ABSTRACT
Objectives: The approval in more than 50 countries of baricitinib, an oral Janus Kinase inhibitor for the treatment of Rheumatoid Arthritis (RA), warrants a framework for corresponding economic evaluations. To develop a comprehensive economic model assessing the cost-effectiveness of baricitinib for the treatment of moderately-to-severely active RA patients in comparison to other relevant treatments, considering the natural history of the disease, real world treatment patterns, and clinical evidence from the baricitinib trials.
Methods: A systematic literature review of previously developed models in RA was conducted to inform the model structure, key modeling assumptions and data inputs. Consultations with rheumatologists were undertaken to validate the modeling approach and underlying assumptions.
Results: A discrete event simulation model was developed to international best practices with flexibility to assess the cost-effectiveness of baricitinib over a lifetime in a variety of markets. The model incorporates treatment sequencing to adequately reflect treatment pathways in clinical practice. Outcomes assessed include cost and quality-adjusted life years, allowing for a full incremental analysis of cost-effectiveness of competing treatments and treatment sequences.
Conclusion: The economic model developed provides a robust framework for future analyses assessing the cost-effectiveness of baricitinib for the treatment of RA in specific country settings.
1. Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory joint disease and affects approximately 2.5% of the population worldwide [Citation1,Citation2], amounting to an estimated 6.1 million cases of RA globally and an annual incidence rate of 1.36% [Citation3]. It is associated with multiple comorbidities within and outside of the musculoskeletal system [Citation4–Citation7] as well as with psychosocial impairments [Citation8]. RA imposes a substantial economic burden, with an estimated average direct medical cost per RA patient of 5,720, USD equating to a global economic burden of over 32 USD billion annually [Citation9].
Current therapeutic targets for treatment of RA include remission, controlling disease activity, minimizing loss of function and improving the quality of life of affected patients [Citation10]. Several treatment options are available to RA patients [Citation11]: Treatment recommendations focus on early diagnosis, followed a step-wise pharmacotherapeutic approach that is commonly initiated with nonsteroidal anti-inflammatory drugs (NSAIDs) and/or glucocorticoids. Conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) such as methotrexate (MTX) are typically added if therapeutic targets are not achieved. If csDMARDs also fail to adequately control disease activity, a biologic DMARD (bDMARD) is typically added to the regimen, such as a tumor necrosis factor inhibitor (TNFi) [Citation12]. If this regimen also fails to adequately control disease activity, a switch to another TNFi or to a bDMARD with a different mechanism of action is usually considered.
Since a substantial proportion of patients seldom achieves sustained disease remission with currently available therapies, there remains a considerable unmet need for effective treatments for patients suffering with RA [Citation13]. Baricitinib is an orally administered, selective and reversible Janus kinase (JAK) inhibitor that belongs to the new drug class of targeted synthetic DMARDs (tsDMARDs). It is rapidly absorbed, has an elimination half-life of 12.5 hours and is dosed once daily. Its efficacy and safety as monotherapy or in combination with csDMARDs for treatment of moderately-to-severely active RA has been demonstrated in 52-week, phase 3, double-blind, placebo- and active-controlled trials in csDMARD-naïve patients (RA-BEGIN [Citation14]), patients with prior inadequate response or intolerance to csDMARD therapy (csDMARD-IR) (RA-BUILD [Citation7]), patients with prior inadequate response or intolerance to MTX (RA-BEAM [Citation15]), and prior inadequate response or intolerance to bDMARD therapy (RA-BEACON [Citation16]).
To assess the cost-effectiveness of baricitinib, a robust model was developed. While previous economic models in RA were considered for the framework development, a new model build was required given the economic evaluation focused on a first-in-class product which warranted a tailored design to appropriately model the product characteristics.
In this paper, we present the modeling approach developed for assessing the cost-effectiveness of baricitinib in various RA populations. The approach adopted in developing the baricitinib model was multi-faceted. It incorporated findings from a systematic review of economic models and from critical appraisals by health technology assessment (HTA) agencies on previously developed models and their methodologies. The complexity of the natural history of the disease, common treatment patterns in RA, and the heterogeneity of patient populations in RA were further considerations for the model framework development. Evidence from the baricitinib clinical trial programme and real world evidence from registries and databases, along with clinical expertise to overcome any evidence gaps, also informed the model concept. This paper presents the conceptual model framework and its assumptions, and will discuss its value as a comprehensive model to assess cost-effectiveness of RA treatments.
