ABSTRACT
Objectives: Utilization of multisource biological (off-patent originator and its biosimilar) medicines can improve the efficiency of resource allocation by 1) generating savings while maintaining health outcomes or 2) increasing the number of patients treated with more affordable treatments. This study evaluates the efficiency of the Hungarian biosimilar drug policy on the case of biosimilar infliximab.
Methods: We analyzed the utilization of biologicals in all reimbursed indications of infliximab including initial therapy of new patients and switching patterns retrospectively based on patient-level payer’s data between September 2012 and December 2016.
Results: Despite the economic rationale, patent expiry did not manifest in increased utilization of multisource infliximab in an access-restricted environment: 1) Patients previously treated with original biologicals were switched mainly to other original biologicals instead of more affordable biosimilar alternatives. 2) Although some treatment-naive patients started on more affordable multisource infliximab with price competition, the majority of new patients started on other original biologicals with monopolistic price.
Conclusion: Policy tools and measures should be developed to facilitate first-line use of multisource biologicals for treatment-naive patients and promoting the use of more affordable multisource biologicals in case of switching.
1. Introduction
Biological medicines are complex molecules produced through biotechnological processes [Citation1,Citation2]. Their launch has changed the treatment algorithms in different disease areas like rheumatology, dermatology, oncology, gastroenterology, and endocrinology. Utilization of biological medicines has been increasing constantly and these medicines are gaining market share in both volume and value [Citation3–7]. The price of biologicals is high partly due to increased development costs stemming from the more complex requirements of authorities and manufacturing procedure [Citation8–10].
Sustainability of financing pharmaceuticals is more and more challenging for third-party health-care payers; hence, patient access to high-priced biologicals could be limited [Citation11]. In several lower-income European countries with severe resource constraints, a wide spectrum of access restrictions have been implemented to ensure sustainability of pharmaceutical expenditures [Citation12–14]. Such restrictions specified by financial guidelines and protocols include volume restrictions on the number of patients treated with reimbursed biological treatment per treatment centers, limited duration of treatment with reimbursement, or the use of biologicals only in subsequent treatment lines after the failure of multiple non-biologic standard treatments. These public and hidden access restrictions, however, may prevent many eligible patients with chronic diseases from maximizing their health benefits. Evidence suggests, that standardized utilization figures of biological medicines for Central and Eastern European (CEE) countries are less than those in Western or Northern Europe in cancer and in rheumatoid arthritis [Citation15–17].
In the upcoming years, many biological medicines with high utilization and significant budget impact are going to lose market exclusivity [Citation18,Citation19]. After patient expiry, new biosimilar medicines come to the market with price discount. Biosimilars with EMA/FDA approval have similar safety and efficacy profile as the original biological medicines in case of new patients [Citation20–22]. In an internal price referencing system, such price discount may also incentivize the manufacturer of the off-patent originator product to decrease price. Therefore, multisource biological medicines (off-patent originator and its biosimilars) after patent expiry will become more affordable compared to still patent-protected originators maintaining their monopolistic position, but often with no or only limited added therapeutic value. Multisource biologicals with lower price carry the opportunity to generate savings in pharmaceutical budget (by maintaining the number of treated patients) or increase the number of patients treated with more cost-effective treatments in de-novo cases.
However, full potential of biosimilars in maintenance care has not been exploited due to concerns raised by different stakeholders related to the efficacy and safety of switching patients to biosimilar medicines. The European Medicines Agency (EMA) has not implemented any regulatory barriers toward switching of a reference medicine by its biosimilars. EMA lets EU member states to set up their own regulation [Citation23]. It is important to know that recent evidence suggests that majority of concerns related to switching have been overestimated [Citation24] and so increasing evidence supports that single switch from an originator to a biosimilar medicine under medical supervision is not associated with significantly increased risk of immunogenicity for patients [Citation25–27]. From the societal perspective, the risk of switching under medical supervision seems to be disproportional compared to the expected benefit [Citation11,Citation24,Citation28].
