M. The half-cycle correction explained: two alternative pedagogical approaches. Med Decis Making. 2008 Sep-Oct;28(5):706–712. . PubMed PMID: 18448700; eng.
- Veling W, van der Wal M, Jansen S, et al. Handboek Vroege Psychose. Amsterdam: Uitgeverij SWP; 2012.
- Vemer P, Ramos IC, Van Voorn G, et al. AdViSHE: a validation-assessment tool of health-economic models for decision makers and model users. Pharmacoeconomics. 2016;34(4):349–361.
- Statistics Netherlands. Sterfte; geslacht, leeftijd (op 31 december) en burgerlijke staat 1950-2014. 2014 [2018 Nov 12]. Available from http://statline.cbs.nl/Statweb/publication/?DM=SLNL&PA=37530ned&D1=1&D2=101-120&D3=0&D4=l&HDR=T,G1&STB=G2,G3&VW=T
- Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012 Mar;69(3):220–229. . PubMed PMID: 22393215; eng.
- Austin SF, Mors O, Secher RG, et al. Predictors of recovery in first episode psychosis: the OPUS cohort at 10 year follow-up. Schizophr Res. 2013 Oct;150(1):163–168.
- Fusar-Poli P, Cappucciati M, Bonoldi I, et al. Prognosis of brief psychotic episodes: a meta-analysis. JAMA Psychiatry. 2016;73(3):211–220.
- Lokkerbol J, Lokman S, Janssen R, et al. Rendeert zorgonderzoek in de GGZ? Ned Tijdschr Evidence Based Pract. 2017;15(2):8–10.
- Hamilton SH, Brown RE. A cost-effectiveness clinical decision analysis model for schizophrenia. Am J Man Care. 1998;4:345–355.
- Palmer CS, Brunner E, Ruı́z-Flores LG, et al. A cost-effectiveness clinical decision analysis model for treatment of schizophrenia. Arch Med Res. 2002;33(6):572–580.
- Perlis RH, Ganz DA, Avorn J, et al. Pharmacogenetic testing in the clinical management of schizophrenia: a decision-analytic model. J Clin Psychopharmacol. 2005;25(5):427–434.
- De Graeve D, Smet A, Mehnert A, et al. Long-acting risperidone compared with oral olanzapine and haloperidol depot in schizophrenia: a Belgian cost-effectiveness analysis. Pharmacoeconomics. 2005;23(1):35–47.
- Vera‐Llonch M, Delea TE, Richardson E, et al. Outcomes and costs of risperidone versus olanzapine in patients with chronic schizophrenia or schizoaffective disorders: a Markov model. Value Health. 2004;7(5):569–584.
- van der Lee A, de Haan L, Beekman A. Schizophrenia in the Netherlands: continuity of Care with Better Quality of Care for Less Medical Costs. PloS One. 2016;11(6):e0157150–e0157150. . PubMed PMID: 27275609
- Yung AR, Phillips LJ, Yuen HP, et al. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophr Res. 2004 Apr 01;67(2):131–142. .
- Morrison AP, French P, Walford L, et al. Cognitive therapy for the prevention of psychosis in people at ultra-high risk: randomised controlled trial. Br J Psychiatry. 2004;185(4):291–297.
- Lee TY, Lee J, Kim M, et al. Can we predict psychosis outside the clinical high-risk state? A systematic review of non-psychotic risk syndromes for mental disorders. Schizophr Bull. 2018;44(2):276–285.