ABSTRACT
Introduction: Early biological treatment of rheumatoid arthritis (RA) may reverse the autoimmune response in some patients resulting in favorable long-term outcomes. Although the cost-effectiveness of this strategy has been questioned, biosimilar entries warrant the revision of clinical and pharmaco-economic evidence.
Areas covered: We conducted a systematic review of randomized controlled trials (RCTs) published up to 24 May 2018 in Pubmed, EMBASE and Cochrane CENTRAL, comparing infliximab with non-biological therapy in patients with RA naïve to methotrexate. We performed meta-analyses for efficacy outcomes at month 6 and years 1 and 2. Six RCTs were identified, involving 1832 patients. At month 6 ACR70 response and remission, and at year 1 ACR20/ACR70 responses and remission were improved significantly with first-line infliximab versus control. The differences were not significant at year 2. We reviewed cost-utility studies, up to 31 October 2018 in PubMed, Cochrane CENTRAL and the CRD HTA databases. Four studies indicated that first-line use of originator infliximab calculated at 2005–2008 prices was not cost-effective.
Expert opinion: We demonstrated the efficacy benefits of first-line infliximab therapy up to 1 year in methotrexate-naïve RA. We highlighted the need for standardized reporting of outcomes and conducting cost-effectiveness analyses of first-line biosimilar therapy in RA.
1. Introduction
Rheumatoid arthritis (RA) affects the synovial joints, causing inflammation and pain. Without treatment, progressive destruction of the joints can lead to considerable disability [Citation1–Citation3]. Patients with early or ‘recent-onset’ RA can be defined as individuals with a disease duration of 2 years or less [Citation1,Citation2]. Of late, there has been increasing emphasis on the prompt referral of patients with suspected early RA to rheumatologists in order to prevent irreversible joint damage [Citation1,Citation2]. Effective early treatment within the therapeutic ‘window of opportunity’ for RA may reverse the autoimmune response in some patients, leading to more favorable long-term outcomes compared with patients who are treated later in the disease course [Citation4,Citation5]. Moreover, achievement of early response may allow eventual tapering of RA treatment and potential drug-free remission in some patients [Citation6].
Tumor necrosis factor inhibitors (TNFis) and other biological disease-modifying antirheumatic drugs (bDMARDs) have traditionally been used to treat patients with long-standing RA who have experienced inadequate response to previous conventional synthetic DMARDs (csDMARDs) such as methotrexate (MTX) or leflunomide. However, a number of studies have demonstrated that TNFi treatment is also effective in patients with early RA [Citation7–Citation15]. Indeed, several bDMARDs, including infliximab and other TNFis, have now been approved for the first-line treatment of patients with severe, active and progressive disease that has not been treated with MTX or other csDMARDs [Citation16]. Recent meta-analyses have demonstrated the short-term benefits of biologic agents over csDMARDs in early, MTX/csDMARD-naïve RA, suggesting that more patients may achieve remission with first-line biological therapy than with csDMARDs [Citation3,Citation17–Citation19]. However, the selection of studies in these meta-analyses were diverse and sometimes incomplete [Citation3,Citation17,Citation18].
At the current time, bDMARDs such as infliximab are not widely used in first-line treatment of patients with early RA [Citation16,Citation20]. This fact is likely to be at least partly related to the most recent (2016) guidance on this topic issued by the European League Against Rheumatism (EULAR) [Citation21] and National Institute for Health and Care Excellence (NICE) [Citation16], both of which do not recommend first-line treatment with bDMARDs in patients with RA. The EULAR recommendations note that the early use, and potential for subsequent withdrawal, of bDMARDs was discussed, but was not supported by the majority of members of the recommendations task force [Citation21]. One reason for this was the lack of evidence for superiority of bDMARDs compared with the combination of MTX plus glucocorticoid. Furthermore, the task force commented that in the context of a treat-to-target strategy, initial use of csDMARDs has equivalent long-term efficacy. The cost-effectiveness of first-line bDMARD therapy was also questioned and considered to be poor given the lack of evidence supporting its superior long-term efficacy [Citation21]. EULAR therefore recommend the use of MTX in combination with short-term glucocorticoid as a first-line strategy for the treatment of RA. Following treatment failure with initial csDMARD therapy, additional csDMARDs or bDMARDs/targeted synthetic DMARDs may be initiated, depending on the prognostic status of each patient. The EULAR recommendations further state that tapering of bDMARDs should be considered if the patient is in sustained remission [Citation21]. NICE recommend the use of bDMARDs in combination with MTX for patients with severe RA (disease activity score [DAS] 28 > 5.1) following csDMARD therapy [Citation16].
The lack of uptake of biologic agents for the first-line treatment of RA may also be due to high drug costs [Citation20]. However, the introduction of biosimilar versions of RA-approved drugs may help to reduce treatment costs, with the availability of biosimilar infliximab since 2013 already leading to considerable price discounts in some regions [Citation22]. Budget impact analyses have predicted large healthcare savings following the uptake of biosimilar infliximab in RA across multiple markets [Citation23–Citation25]. There is, however, a need for more recent cost-effectiveness analyses that take into account recent reductions in the cost of some bDMARDs following the introduction of biosimilars. Because of its comparable efficacy and lower cost versus originator infliximab, biosimilar infliximab may be an ideal candidate with which to reassess the available medical and economic evidence for first-line biological therapy in MTX-naïve, early RA. The aims of our study, therefore, were to systematically review the literature and analyze the outcomes of first-line infliximab treatment versus other treatment strategies in patients with MTX-naïve RA, with a special focus on long-term efficacy outcomes. We also reviewed available health economic literature for infliximab cost-utility studies in this patient population.
2. Methods
2.1. Search strategy for clinical studies
We conducted a systematic literature search in PubMed, EMBASE and the Cochrane Central Register of Controlled Trials (CENTRAL) up to 24 May 2018. Our search included the Cochrane filter for clinical trials [Citation26], all known development codes of infliximab [Citation27–Citation29], and the MeSH term for RA. Full details of the PubMed, Embase and CENTRAL search criteria are shown in Supplementary Tables S1, S2 and S3, respectively. To identify papers not yet indexed by MeSH terms, we conducted another search in PubMed without including RA as a keyword for the period of January 1 to 24 May 2018 (Supplementary Table S1).