The main objective of health policymakers is to maximize health gain at population-level by improving the allocative efficiency of the limited resources [Citation29,Citation30]. The value proposition of off-patent biologicals can be approached from two different perspectives: If accessibility to original biologicals is not restricted, after patent expiry multisource biologicals should generate savings without compromising health outcomes (i.e. ‘disinvestment’ scenario) [Citation31,Citation32]. However, the policy objective is different in those lower-income European countries where patient access to original biologicals is restricted. In this case after patent expiry multisource medicines may provide additional health gain (i.e. wider patient access) with better cost-effectiveness ratio (i.e. ‘investment’ scenario) [Citation33].
This study aims to evaluate the efficiency of the Hungarian biosimilar drug policy by investigating utilization patterns of biologicals in the indications of infliximab based on data received from the National Institute of Health Insurance Fund Management (NIHIFM).
In Hungary the first biosimilar alternative of an originator biological needs to offer at least 30% price reduction compared to the ex-factory price of the originator, the second product an additional 10%, and the third product a further 10% [Citation34]. In 2012 an annual blind-bidding process was introduced for erythropoetins and granulocyte colony-stimulating factors. Based on the results of the bidding process there are preferred and non-preferred products with different co-payment levels, products with much higher price than the preferred alternative are delisted from the reimbursement system. Monoclonal antibodies are reimbursed through central public procurements where the key decisive factor is price.
After the originator infliximab (Remicade) lost its patent protection in 2013, two biosimilar infliximab alternatives (Remsima and Inflectra, both manufactured by Celltrion, distributed by Egis and Hospira/Pfizer, respectively) were granted marketing authorization for the same indications by EMA in September 2013 [Citation35–37]. Several studies confirmed the efficacy and safety of biosimilar infliximabs [Citation38,Citation39]. In Hungary, both biosimilar infliximabs have been reimbursed since November 2013 [Citation40]. (During the study period, national procurement tenders were won by Inflectra; thus, there were no utilization records for Remsima in the payer’s database.)
2. Data and methods
Out of eight therapeutic indications of infliximab registered by EMA (rheumatoid arthritis – RA; adult Crohn’s disease – CD; pediatric Crohn’s disease – PCD; ulcerative colitis – UC; pediatric ulcerative colitis – PUC; ankylosing spondylitis – AS; psoriatic arthritis – PA; psoriasis – P) seven – except PUC – were reimbursed during the observational period in Hungary. We analyzed patient-level utilization data of all biological medicines in the seven reimbursed indications of infliximab between September 2012 and December 2016 from the public payer’s database (NIHIFM). Those patients were involved into this claims data analysis, who had utilization record of at least one biologic treatment within the study period. Individual utilization records were collected and processed by employees of the NIHIFM and only aggregated data were made available for academic investigators. Indications were identified through ICD codes linked to utilization of biological medicine in payer records. Foreign patients were excluded from the analysis, since completeness of utilization patterns was less likely for them.
We evaluated the efficiency of the Hungarian biosimilar drug policy by (1) investigating the uptake of multisource infliximab after the originator infliximab has lost market exclusivity, (2) assessing prescribing patterns of patients without prior biological treatment (multisource infliximab vs. patent-protected biologicals) and (3) analyzing the switching patterns among different biological medicines.
To answer our research questions, we conducted the following analyses: (1) We prepared a longitudinal analysis of the number of patients on biological medicines in each reimbursed indication of infliximab. We aggregated independent patient utilization records at monthly-level and we calculated number of patients on treatment. (2) We calculated the market share of multisource infliximab compared to other patent-protected biologicals in the initial biological therapy of treatment-naive patients. (3) We calculated and compared switching rates a) from original infliximab to biosimilar infliximab; b) from original infliximab to other patented biologicals; c) from other patented biologicals to multisource infliximab; d) between other patented biologicals.
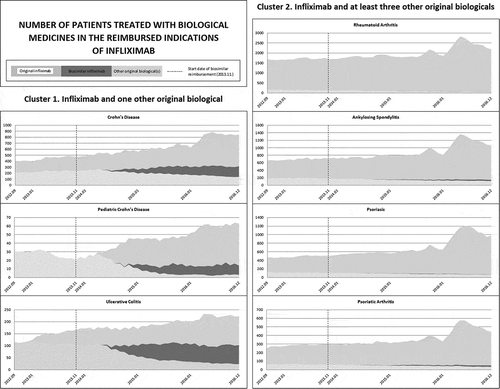
Initial assessment of utilization patterns suggested to create and separately analyze two indication clusters, based on the number of reimbursed active substances per indication (Cluster 1: Infliximab and one other original biological, Cluster 2: Infliximab and at least three other original biologicals) ().