2. Methods
2.1. Systematic literature review of published economic evaluations
To understand previous modeling approaches employed in RA, published economic models and evaluations were first identified and reviewed through an internal systematic literature review (SLR) and a pragmatic search that followed guidelines published by the Cochrane Collaboration and the United Kingdom’s (UK) National Institute for Health and Care Excellence (NICE) [Citation17,Citation18]. The purpose of the SLR was to inform the model structure and to identify relevant data inputs for the economic model. The SLR was conducted in November 2014 and updated in October 2016, and covered all pharmacological treatments used in RA. Searches were run in the following databases: Embase, MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations, EconLit, and NHS EED.
The SLR identified key elements for consideration throughout the model development phase, including model types, cohort heterogeneity, model outcomes, quality of life (QOL) modeling and mapping techniques, treatment comparisons, clinical endpoints, resource use inputs and incorporation of adverse events (AEs) in RA modeling.
A large pool of literature on cost-effectiveness modeling in RA was identified to inform the model type, which generally fit into one of the following four groups:
2.1.1. Decision tree model
Decision trees (see references 2, 25–26 in Table 3 of supplementary material) are a popular form of aggregate level ‘cohort’ models, examining the proportions of the population undergoing different events with associated costs and benefits. The most important limitation of the decision trees is that recursion, or looping, is not allowed and thus these models represent a static ‘frameshot’ of the condition under consideration, with assessment of singular or a limited set of treatment decisions. More complex models are typically needed when modeling chronic diseases and if timing is an important factor in the analysis [Citation19].
2.1.2. Cohort Markov model
Cohort Markov models (see references 4, 21–23, 33-34-39, 43–48, 50, 62, 64, 66, 69–70, 75–77 in Table 3 of supplementary material) are better suited than decision trees to accommodate modeling of disease progression as well as sequential or repetitive outcomes. In a traditional Markov model, a cohort of ‘average’ patients progress through mutually exclusive health states. The model is run for several cycles in which there are sub-cohort transitions between the states determined by transition probabilities. An important limitation of the Markov model is that transition probabilities and rewards cannot vary with patient history (Markovian assumption) [Citation19].
2.1.3. Individual patient simulation model
Individual patient simulation (IPS) models (see references 27, 31–32, 42, 44, 51–57, 59 67–68 in Table 3 of supplementary material) track unique individuals as they progress through the model independently of each other. Modeled individuals have potentially heterogeneous characteristics that affect their progression. A common type of IPS model is a Markov model that simulates different individuals rather than an ‘average’ cohort of patients. This allows for more accurate modeling of heterogeneous patient cohorts, especially where such heterogeneity affects patient outcomes, albeit at the expense of computational complexity. A limitation of the Markov IPS is the need to make specific assumptions about the time cycles used in the model. Moreover, a Markov IPS can become unmanageable in cases where a large number of different health states need to be modeled [Citation19].
2.1.4. Non-Markov IPS model
Non-Markov IPS models (see in Table 3 of supplementary material references 1, 3, 5–20, 27–30, 40–41, 49, 63, 65, 71–74) are a subset of IPS models that do not have an underlying Markov structure. The two main model types that fit into this category are discrete time models and discrete event simulation models (DES). The key difference between the two is the modeling of time. In discrete time models, regular intervals of time elapse at which patients may or may not experience an event or an update to their characteristics. These updates may influence patients’ probability of experiencing events in future intervals. In DES models, the sequence of events are tracked along with the gap (i.e. time) between events, therefore representing a fixed system until each event is simulated, at which point the system is updated and used to simulate the next event. Since time is not simulated between the occurrence of events, DES models are less burdensome computationally [Citation19].
The results of the SLR found that a number of key decision analytic models were developed using a DES approach [Citation20]. Notably, this included the model developed by NICE in the UK for its multiple technology assessment in RA [Citation18], the Birmingham Rheumatoid Arthritis Model, BRAM [Citation21], and the economic model developed by the Institute for Clinical and Economic Review (ICER) in the United States (US) for its review of targeted immune modulators for treatment of RA [Citation22]. In addition, an independent SLR of decision analytic models in RA endorsed DES as the methodology of choice for modeling cost-effectiveness in RA wherever sufficient data are available [Citation23]. Other sources to inform the modeling approach included model conceptualization and consensus statements [Citation24]. The open source RA model developed by the Innovation and Value Initiative (IVI) in the statistical packages R and C++ was only published after the baricitinib model development and hence not considered (25).