2.2. Selection of clinical studies
Eligible studies included published randomized controlled trials (RCTs) involving patients with RA who were MTX-naïve at randomization. The treatment intervention of interest was infliximab. Control treatments could include any pharmacological treatment (i.e. placebo, csDMARDs and/or steroids) with or without concomitant MTX that did not involve any biological therapy at study initiation, although biological treatment could be included per protocol in a later phase of the study. Multiple publications from the same RCT were retained, including RCT extension studies. We did not impose any language restrictions on our search, but we excluded case studies, pooled analyses, review articles and conference abstracts.
For mapping available evidence, outcomes of interest included any physician- or patient-reported clinical efficacy, economic or radiological outcome, as well as any key safety outcomes such as occurrence of adverse events (AEs), discontinuations due to AEs, deaths, infusion reactions, severe infections, tuberculosis, malignancy, cardiovascular events and immunogenicity (i.e. the presence of anti-drug antibodies [ADAs]). For quantitative syntheses, we included studies reporting American College of Rheumatology (ACR) response criteria (ACR20/ACR70) or clinical remission (see ‘Data extraction’ section below for definitions) measured at month 6, year 1 and year 2. The abstract of each potential publication was reviewed by two independent researchers to confirm relevance and, if needed, a third independent researcher resolved any differences.
2.3. Data extraction from clinical studies
All reported outcomes (detailed above) and corresponding time points for all relevant primary and secondary publications of the selected studies were recorded. For quantitative synthesis, a Microsoft Excel spreadsheet was developed to capture the following details of relevant studies by treatment arm: study name; reference; treatment and dosing; number of randomized patients, risk of bias assessment; ACR20, ACR70 and remission based on any criteria (ACR, EULAR, DAS in 28 or 44 joints [DAS28/DAS44], Simple Disease Activity Index [SDAI] or Clinical Disease Activity Index [CDAI]) at month 6, year 1 and year 2. If multiple remission measures were reported, we used DAS28-erythrocyte sedimentation rate (ESR) if available, or the measure with highest number of patients in remission in the treatment arms combined.
2.4. Risk of bias assessment of clinical studies
The Cochrane Risk of Bias Tool [Citation26] was applied to all studies. In short, based on the overall assessment of available information on randomization, treatment allocation concealment, blinding of participants, personnel and outcome assessments, the attrition of participants as well as the reporting of outcomes and other potential sources, we categorized studies as having a low, high or unknown risk of bias. Due to the transitions between double-blind and open-label observation strategies at various time points, risk of bias was assessed separately at month 6, year 1 and year 2.
2.5. Meta-analysis of clinical endpoints
All endpoints were included on an intent-to-treat basis, using the number of patients initially randomized. Calculations were performed using the Stata14 statistical software package (StataCorp LLC., College Station, Texas, USA). Due to the heterogenous design of studies, we chose different quantitative synthesis methods for pre-defined short- and long-term outcomes. For month 6 outcomes, a network meta-analysis using the Stata network meta package was performed comparing the infliximab plus MTX combination, multiple DMARD combinations with corticosteroid with or without infliximab, steroid and MTX combinations, and MTX monotherapy, using MTX monotherapy as control [Citation30]. There was no source of loop-inconsistency (i.e. incomparable results from direct and indirect comparisons) within the network, therefore we applied the consistency network meta-analysis model (assuming random effects for study heterogeneity but no inconsistency between direct- and indirect comparisons). Due to the changes in applied treatments and observation strategies from month 6, treatment arms used in the network meta-analysis were not feasible for later outcomes. Rather, treatments were dichotomized according to a first-line infliximab treatment versus other strategies (control) at randomization, and, – considering the heterogeneity of studies – outcomes at year 1 and 2 were assessed using a random effects meta-analysis according to the method of DerSimonian and Laird [Citation31]. Month 18 outcomes were included in the meta-analysis at year 2, if no year 2 outcomes were available from the same study. For ACR20, ACR70 and remission, log odds ratios (ORs) with 95% confidence intervals were applied for the comparisons. The significance threshold was set at p < 0.05.
We also investigated how the results of our analysis at year 2 would have changed if the ASPIRE trial [Citation15], a 1-year parallel design study of first-line infliximab versus placebo in combination with MTX, had included a long-term extension study. We projected hypothetical year 2 ACR70 results in the ASPIRE study using published year 1 ACR70 data and included the simulated results in our meta-analysis.
2.6. Search for cost-utility studies
We also searched for cost-utility studies of first-line infliximab in PubMed, Cochrane CENTRAL and the Centre for Reviews and Dissemination (CRD) Health Technology Assessment (HTA) database of the University of York up to 31 October 2018. The search-terms included keywords for economic analyses, infliximab and the MeSH terms for rheumatoid arthritis. Full details of the PubMed, CENTRAL and CRD search criteria are shown in Supplementary Tables S4, S5 and S6, respectively. Eligible studies included those that reported incremental cost-effectiveness ratio (ICER) values for infliximab in patients with MTX-naïve RA. Full text manuscripts were considered. Data extraction for main characteristics of the evaluations (publication year, country, type of model, time horizon applied, data sources used, study sample, applied treatments and comparators, year of costs, study perspective, ICER) was performed.