Table 1. Number of active biological substances in the reimbursed indications of infliximab
3. Results
3.1. Longitudinal patient numbers in each reimbursed indication
Results of the longitudinal data analysis provide an overall picture about major utilization trends of biological medicines within the reimbursed indications of infliximab (). In case of Cluster 1 the original infliximab had high market share before the patent expiry. In UC and PCD infliximab was the dominant choice compared to adalimumab. Regarding UC, the average monthly market share of original infliximab was 81.7% (decreasing from 100% to 63% at patent expiry); while adalimumab had an 18.3% average market share increasing from 0% to 37% at infliximab patent expiry. As for PCD, the average monthly market share of original infliximab before patent expiry was 100% since it was the only reimbursed biological medicine within the indication. In case of CD, infliximab and adalimumab had almost the same average market share at patent expiry, 51% and 49%, respectively. Interestingly, after patent expiry, the market share of multisource infliximab showed a decreasing trend in each indication. As for Cluster 2, infliximab utilization was much less dominant even before patent expiry: the average monthly market share was in the range of 9% and 27% in different indications. Furthermore, the utilization of multisource infliximab was even further reduced in Cluster 2 after the original infliximab lost its market exclusivity.
3.2. Treatment for new patients
contains the average market share of reimbursed biologicals (original and biosimilar infliximab and other, still patent-protected biologicals) before and after patent expiry. Calculations were done to appraise the prescribing patterns in biological treatment-naive patients before and after the patent expiry of infliximab.
Table 2. Average share of initial biological therapy for treatment-naive patients in different indications before and after patent expiry of infliximab
In Cluster 1, before patent expiry infliximab was the dominant choice for biological treatment-naive patients in UC (59.6%), in CD (49.7%) and in PCD (100.0%). After patent expiry, the overall utilization of multisource infliximab (i.e. the sum of original and biosimilar infliximab utilization) increased to 63.8% in UC, and to 51.3% in CD. On the contrary, a declining trend was observable in case of PCD, where utilization of multisource infliximab decreased to 35.5% after patent expiry. (Originator infliximab maintained a market share between 13.5% and 17.1% in all three indications even after patent expiry.)
As for the indications in Cluster 2 original infliximab utilization was marginal compared to Cluster 1, both before and after patent expiry. In RA, the market share of original infliximab was only 2.6% during the patent protection period, interestingly price erosion due to patent expiry did not affect positively the utilization of more affordable multisource infliximab. In AS and P higher utilization of infliximab was observed before patent expiry (11.0% and 8.9%, respectively); however, the utilization of more affordable multisource infliximab after patent expiry decreased further to 5.2% and 3.0%, respectively. In PA a slight increase from 6.3% (original infliximab) to 6.9% (multisource infliximab) was found after the market entry of biosimilar infliximab.
3.3. Switching patterns
Switching patterns of biologicals in different indications are presented in . As for Cluster 1, 70-76% of switches occurred from infliximab to the other, still patented biological medicine. In case of Cluster 2, proportion of switching from infliximab to another still patent protected original biological was much smaller, between 9%-23% in different indications.
Table 3. Switching patterns in the reimbursed indications of infliximab (2012.09. – 2016.12.)
Switching from original infliximab to biosimilar infliximab happened rarely. In case of Cluster 1 switching from original infliximab to biosimilar infliximab occurred only in 7-16% of switches. In those indications with three or more patented biologicals (Cluster 2), only 0%-3% of patients were switched from original infliximab to the biosimilar alternative. Patients in both clusters were switched mainly to other, still patented original biologicals.