2.2. Economic modeling approach
Based on the findings of the RA modeling SLR, the modeling framework adopted a DES approach to evaluate the cost-effectiveness of treating patients with baricitinib. Key advantages driving the decision for adoption of a DES approach included its capacity and flexibility to incorporate marked population heterogeneity in RA [Citation24] by considering individual patient characteristics and disease history, to decrease simulation time, and to capture the patient pathway in conditions where sequences of treatments need to be modeled (as is the case in RA). The ISPOR Task Force good research practices in DES also argue that a DES model is appropriate when patient heterogeneity needs to be taken into account, especially if they are likely to change over time – as they do with RA patients [Citation25]. Given that a cohort model does not possess this flexibility when patient heterogeneity is an important characteristic of the disease, it was decided that the best approach to model baricitinib cost-effectiveness was the DES approach.
2.3. Model structure and inputs
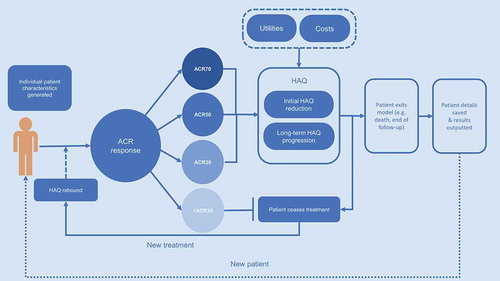
The model was developed in Microsoft Excel (© Microsoft Corporation) with Visual Basic for Applications, following international guidance and best practices in DES modeling [Citation26]. The model schematic in depicts the flow of each patient through the model simulation. In total, up to 18 comparator treatment sequences with up to 10 treatments within each sequence can be accommodated. The model hence has extensive capacity to assess cost-effectiveness in various markets, since countries may differ in terms of standard treatment pathways and sequencing of therapies.
2.3.1. Patient populations
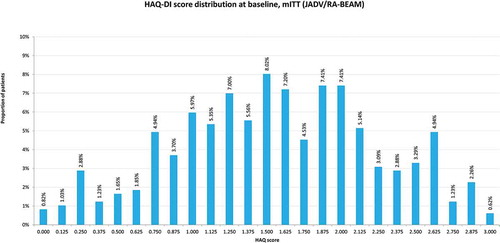
Within the DES modeling framework, individual patients were generated with a range of characteristics, capturing the heterogeneity of RA patients. Patient baseline characteristics on the Health Assessment Questionnaire–Disability Index (HAQ-DI, from now on referred to as HAQ), age and gender were generated from distributions around mean values and associated confidence intervals derived from the respective baricitinib clinical trials, depending on whether csDMARD-naïve RA populations, csDMARD-IR RA populations, or bDMARD-IR RA populations are modeled. Different baseline data were applied to populations with moderately active, severely active, or moderately-to-severely active RA. For comparisons based on the RA-BEAM head-to-head study comparing baricitinib and adalimumab, the exact distribution of the patient level data from the trial was used in the model to replicate the patient baseline heterogeneity ().
2.3.2. Treatment sequences
The model was built to incorporate both head-to-head single comparator analyses and different potential treatment sequences, consistent with the RA modeling landscape investigated in the SLR. The inclusion of sequences is pivotal to the modeling methodology, with the flexibility to run multiple treatment sequences reflecting common local treatment pathways. Treatment sequencing also allows investigating the impact of initiating treatment with baricitinib at different stages of the RA pathway.