3. Results
3.1. Literature search of clinical studies
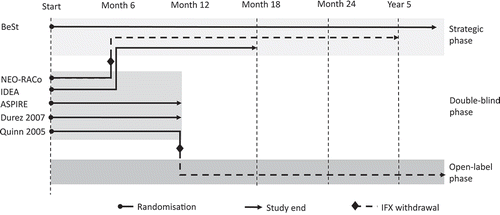
A PRISMA flow diagram for our systematic review is shown in and includes the number of citations found and screened. We identified six RCTs (reported in 26 full text publications) of first-line infliximab in RA: ASPIRE (3 publications) [Citation15,Citation32,Citation33], BeSt (15 publications) [Citation34–Citation48], Durez 2007 (1 publication) [Citation49], IDEA (1 publication) [Citation12], NEO-RACo (3 publication) [Citation50–Citation52] and Quinn 2005 (3 publications) [Citation53–Citation55]. The sample size of these RCTs ranged from 20 to 1,049 patients; follow-up times were between 1 and 10 years. Key details of the studies are shown in . Data from six studies (reported in eight publications [Citation12,Citation15,Citation34,Citation35,Citation38,Citation49,Citation51,Citation53]) were included in the quantitative synthesis. The studies included 1,832 patients at baseline, of whom 1,009 (55%) received first-line infliximab. Inclusion criteria for RA diagnosis was based on ACR criteria in all six RCTs. Disease activity criteria was defined in most studies as having ≥6 tender and swollen joints. Disease duration was limited to between 3 months and 3 years.
Figure 1. PRISMA flow diagram of the systematic review.
CENTRAL Cochrane Central Register of Controlled Trials; IFX infliximab, RA rheumatoid arthritis, RCT randomized controlled trial.
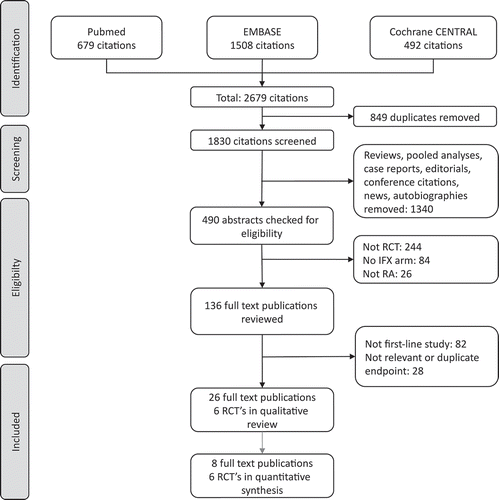
Table 1. RCTs of first-line infliximab treatment in patients with RA.
Results were reported for 19 different time points and for 57 relevant endpoints, allowing 244 different time point–outcome combinations (Supplementary Tables S7 and S8). Of these, 188 (77%) were reported only by a single study, 39 (16%) by at least two studies and 17 (7%) by more than two studies. Results of ACR response and remission rates by any criteria were reported at month 6 by three [Citation12,Citation34,Citation49] and four [Citation12,Citation34,Citation49,Citation51] studies, respectively, and at year 1 by five and four studies, respectively [Citation12,Citation15,Citation34,Citation49,Citation51,Citation53]. ACR20 and ACR70 at year 1 were reported by four studies; all other measures were reported by no more than three studies at the same time point. Apart from three studies reporting ACR70 outcomes at year 2 [Citation35,Citation51,Citation53], longer-term results (>1 year) for each measure were reported by a maximum of two studies at the same time point (Supplementary Tables S7 and S8). Although the presence of ADAs was included among the pre-defined outcomes, none of the studies reported ADA results.
3.2. Methodological heterogeneity of clinical studies
The study designs showed great heterogeneity. The methodological heterogeneity of studies and the treatment arms are summarized in and Supplementary Table S9, respectively. Patients were naïve to any DMARDs in three studies [Citation12,Citation51,Citation53], and were exposed to csDMARDs other than MTX in two studies [Citation34,Citation49]. In ASPIRE [Citation15], minimal exposure to MTX was allowed. All patients were naïve to bDMARDs. Two studies had a duration of 1 year [Citation15,Citation49] and four studies had patient follow-up beyond 1 year [Citation12,Citation34,Citation51,Citation53]. Randomized treatments changed substantially between month 6 and year 2 in all studies. The BeSt [Citation34] study was an open-label, strategic, treat-to-target study from the onset. In the NEO-RACo [Citation51] and IDEA [Citation12] studies, patients continued an open-label, treat-to-target protocol following 6 months of double-blind treatment. Patients in the Quinn 2005 et al. [Citation53] study could continue open-label MTX treatment after cessation of infliximab at 1 year. Infliximab was also discontinued in the NEO-RACo study after 6 months [Citation51].
3.3. Risk of bias analysis of clinical studies
At month 6 relevant outcomes were reported from four studies. One study was a randomized, open-label, strategic trial and was assessed as having a high risk of bias due to the lack of blinding [Citation34]. Three double-blind, randomized controlled studies had a low risk of bias [Citation12,Citation49,Citation51]. At year 1, three studies were categorized as high risk of bias due to their open-label design [Citation12,Citation34,Citation51], while outcomes were measured during the double-blind randomized phase of three studies categorized as having a low risk of bias [Citation15,Citation49,Citation53]. At year 2, all studies were open-label and were assessed as having a high risk of bias [Citation12,Citation34,Citation51,Citation53].
3.4. Meta-analyses of clinical studies
We performed network meta-analysis for efficacy outcomes at month 6. The network map for 6-month ACR and remission outcomes are displayed in Supplementary Figure S10. There was no source of inconsistency in the network structure of the included treatments, therefore we performed the network meta-analysis using the consistency model assuming no treatment effect variation between designs [Citation30]. ACR70 and remission outcomes at 6 months were improved significantly with first-line infliximab plus MTX compared with MTX control (). ACR20 at 6 months was not significantly different between the infliximab and control groups ().
Table 2. Results of meta-analysis for efficacy outcomes at month 6, year 1 and year 2.
At year 1 and year 2, first-line infliximab was compared with other strategies via random effects meta-analysis. Heterogeneity between studies was low and was not significant in the year 1 models. Our analyses showed that ACR20, ACR70 and remission at year 1, significantly favored the infliximab group compared with the control group (); ACR70 response at year 1 is also shown in ). At year 2, there were no significant differences in outcomes (ACR20, ACR70 and remission) between the infliximab and control groups (; )). Heterogeneity was significant in the year 2 ACR70 (I2 = 79.2%; p = 0.002) and remission models (I2 = 75.8%; p = 0.006).
Figure 3. Random effects meta-analysis ACR70 response (a) at year 1 and (b) at year 2 with and without simulated ASPIRE results*.