There were only a few cases when patients switched from other patented biologicals to multisource infliximab in Cluster 1, with more patients switching to the biosimilar alternative compared to those switching to the original infliximab. In Cluster 2 the proportion of switching from other patented biologicals to multisource (original and biosimilar) infliximab was between 1%-5% and 1%-12%, respectively, in different indications. In Cluster 2 patients were dominantly switched from a patented biological to other, still patented biologicals (86%-98%).
4. Discussion
Results of the longitudinal analysis demonstrated an increasing utilization of biologicals during the study period in all indications. This implicitly confirms the existence of hidden access barriers toward biologic medicines with high budget impact in Hungary [Citation14]. Economic rationale suggests that increased utilization of more affordable, multisource biologicals is an appropriate policy intervention to tackle access barriers in countries with limited resources [Citation41]. However, as suggested by the case of infliximab in Hungary, often other patent-protected original biologicals contribute to the increased utilization of biologicals instead of the more affordable multisource products.
If an active substance loses its market exclusivity and other reimbursed original biologicals do not have-, or have only minimal proven added benefit compared to the multisource alternatives, multisource biologicals are more cost-effective; thus, they should be recommended as first-line therapy for treatment-naive patients in financial protocols describing how medicines with reimbursement can be prescribed [Citation14]. In this case all other, still patent-protected biologicals should be used in second-line treatment, after the failure of the multisource product. This would be highly similar to many other diseases with great public health burden, where small molecule generic medicines are the first-line treatments [Citation42,Citation43], and innovative therapies are used only in latter treatment lines. However, despite the economic rationale, the increased utilization of multisource infliximab was not observed either in Cluster 1 or in Cluster 2; moreover, results of Cluster 2 indicates that physicians in 95% of the cases do not even try to initiate the multisource infliximab as a first-line treatment for naive patients. This result indicates that market launch of infliximab biosimilars in Hungary was not translated to increased utilization of more affordable multisource biologicals in the field of monoclonal antibodies.
In case of maintenance care patients, a single switch from the original biological to its more affordable biosimilar alternative should be promoted under medical supervision [Citation41]. Also, in case of a treatment failure on patented biologicals, switching patients to more affordable multisource biologics with competing price could increase allocative efficiency. However, our real-world utilization data indicated that physicians had limited concerns with switching their patients between other original biologicals, but they were reluctant to switch their patients to multisource infliximab, if there were any other alternative patented biologicals on the reimbursement list (in case of Cluster 2).
4.1. Study limitations
Several factors may influence the utilization pattern of different biologicals, hence limit the generalizability of our findings. We could not obtain information on actual prices, and so we did not take into account confidential price discounts in our analysis. We also have not considered the impact of perceived differences in relative effectiveness and different administration route (i.e. subcutaneous vs. intravenous) on therapy selection of biological therapies. Finally, observations in autoimmune diseases may not be transferable to other therapeutic areas, such as malignancies.
5. Conclusion
Our results suggest that the full potential of biosimilars has not been exploited in Hungary yet. In lower-income countries such as the CEE countries, successful biosimilar drug policies should incentivize the utilization of more affordable multisource biological pharmaceuticals to improve patient access.
6. Expert commentary
In line with conclusions of other recent studies [Citation44–46], we believe that education of policymakers, health-care professionals and patients on the opportunity cost of not using biosimilars would be essential in lower-income countries to improve patient access biological medicines.
A recent publication [Citation41] recommended policy interventions in four different areas – 1) public administration, 2) clinical guidelines, 3) evidence base of policymaking and 4) management of uncertainty – to maximize the social benefits of multisource biologicals. We evaluated based on our study findings whether these recommendations (marked with italic) are applied in Hungary. Lessons learned from this study may be generalizable to other lower-income countries as well.
6.1. Recommendations for public administration
Policymakers should develop incentives and administrative tools to enhance the increased utilization of more affordable medicines: analysis of the biologicals’ utilization data in the indications of infliximab suggests that the current Hungarian biosimilar policy could not facilitate the uptake of more affordable multisource biologicals effectively.
Simplified ‘fast-track’ inclusion process should be provided for biosimilars: In Hungary, biosimilar infliximab brands entered the market on 1 November 2013. Despite the fast-track reimbursement decision and that biosimilar infliximab was already available on the positive list, the first utilization record was detected only at the end of May 2014, due to the slow tendering procedure. The opportunity cost of 6-month delay in the utilization of biosimilar infliximab could be considered fairly significant.
6.2. Recommendations for clinical guidelines
Multisource biologicals should be used in first line among biological therapies: When multiple reimbursed biologicals are available within a therapeutic area, priority order of different therapies should be clearly determined in a financial protocol (i.e. mandatory clinical guideline developed jointly by clinical societies and payer, which considers both clinical and economic aspects) in order to facilitate the increased uptake of more affordable multisource biological medicines. Off-patent products on a competitive market with access restrictions may provide more health gain for the same budget by increasing the number of patients treated with biologicals. Based on the current financial protocols, NIHIFM considers that there are no notable differences between the available reimbursed biologicals in terms of efficacy and safety; however, the financial protocol does not consider the price differential between single source medicines with monopolistic price and multisource medicines with price erosion. Consequently, the more affordable multisource infliximab was not a dominant choice as a first-line treatment for prescribers and patients.
Single switch from original to the more affordable biosimilar alternatives under medical supervision should be mandatory: During the research period of our analysis (2012–2016), switching from maintenance treatment to the preferred tender winner brand was not mandated or at least incentivized by the public payer. Using the lower priced biosimilar infliximab was only mandated for biological treatment-naive patients, but not for patients on maintenance therapy.
6.3. Recommendations for evidence-based policymaking and for managing uncertainty
Collect pharmacovigilance data and develop risk-management plans due to increased risk of immunogenicity: Currently, NIHIFM has highly limited resources to manage the complex tasks of 1) evidence-based pharmaceutical policymaking and 2) monitoring the impact of previous policy decisions in the arena of biological products. In the last years, there have been multiple restructuring and cutbacks in all fields of the public administration in Hungary. As a consequence, turnover of human resources at the public payer is outstanding, and analytical capacities are constantly decreasing. Lack of trained experts prevents collecting real-world evidence on the consequences of switching and applying outcomes-based risk-sharing agreements to manage any potential uncertainties related to switching.
7. 5-years view
Evidence-based pharmaceutical policy has become the standard approach for investment decisions of health care including coverage decisions of new pharmaceuticals. On the contrary, disinvestment decisions like the rational use of off-patent pharmaceuticals including biosimilars are rarely substantiated with convincing scientific evidence. In the next five years, more policy research is needed to facilitate effective biosimilar policies. Decision-makers are expected to recognize that the opportunity cost of suboptimal biosimilar medicine policies is even higher in countries with limited resources and poorer overall health status. Countries with access restrictions toward modern medicines should not approach biosimilars only from a disinvestment perspective. Payers are expected to be more proactive in promoting rational use of multisource biologicals as preferred first-line treatments for naive patients. Switching patients on maintenance treatment to biosimilars are expected not to be prevented based on hypothetical concerns. Manufacturers of biosimilar products are expected to be more involved in managing uncertainties related to biosimilars and increasingly taking over risks of payers.
Article highlights
The value proposition of off-patent pharmaceuticals can be approached from two different perspectives based on accessibility of patients to original medicines before the patent expiry. Hence, lower priced biosimilar alternatives can either generate savings in the pharmaceutical budget or increase the number of patients treated by biologicals without the need for extra budget.
In lower-income countries with economic constraints (such as countries in Central and Eastern Europe), biosimilar drug policies should incentivize the utilization of more affordable multisource biological pharmaceuticals to improve patient access.
In Hungary, the biosimilar policy did not result in increasing utilization of more affordable multisource biological medicines compared to other original biologicals in the indications of infliximab.
Policymakers may choose from several options in different areas, including public administration and clinical guidelines, to facilitate biosimilar use. The efficiency of policy interventions should be monitored by reviewing the initial therapy of new patients and switching patterns.
Author contribution statement
All authors contributed to the design and implementation of the research, to the analysis of the results and to the writing of the manuscript. Authors summarized their independent professional opinion and take full responsibility for potential errors in the manuscript.
Declaration of interest
Data were provided by the National Institute of Health Insurance Fund Management. The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Morrow T, Felcone LH. Defining the difference: what makes biologics unique. Biotechnol Healthc. 2004;1(4):24–29.