*ASPIRE 2-year ACR70 response was projected from 1 year-long double-blind, parallel-group RCTsACR American College of Rheumatology, CI confidence interval, D + L DerSimonian & Laird random effects meta-analysis, OR odds ratio, RCT randomized controlled trial
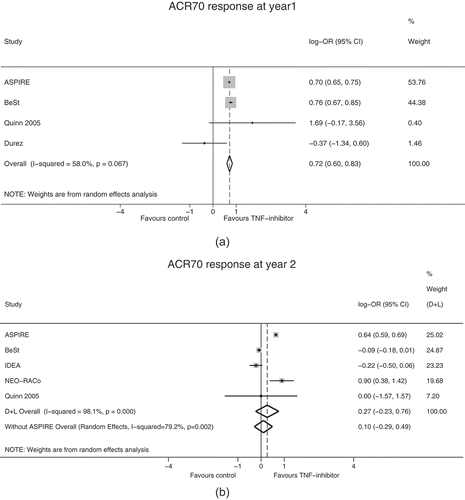
In studies with TNFis that had at least 1 year of a double-blind, parallel phase and reported year 2 results (etanercept: COMET [Citation9], Ebrel ERA [Citation56]; golimumab: GO-BEFORE [Citation57,Citation58]; adalimumab: PREMIER [Citation8]; infliximab: Quinn 2005 [Citation53]), there was an additional 8.9% and 9.3% of patients with an ACR70 response at year 2 versus year 1 in the control group and TNFi group, respectively. We assumed that ACR70 response rates of the MTX alone and infliximab plus MTX combination arms in ASPIRE would change from 21% and 38% in year 1 to 21% and 35.8% by year 2, respectively. Based on these results, projected hypothetical year 2 ACR70 outcomes from the 1-year parallel design ASPIRE study were included in our meta-analysis of ACR70 response at year 2 ()). Although projected year 2 ACR70 data from ASPIRE increased the between-group difference in favor of infliximab, the difference remained non-significant ()).
3.5. Summary of cost-effectiveness studies
Our searches yielded 96 abstracts in PubMed, 37 abstracts in the Cochrane CENTRAL database and 123 abstracts in the CRD HTA database. We removed 27 duplicates, 171 records based on their title and two conference posters. From the remaining 56 records we identified four full text journal publications reporting cost-utility studies of infliximab as first-line treatment in patients with RA (). Two studies were conducted in the United States, and the other studies were conducted in the United Kingdom and the Netherlands. ICERs ranged between €22,000/quality-adjusted life-year (QALY) and £650,000/QALY (). The cost effectiveness of first-line infliximab treatment was above the usually acceptable financial threshold of the respective countries in most models. All models were calculated with prices prior to the registration of biosimilar infliximab by the European Medicines Agency in 2013.
Table 3. Cost-utility studies of infliximab as first-line treatment in patients with RA.
4. Conclusions
In summary, our systematic review and meta-analysis has demonstrated that 1-year efficacy outcomes (ACR20, ACR70 and remission) were improved significantly with first-line infliximab versus control strategies in patients with csDMARD-naïve RA. These efficacy differences were not significant in a longer follow-up of 2 years. The diminished effect size and precision of long-term estimates may partly be explained by the highly diverse methodology of studies. The long-term effects of first-line biological therapy on disease progression should be further analyzed using all TNFi agents.
Analysis of cost-utility studies performed before the availability of lower cost infliximab biosimilars revealed that first-line use of originator infliximab was not cost effective. New cost-effectiveness analyses are needed in early RA that consider the sizable potential savings resulting from the uptake of biosimilar infliximab.
Given the potentially considerable savings due to biosimilar infliximab, the evident short-term efficacy benefits, the availability of prognostic factors for progressive disease and the potential for biological-free remission if administered early, the cost-effectiveness of first-line infliximab in early, treatment-naïve RA should be re-assessed using biosimilar prices.
5. Expert opinion
The results of this systematic review and network meta-analysis show that ACR70 and remission outcomes at 6 months in patients with MTX-naïve RA were significantly improved with infliximab versus MTX control. At year 1, ACR20, ACR70 and remission outcomes were also significantly improved with infliximab versus control strategies. However, at year 2, no statistically significant differences in ACR20, ACR70 or remission outcomes were observed between treatment groups. Long-term (>2 years) results on clinical remission were available only from two RCTs (BeSt [Citation34], NEO-RACo [Citation51]) and patient-reported outcome data were presented only from the BeSt [Citation34] and Quinn 2005 [Citation53] studies.
We have demonstrated the need for improved standardized reporting of studies. Despite the 244 reported time point-outcome combinations, neither ACR response nor remission rates were reported by all studies at 6 months and 1 year, and apart from ACR70, only two studies reported these efficacy outcomes at the same time point after 1 year. For a given time point only two outcomes were reported in four studies and 13 outcomes were reported in three studies. The diverse reporting of results is a major barrier for effective evidence synthesis.
The included studies showed increasingly heterogenous designs over time, which partly explains the smaller effect sizes and increasing variance at year 2 compared with year 1 outcomes. However, due to the small number of studies, the decomposition of study heterogeneity via meta-regression was not feasible [Citation26]. The randomized treatments were modified substantially beyond month 6 in all studies. In NEO-RACo [Citation51], infliximab treatment was discontinued after 6 months, and in Quinn 2005 [Citation53], after 12 months. In the BeSt study [Citation34,Citation35] at year 2 nearly three quarters of initially randomized patients received infliximab plus MTX combination therapy, while in the DMARD monotherapy arm more than one quarter of patients received infliximab. In IDEA [Citation12] two thirds of patients in the control arm received dose escalation, while DMARDs were adjusted in more than half of the patients in the infliximab arm, and infliximab was discontinued in every fourth patient due to sustained low disease activity.