- EMA [Internet]. Biosimilars in the EU – information guide for healthcare professionals. European Medicines Agency; [cited 2018 Jul 28]. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/general/general_content_001832.jsp
- Bennett CL, Chen B, Hermanson T, et al. Regulatory and clinical considerations for biosimilar oncology drugs. Lancet Oncol. 2014 Dec;15(13):e594–e605.
- GaBi Online [Internet]. Biologicals sales have almost doubled since 2006. Pro Pharma Communications International; [cited 2018 Jul 28]. Available from: http://www.gabionline.net/Biosimilars/General/Biologicals-sales-have-almost-doubled-since-2006
- PipelineReview.com [Internet]. Blockbuster biologics 2012. R&D Pipeline News, Special Edition 1/2013; [cited 2018 Jul 28]. Available from: https://pipelinereview.com/index.php/2013050850905/FREE-Reports/Blockbuster-Biologics-2012.html
- Aitken M. Delivering on the potential of biosimilar medicines. The role of functioning competitive markets. IMS Health; 2016.
- Aitken M. Outlook for global medicines through 2021. Balancing cost and value. QuintilesIMS Institute; 2016.
- IMS Health. Shaping the biosimilars opportunity: a global perspective on the evolving biosimilars landscape. IMS Health; 2011.
- DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22(2003):151–185.
- Crommelin D, Bermejo T, Bissig M, et al. Pharmaceutical evaluation of biosimilars: important differences from generic low-molecular weight pharmaceuticals. Eur J Hosp Pharm. 2005;11(1):11–17.
- Moorkens E, Jonker-Exler C, Huys I, et al. Overcoming barriers to the market access of biosimilars in the European Union: the case of biosimilar monoclonal antibodies. Front Pharmacol. 2016;7:193.
- Kawalec P, Stawowczyk E, Tesar T, et al. Pricing and reimbursement of biosimilars in central and eastern European countries. Front Pharmacol. 2017;8:288.
- Kalo Z, Voko Z, Ostor A, et al. Patient access to reimbursed biological disease-modifying antirheumatic drugs in the European region. J Mark Access Health Policy. 2017;5(1):1345580.
- Inotai A, Csanadi M, Petrova G, et al. Patient access, unmet medical need, expected benefits, and concerns related to the utilisation of biosimilars in Eastern European countries: a survey of experts. Biomed Res Int. 2018;2018. p. 1–9. Article ID 9597362.
- Jönsson B, Hofmarcher T, Lindgren P, et al. Comparator report on patient access to cancer medicines in Europe revisited. IHE Report. Lund: IHE; 2016:4.
- Kobelt G, Kasteng F. Access to innovative treatments in rheumatoid arthritis in Europe. European Federation of Pharmaceutical Industry Associations (EFPIA); 2009.
- Orlewska E, Ancuta I, Anic B, et al. Access to biologic treatment for rheumatoid arthritis in Central and Eastern European (CEE) countries. Med Sci Monit. 2011 Apr;17(4):SR1–13.
- GaBi Online [Internet]. Biologicals dominate Europe’s best sellers. Pro Pharma Communications International; [cited 2018 Jul 28]. Available from: http://www.gabionline.net/Reports/Biologicals-dominate-Europe-s-best-sellers
- GaBi Journal Editor. Patent expiry dates for biologicals: 2017 update. GaBI J. 2018;7(1):29–34.
- EMA. Biosimilars in the EU. Information guide for healthcare professionals. European Medicines Agency; 2017.
- EMA. Guideline on similar biological medicinal products. European Medicines Agency; 2014. CHMP/437/04 Rev.1.
- Lamanna WC, Holzmann J, Cohen HP, et al. Maintaining consistent quality and clinical performance of biopharmaceuticals. Exp Opin Biol Ther. 2018;18(4):369–379.
- EMA, European Commission. Biosimilars in the EU. Information guide for healthcare professionals; [cited 2019 Sept 3]. Available from: https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_en.pdf
- Inotai A, Prins CPJ, Csanadi M, et al. Is there a reason for concern or is it just hype? – A systematic literature review of the clinical consequences of switching from originator biologics to biosimilars. Exp Opin Biol Ther. 2017;17(8): 915–926.