While five of the six studies measured endpoints in a double-blind setting at month 6, year 2 efficacy outcomes were available only from open-label studies. We hypothesize that longer-term benefits of biologic combinations and MTX monotherapy would be demonstrated in studies that remain blinded for longer periods. Therefore, using ACR70 data, we investigated how the results of our analyses at year 2 might have changed if the 1-year long ASPIRE study [Citation15] had included a long-term extension study. The absolute risk difference in ACR70 response at year 2 between the treatment and control arms in the PREMIER [Citation8] and COMET [Citation9] studies were 26% and 25%, respectively. However, in our analysis of hypothetical year 2 ASPIRE results, we assumed a conservative 15% difference between infliximab plus MTX and MTX monotherapy. Although the projected year 2 ACR70 data from ASPIRE increased the between-group difference in favor of infliximab, the difference remained non-significant. Although loss of sustained clinical response due to immunogenicity [Citation63] may be an alternative explanation for the moderate long-term effect size, we found no reports of anti-drug-antibody levels in the included studies (Supplementary Table S7).
A key strength of our study is that we provide a comprehensive evaluation of efficacy endpoints that are relevant for pharmacoeconomic analyses – ACR20, ACR70 and remission – at different time points from 6 months up to long-term outcomes at 2 years. While the benefit of immediate infliximab in preventing joint damage has been shown over other treatment strategies [Citation15,Citation34], radiographic progression was out of the scope of this review. Several other recent meta-analyses have compared the combination of biologic agents plus MTX over csDMARDs in early MTX-naïve RA [Citation3,Citation17–Citation19,Citation64]. The Cochrane report from Singh et al. [Citation3] included analyses of ACR50, Health Assessment Questionnaire (HAQ) scores and remission rates at <6 months, 6–12 month and >12 month time points. From the included 19 RCTs seven were infliximab studies; however, in one of them all patients received MTX at baseline [Citation3]. Cai et al. [Citation17] included 20 RCTs in the analysis of ACR20, ACR50 and ACR70 endpoints. In one of the five included infliximab studies, all patients received three months of MTX induction therapy before randomization [Citation17]. Stevenson et al. [Citation64] in a comprehensive HTA report commissioned by NICE, synthesized month 6 ACR and EULAR response outcomes for seven biological drugs, including two infliximab trials. Albert et al. [Citation18] included one infliximab study in an indirect pairwise meta-analysis of year 1 ACR20, ACR50 and ACR70 endpoints from six RCTs of biologics + DMARD therapy. Donahue et al. [Citation19] reported less radiological progression and higher ACR50 response rates at year 1 with four TNFis in combination with MTX versus MTX monotherapy. Three of the 13 trials included infliximab. Taken together, data from these studies support the results of our own analysis that, in the short-term, biologics combined with MTX are superior to MTX monotherapy in terms of ACR and remission endpoints in early RA. To date, no consistent evidence supports the superiority of one biologic therapy over another.
In all the six RCTs, RA diagnosis was based on the 1987 ACR diagnostic criteria for RA [Citation65], which have been criticized for their lack of sensitivity in early disease [Citation66]. The new ACR/EULAR classification system introduced in 2010, focuses on symptoms at earlier stages of the disease rather than establishing the diagnosis by its late-stage features [Citation66]. Further studies using the new ACR/EULAR classification system may contribute to a better understanding of the effectiveness of first-line infliximab treatment in early-stage RA. Moreover, we believe that there is a need for new outcomes to detect the long-term effects of deteriorated health patients may suffer due to the lack of early elimination of symptoms. These may include measures to assess the impact on internalization and legitimization of societal stereotypes of RA, subjective expectations regarding future health and treatment goals, acceptability of symptoms, uncertainty about the future, work capacity, long-term life planning and wellbeing in terms of the ability to do things that are important in patients’ lives [Citation67–Citation71].
In our cost-utility analysis, the cost-effectiveness of originator infliximab was deemed unacceptable in the published economic analyses we reviewed, with ICERs of between €22,000/QALY and £650,000/QALY reported. Due to the broad variety of modelling assumptions of long-term outcomes, the results of cost-effectiveness analyses need to be interpreted with caution. Although systematic reviews did not demonstrate a substantial difference between branded TNFi drugs in terms of efficacy, ICERs between individual drugs differed considerably. In a review of six cost-utility analyses by Stevenson et al. [Citation64], ICERs were >US$100,000/QALY in three of four infliximab studies, two of five adalimumab studies, and five of six etanercept studies. Treating biological agents as a homogenous group, Stevenson et al. estimated that the ICER for a first-line biological strategy versus MTX monotherapy in MTX-naïve, early RA was £58,300/QALY, still above the acceptable cost-effectiveness threshold.
However, the available cost-effectiveness studies with infliximab in MTX-naïve RA were performed before the market entry of biosimilar competitors. The most recent economic evaluation of MTX-naïve, early RA reported base case results using 2013 innovator etanercept prices [Citation72]. Reductions in the price of infliximab therapy due to the entry of biosimilar products in the RA market could improve the cost-effectiveness of first-line infliximab therapy for patients with RA. The considerable cost saving achievable with biosimilar infliximab along with the potential to improve access to RA has been demonstrated in several budget impact analyses [Citation23–Citation25,Citation73]. We hypothesize that given the considerable price reductions driven by biosimilar competition, the cost-effectiveness of first-line infliximab therapy may fall under the financing threshold in many countries and allow use of this agent in the first-line setting for the treatment of patients with early RA. If proven to be cost effective, first-line therapy with biologic agents could provide a valuable opportunity to test the window of opportunity hypothesis in a real-world setting and increase the possibility for drug-free remission in some patients with RA.
Article highlights
We performed a systematic search of the literature for RCTs of infliximab in early methotrexate-naïve rheumatoid arthritis, up to 24 May 2018
We identified six RCTs with diverse designs, involving 1,832 patients. The sample size ranged between 20-1,049, and follow-up times between 1-10 years.
The reporting of results was heterogenous: in 26 journal articles we identified 57 endpoints for 19 time-points, allowing 244 time-point outcome combinations. 77% of outcomes were reported by a single study, 16% by at least two studies and only 7% by more than two studies. These results highlight the need for standardized reporting of outcomes.
The meta-analysis demonstrated that first-line infliximab was more effective than delayed biological therapy at month 6 (ACR70, remission) and at year 1 (ACR20, ACR70, remission). At year 2 the difference was not significant.
Economic evaluations using originator prices from years 2005–2008 did not demonstrate the cost-effectiveness of first-line infliximab therapy in early RA. The cost-effectiveness of this strategy at biosimilar prices is yet to be determined.
Author contribution statement
LG conceived the study, ZZ, LG, VB and MP performed the systematic literature search; ZZ, LG, VB, FR and MP contributed to data collection; ZZ performed the data analysis; ZZ, LG, VB, FR, RA, ZS and MP contributed to interpretation of data and writing the manuscript. All authors take responsibility for the integrity of the work as a whole, and have given final approval for the article to be published. The conception, design, analysis and interpretation of results of this study are a product of independent research performed by the authors.
Declaration of interest
Z Zrubka used to be a full-time employee of Egis Pharmaceuticals, Janssen Cilag, Sandoz and Pfizer. L Gulácsi has received consultancy and lecturing fee from Astellas, BMS, Celltrion, Egis Pharmaceuticals, GSK, Hikma, Hospira, Lilly Hungaria Ltd, MSD Hungary, Pfizer, Roche, Sandoz and UCB. V Brodszky has received grants and personal fees from Celltrion, Egis Pharmaceuticals, Pfizer and Sager Pharma. F Rencz has received consultancy fees from Celltrion and Hospira. R Alten has received honoraria from Celltrion. Z Szekanecz has received consultancy and lecturing fees from Abbvie, Amgen, BMS, Lilly, MSD, Novartis, Pfizer, Roche and UCB. M Péntek has received grants and personal fees from Celltrion, Egis Pharma, Merck, Pfizer and Sager Pharma. Z Zrubka, L Gulácsi, V Brodszky, F Rencz and M Péntek received grants from Celltrion during the writing of this article.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download Zip (773.7 KB)Acknowledgments
Medical writing support for this manuscript (including copyediting and fact checking) was provided to the authors by Emma Evans, PhD CMPP at Aspire Scientific Limited (Bollington, UK).
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Scott DL. Early rheumatoid arthritis. Br Med Bull. 2007;81-82:97–114.
- National Clinical Centre for Chronic Conditions. Clinical guidelines. Rheumatoid arthritis: national clinical guideline for management and treatment in adults. London: Royal College of Physicians (UK); 2018.
- Singh JA, Hossain A, Mudano AS, et al. Biologics or tofacitinib for people with rheumatoid arthritis naive to methotrexate: a systematic review and network meta-analysis. Cochrane Database Syst Rev. 2017;5:CD012657.
- van Nies JA, Krabben A, Schoones JW, et al. What is the evidence for the presence of a therapeutic window of opportunity in rheumatoid arthritis? A systematic literature review. Ann Rheum Dis. 2014;73(5):861–870.
- Raza K, Filer A. The therapeutic window of opportunity in rheumatoid arthritis: does it ever close? Ann Rheum Dis. 2015;74(5):793–794.
- Nagy G, van Vollenhoven RF. Sustained biologic-free and drug-free remission in rheumatoid arthritis, where are we now? Arthritis Res Ther. 2015;17(181).
- Breedveld FC, Emery P, Keystone E, et al. Infliximab in active early rheumatoid arthritis. Ann Rheum Dis. 2004;63(2):149–155.
- Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54(1):26–37.
- Emery P, Breedveld FC, Hall S, et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet. 2008;372(9636):375–382.
- Emery P, Kvien TK, Combe B, et al. Combination etanercept and methotrexate provides better disease control in very early (≤4 months) versus early rheumatoid arthritis (>4 months and <2 years): post hoc analyses from the COMET study. Ann Rheum Dis. 2012;71(6):989–992.
- Genovese MC, Bathon JM, Martin RW, et al. Etanercept versus methotrexate in patients with early rheumatoid arthritis: two-year radiographic and clinical outcomes. Arthritis Rheum. 2002;46(6):1443–1450.
- Nam JL, Villeneuve E, Hensor EM, et al. Remission induction comparing infliximab and high-dose intravenous steroid, followed by treat-to-target: a double-blind, randomised, controlled trial in new-onset, treatment-naive, rheumatoid arthritis (the IDEA study). Ann Rheum Dis. 2014;73(1):75–85.
- Nam JL, Villeneuve E, Hensor EM, et al. A randomised controlled trial of etanercept and methotrexate to induce remission in early inflammatory arthritis: the EMPIRE trial. Ann Rheum Dis. 2014;73(6):1027–1036.
- Smolen JS, Emery P, Fleischmann R, et al. Adjustment of therapy in rheumatoid arthritis on the basis of achievement of stable low disease activity with adalimumab plus methotrexate or methotrexate alone: the randomised controlled OPTIMA trial. Lancet. 2014;383(9914):321–332.
- St Clair EW, van der Heijde DM, Smolen JS, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum. 2004;50(11):3432–3443.
- National Institute for Health and Care Excellence. Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for rheumatoid arthritis not previously treated with DMARDs or after conventional DMARDs only have failed. 2016 [cited 2018 Oct 11]. Available from: https://www.nice.org.uk/guidance/ta375/documents/rheumatoid-arthritis-adalimumab-etanercept-infliximab-certolizumab-pegol-golimumab-abatacept-and-tocilizumab-review-id537-appraisal-consultation-document2
- Cai W, Gu Y, Cui H, et al. The efficacy and safety of mainstream medications for patients with cDMARD-naive rheumatoid arthritis: a network meta-analysis. Front Pharmacol. 2018;9:138.
- Albert DA. Are all biologics the same? Optimal treatment strategies for patients with early rheumatoid arthritis: systematic review and indirect pairwise meta-analysis. J Clin Rheumatol. 2015;21(8):398–404.
- Donahue KE, Gartlehner G, Schulman ER, et al. Drug therapy for early rheumatoid arthritis: A systematic review update. 2018 [cited 2018 Nov 29]. Available from: https://effectivehealthcare.ahrq.gov/topics/rheumatoid-arthritis-medicine-update/final-report-update-2018
- van der Velde G, Pham B, Machado M, et al. Cost-effectiveness of biologic response modifiers compared to disease-modifying antirheumatic drugs for rheumatoid arthritis: a systematic review. Arthritis Care Res (Hoboken). 2011;63(1):65–78.