- Cohen HP, Blauvelt A, Rifkin RM, et al. Switching reference medicines to biosimilars: a systematic literature review of clinical outcomes. Drugs. 2018 Mar;78(4):463–478.
- Ebbers HC, Muenzberg M, Schellekens H. The safety of switching between therapeutic proteins. Expert Opin Biol Ther. 2012 Nov;12(11):1473–1485.
- Gisbert JP, Chaparro M. Switching from an originator anti-TNF to a biosimilar in patients with inflammatory bowel disease: can it be recommended? A systematic review. Gastroenterol Hepatol. 2018 Jun - Jul;41(6):389–405.
- NOR-SWITCH study group, Jorgensen KK, Olsen IC, Goll GL, et al. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet. 2017;389(10086):2304–2316.
- Kutzin J Health financing policy: a guide for decision-makers. Health (Financing Policy Paper 2008/1). WHO; 2008.
- WHO. The world health report 2000. Health systems: improving performance. WHO; 2000.
- Kalo Z, Holtorf AP, Alfonso-Cristancho R, et al. Need for multicriteria evaluation of generic drug policies. Value Health. 2015;18:346–351.
- Aladul MI, Fitzpatrick RW, Chapman SR. Impact of infliximab and etanercept biosimilars on biological disease-modifying antirheumatic drugs utilisation and NHS budget in the UK. BioDrugs. 2017;31:533.
- Elek P, Harsanyi A, Zelei T, et al. Policy objective of generic medicines from the investment perspective: the case of clopidogrel. Health Policy. 2017;121(2017):558–565.
- Inotai A, Csanadi M, Harsanyi A, et al. Drug policy in Hungary. Value Health Reg Issues. 2017 Sep;13:16–22.
- EMA: Inflectra [Internet]. European Medicines Agency; [cited 2018 Jul 28]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/inflectra
- EMA: Remsima [Internet]. European Medicines Agency; [cited 2018 Jul 28]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/remsima
- EMA: Remicade – summary of product characteristics [Internet]. European Medicines Agency; [cited 2018 Jul 28]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000240/WC500050888.pdf
- Farkas K, Rutka M, Ferenci T, et al. Infliximab biosimilar CT-P13 therapy is effective and safe in maintaining remission in Crohn’s disease and ulcerative colitis – experiences from a single center. Exp Opin Biol Ther. 2017;17(11):1325–1332.
- Balint A, Rutka M, Kolar M, et al. Infliximab biosimilar CT-P13 therapy is effective in maintaining endoscopic remission in ulcerative colitis – results from multicenter observational cohort. Exp Opin Biol Ther. 2018;18(11):1181–1187.
- Public database on reimbursement submissions [Internet]. National Institute of Health Insurance Fund Management, Hungary; [cited 2018 Jul 28]. Available from: http://www.neak.gov.hu/felso_menu/szakmai_oldalak/gyogyszer_segedeszkoz_gyogyfurdo_tamogatas/egeszsegugyi_vallalkozasoknak/gyartok_forgalomba_hozok/KERELEM_IND_ELJ_GYOGYSZ_TAPSZ.html
- Inotai A, Csanadi M, Vitezic D, et al. Policy practices To maximise social benefit from biosimilars. J Bioequivalence Bioavailability. 2017;9:467–472
- James PA, Oparil S, Carter BL, et al. Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520.
- Davidson JR. Major depressive disorder treatment guidelines in America and Europe. J Clin Psychiatry. 2010;71:e04.
- Sule A, Jorgensen F, Horak P, et al. Biosimilar medicines. Eur J Hosp Pharm. 2019 Mar;26(2):117–118.
- Giuliani R, Tabernero J, Cardoso F, et al. Knowledge and use of biosimilars in oncology: a survey by the European Society for Medical Oncology. ESMO Open. 2019 Mar 6;4(2):e000460.
- Leonard E, Wascovich M, Oskouei S, et al. Factors affecting health care provider knowledge and acceptance of biosimilar medicines: a systematic review. J Manag Care Spec Pharm. 2019 Jan;25(1):102–112.