- Smolen JS, Landewe R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960–977.
- Generics and Biosimilars Initiative. Huge discount on biosimilar infliximab in Norway. 2015 [updated 2015 Mar 13; cited 2018 Oct 16]. Available from: http://www.gabionline.net/Biosimilars/General/Huge-discount-on-biosimilar-infliximab-in-Norway
- Kim J, Hong J, Kudrin A 5 Year budget impact analysis of biosimilar infliximab for the treatment of rheumatoid arthritis in UK, Italy, France and Germany. American College of Rheumatology Annual Meeting; 2014; Boston, Massachusetts.
- Brodszky V, Baji P, Balogh O, et al. Budget impact analysis of biosimilar infliximab (CT-P13) for the treatment of rheumatoid arthritis in six Central and Eastern European countries. Eur J Health Econ. 2014;15(Suppl 1):S65–S71.
- Jha A, Upton A, Dunlop WC, et al. The budget impact of biosimilar infliximab (Remsima(R)) for the treatment of autoimmune diseases in five European countries. Adv Ther. 2015;32(8):742–756.
- Higgins JPT, Green S Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011. [cited 2018 Oct 25]. Available from: http://handbook.cochrane.org.
- clinicaltrials.gov. NCT00480272 - Prospective study on intensive early rheumatoid arthritis treatment with adalimumab: induction of remission and maintenance - ’CURE’ A phase IV multicenter, randomized, double-blind study; 2017. [cited 2018 Jul 15]. Available from: https://clinicaltrials.gov/ct2/show/NCT00480272
- Hochberg MC, Silman AJ, Smolen JS, et al. Rheumatology E-Book. Philadelphia: Elsevier Health Sciences; 2018.
- Wood L Global clinical insight on 17 biosimilar versions of Remicade (infliximab) drug in clinical pipeline 2016 - Research and markets. PR Newswire; 2016 [cited 2016 Oct 19]. Available from: https://www.prnewswire.com/news-releases/global-clinical-insight-on-17-biosimilar-versions-of-remicade-infliximab-drug-in-clinical-pipeline-2016—research-and-markets-300347760.html
- White I. Network meta-analysis. In: editors, Palmer TM, Jonathan AC. Meta-analysis in stata: an updated collection from the stata journal. 2nd ed. College Staton, Texas: Stata Press; 2015:410–450
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials. 1986;7(3):177–188.
- Smolen JS, Han C, van der Heijde D, et al. Infliximab treatment maintains employability in patients with early rheumatoid arthritis. Arthritis Rheum. 2006;54(3):716–722.
- Smolen JS, Han C, van der Heijde DM, et al. Radiographic changes in rheumatoid arthritis patients attaining different disease activity states with methotrexate monotherapy and infliximab plus methotrexate: the impacts of remission and tumour necrosis factor blockade. Ann Rheum Dis. 2009;68(6):823–827.
- Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum. 2005;52(11):3381–3390.
- Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, et al. Comparison of treatment strategies in early rheumatoid arthritis: a randomized trial. Ann Intern Med. 2007;146(6):406–415.
- Klarenbeek NB, Guler-Yuksel M, van der Kooij SM, et al. The impact of four dynamic, goal-steered treatment strategies on the 5-year outcomes of rheumatoid arthritis patients in the BeSt study. Ann Rheum Dis. 2011;70(6):1039–1046.
- Allaart CF, Breedveld FC, Dijkmans BA. Treatment of recent-onset rheumatoid arthritis: lessons from the BeSt study. J Rheumatol Suppl. 2007;80:25–33.
- Allaart CF, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, et al. Aiming at low disease activity in rheumatoid arthritis with initial combination therapy or initial monotherapy strategies: the BeSt study. Clin Exp Rheumatol. 2006;24(6 Suppl 43):S77–S82.
- Allaart CF, Markusse IM, Lems WF. What have we learned from BeSt? Clin Immunol. 2018;186:74–78.
- Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, et al. Patient preferences for treatment: report from a randomised comparison of treatment strategies in early rheumatoid arthritis (BeSt trial). Ann Rheum Dis. 2007;66(9):1227–1232.
- Guler-Yuksel M, Bijsterbosch J, Goekoop-Ruiterman YP, et al. Changes in bone mineral density in patients with recent onset, active rheumatoid arthritis. Ann Rheum Dis. 2008;67(6):823–828.
- Markusse IM, Akdemir G, Dirven L, et al. Long-term outcomes of patients with recent-onset rheumatoid arthritis after 10 years of tight controlled treatment: a randomized trial. Ann Intern Med. 2016;164(8):523–531.
- van Den Broek M, Lems WF, Allaart CF. BeSt practice: the success of early-targeted treatment in rheumatoid arthritis. Clin Exp Rheumatol. 2012;30(4 Suppl 73):S35–S38.
- van der Bijl AE, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, et al. Infliximab and methotrexate as induction therapy in patients with early rheumatoid arthritis. Arthritis Rheum. 2007;56(7):2129–2134.
- van der Kooij SM, de Vries-Bouwstra JK, Goekoop-Ruiterman YP, et al. Patient-reported outcomes in a randomized trial comparing four different treatment strategies in recent-onset rheumatoid arthritis. Arthritis Rheum. 2009;61(1):4–12.
- van der Kooij SM, le Cessie S, Goekoop-Ruiterman YP, et al. Clinical and radiological efficacy of initial vs delayed treatment with infliximab plus methotrexate in patients with early rheumatoid arthritis. Ann Rheum Dis. 2009;68(7):1153–1158.
- Visser K, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, et al. A matrix risk model for the prediction of rapid radiographic progression in patients with rheumatoid arthritis receiving different dynamic treatment strategies: post hoc analyses from the BeSt study. Ann Rheum Dis. 2010;69(7):1333–1337.
- van der Kooij SM, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, et al. Drug-free remission, functioning and radiographic damage after 4 years of response-driven treatment in patients with recent-onset rheumatoid arthritis. Ann Rheum Dis. 2009;68(6):914–921.
- Durez P, Malghem J, Nzeusseu Toukap A, et al. Treatment of early rheumatoid arthritis: a randomized magnetic resonance imaging study comparing the effects of methotrexate alone, methotrexate in combination with infliximab, and methotrexate in combination with intravenous pulse methylprednisolone. Arthritis Rheum. 2007;56(12):3919–3927.
- Rantalaiho V, Kautiainen H, Korpela M, et al. Targeted treatment with a combination of traditional DMARDs produces excellent clinical and radiographic long-term outcomes in early rheumatoid arthritis regardless of initial infliximabThe 5-year follow-up results of a randomised clinical trial, the NEO-RACo trial. Ann Rheum Dis. 2014;73(11):1954–1961.
- Leirisalo-Repo M, Kautiainen H, Laasonen L, et al. Infliximab for 6 months added on combination therapy in early rheumatoid arthritis: 2-year results from an investigator-initiated, randomised, double-blind, placebo-controlled study (the NEO-RACo Study). Ann Rheum Dis. 2013;72(6):851–857.
- Kuusalo L, Puolakka K, Kautiainen H, et al. High burden of adverse events is associated with reduced remission rates in early rheumatoid arthritis. Clin Rheumatol. 2018;37(6):1689–1694.
- Quinn MA, Conaghan PG, O’Connor PJ, et al. Very early treatment with infliximab in addition to methotrexate in early, poor-prognosis rheumatoid arthritis reduces magnetic resonance imaging evidence of synovitis and damage, with sustained benefit after infliximab withdrawal: results from a twelve-month randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2005;52(1):27–35.
- Bejarano V, Conaghan PG, Quinn MA, et al. Benefits 8 years after a remission induction regime with an infliximab and methotrexate combination in early rheumatoid arthritis. Rheumatology (Oxford). 2010;49(10):1971–1974.
- Haugeberg G, Conaghan PG, Quinn M, et al. Bone loss in patients with active early rheumatoid arthritis: infliximab and methotrexate compared with methotrexate treatment alone. Explorative analysis from a 12-month randomised, double-blind, placebo-controlled study. Ann Rheum Dis. 2009;68(12):1898–1901.
- Bathon JM, Martin RW, Fleischmann RM, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med. 2000;343(22):1586–1593.
- Emery P, Fleischmann RM, Moreland LW, et al. Golimumab, a human anti-tumor necrosis factor alpha monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: twenty-four-week results of a phase III, multicenter, randomized, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum. 2009;60(8):2272–2283.
- Emery P, Fleischmann RM, Doyle MK, et al. Golimumab, a human anti-tumor necrosis factor monoclonal antibody, injected subcutaneously every 4 weeks in patients with active rheumatoid arthritis who had never taken methotrexate: 1-year and 2-year clinical, radiologic, and physical function findings of a phase III, multicenter, randomized, double-blind, placebo-controlled study. Arthritis Care Res (Hoboken). 2013;65(11):1732–1742.
- Spalding JR, Hay J. Cost effectiveness of tumour necrosis factor-alpha inhibitors as first-line agents in rheumatoid arthritis. PharmacoEconomics. 2006;24(12):1221–1232.
- Chen YF, Jobanputra P, Barton P, et al. A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness. Health Technol Assess. 2006;10(42):iii–iv, xi–xiii, 1–229.
- Davies A, Cifaldi MA, Segurado OG, et al. Cost-effectiveness of sequential therapy with tumor necrosis factor antagonists in early rheumatoid arthritis. J Rheumatol. 2009;36(1):16–26.
- van Den Hout WB, Goekoop-Ruiterman YP, Allaart CF, et al. Cost-utility analysis of treatment strategies in patients with recent-onset rheumatoid arthritis. Arthritis Rheum. 2009;61(3):291–299.
- Spinelli FR, Valesini G. Immunogenicity of anti-tumour necrosis factor drugs in rheumatic diseases. Clin Exp Rheumatol. 2013;31(6):954–963.
- Stevenson M, Archer R, Tosh J, et al. Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for the treatment of rheumatoid arthritis not previously treated with disease-modifying antirheumatic drugs and after the failure of conventional disease-modifying antirheumatic drugs only: systematic review and economic evaluation. Health Technol Assess. 2016;20(35):1–610.
- Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324.
- Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581.
- Corker E, Henderson RC, Lempp H, et al. Internalised stigma in people with rheumatoid arthritis: a cross sectional study to establish the psychometric properties of the ISMI-RA. BMC Musculoskelet Disord. 2016;17:244.
- Pentek M, Gulacsi L, Rojkovich B, et al. Subjective health expectations at biological therapy initiation: a survey of rheumatoid arthritis patients and rheumatologists. Eur J Health Econ. 2014;15(Suppl 1):S83–S92.
- Pentek M, Rojkovich B, Czirjak L, et al. Acceptability of less than perfect health states in rheumatoid arthritis: the patients’ perspective. Eur J Health Econ. 2014;15(Suppl 1):S73–S82.
- Mitchell PM, Venkatapuram S, Richardson J, et al. Are quality-adjusted life years a good proxy measure of individual capabilities? PharmacoEconomics. 2017;35(6):637–646.
- Tang K, Beaton DE, A B, et al. Measures of work disability and productivity: rheumatoid arthritis specific work productivity survey (WPS-RA), workplace activity limitations scale (WALS), work instability scale for rheumatoid arthritis (RA-WIS), work limitations questionnaire (WLQ), and work productivity and activity impairment questionnaire (WPAI). Arthritis Care Res (Hoboken). 2011;63(Suppl 11):S337–S349.
- Jalal H, O’Dell JR, Bridges SL Jr., et al. Cost-effectiveness of triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68(12):1751–1757.
- Han S, Kim S, Kim J, et al. The pharmacoeconomic impact of biosimilar infliximab (CT-P13) in Europe from January 2015 to June 2016. J Crohns Colitis. 2017;11(Issue suppl_1):S377.