2.3.3. Efficacy
Treatment efficacy estimates were defined based on the American College of Rheumatology (ACR) criteria, a validated categorical variable widely used in RA and found in an internal clinical SLR [Citation27] to be the most commonly reported clinical efficacy endpoint in RCTs investigating treatments for RA. ACR was also used as the primary clinical response outcome across the baricitinib clinical trials programme. Each patient was categorized into the appropriate ACR response category (ACR<20; ≥20 to <50; ≥50 to <70; ≥70). To enhance flexibility for model adaptations to various country settings, EULAR response criteria were also considered. In the model, response data were derived either from the baricitinib clinical trial programme or, for treatment comparisons where no head-to-head data is available, from a network meta-analysis (NMA). The Bayesian NMA, which was conducted following best practices in evidence synthesis [Citation28–Citation30], generated relative treatment effects for all comparator treatments and across all RA patient populations (csDMARD-naïve, csDMARD-IR and bDMARD-IR). Clinical data from the RA-BEAM study is used for analyses comparing baricitinib to adalimumab, whereas NMA data is used for comparisons involving other treatments. In the model, if a patient does not achieve at least ACR20 at the primary assessment time point, the treatment is terminated and the patient is assumed to move to the next treatment in a given sequence. Alternatively, as a proxy for last line of therapy, the patient moves to palliative care, which is defined as a mix of leflunomide and cyclosporine, for the remainder of the model time horizon.
2.3.4. HAQ
In the model, physical function was captured by HAQ. A patient’s initial change in HAQ score was assigned based on ACR or EULAR response category achieved at the primary assessment time point, as informed by the published literature and data from the baricitinib clinical trials [Citation31].
Long-term HAQ change based on the calculated HAQ trajectory was then applied for the period beyond the initial time of the primary endpoint assessment to the point at which either treatment discontinuation or death occurs. HAQ was assumed to remain flat while patients were on therapy with bDMARDs or baricitinib. This was consistent with previous economic analyses in RA [Citation18] and was supported by data from the RA-BEYOND trial, a long-term extension study [Citation32]: In patients who continued treatment with baricitinib 4 mg (N = 303) after end of follow-up in the RA-BEAM study, mean (SD) baseline HAQ in RA-BEYOND was 0.73 (0.65), compared to 0.75 (0.68) in patients who were switched from adalimumab 40 mg to baricitinib 4 mg (N = 186) at the start of RA-BEYOND. No statistically significant changes in HAQ change from baseline were observed at Week 24 (i.e. Week 76 overall) for any of the treatment groups [Citation32]. For patients being switched to palliative care, the HAQ trajectory was assumed to deteriorate by replicating the latent class approach derived from a growth mixture model, which used a large longitudinal dataset to identify four trajectories for HAQ score progression over time and the patient baseline characteristics predictors associated with these trajectories as well as their prognostic value for mortality [Citation20]. The model has, however, functionality to consider an alternative assumption of linear HAQ progression (mean rate of HAQ increase of 0.06/year) as derived from the published literature [Citation21]. This functionality allows testing the sensitivity around alternative long-term HAQ change estimation methods.
2.3.5. Quality of life
Initially, the possibility of applying direct estimates of QOL using EQ-5D data derived from RCTs was explored. An assessment of studies included in an internal clinical SLR to identify EQ-5D data for comparator treatments found that direct estimates were not sufficiently reported in included RCTs. As such, the NICE recommendation was followed on using mapping algorithms to convert HAQ score into utility, as adopted by NICE in their multiple technology appraisals of biologics treatments in RA (TA375) [Citation33]. As a result, a number of different algorithms were built into the model to map from the disease-specific HAQ measure to generic EQ-5D estimates. First, the three-class mixture model by Hernandez et al is included as this study has been found to perform better than linear models for estimating EQ-5D [Citation34]. The three-class model was used in the model as the predicted values for selected combinations of covariates were reported in the published article, thus allowing for direct validation of these results with those generated from the replicated mapping algorithm. Model functionality is also provided to alternatively use a quadratic mapping mechanism that was used in the BRAM [Citation21]. Finally, to consider the QOL data collected in the active-controlled RA-BEAM study, a novel algorithm was developed to map from HAQ to EQ-5D, using the trial-derived individual patient data comprised of HAQ scores and ‘crosswalked’ EQ-5D-5 L index values. The model allows the testing of all the above-mentioned mapping algorithms and their impact on cost-effectiveness results.
2.3.6. Discontinuation
For assessment of treatment discontinuation, time on treatment was calculated considering the rate of treatment discontinuation conditional on the initial treatment response. It was also assumed that start and end effects can be modeled as one-off deductions, proportional to the change in QOL score. Long-term treatment discontinuation was modeled by applying the best fitting parametric model that follows Kaplan-Meier data of an RA registry analysis on continuation of first-line biologic use in RA [Citation35]. No long-term discontinuation data was identified for JAK inhibitors, hence the data from the registry analysis of bDMARDs was applied to both bDMARDs and baricitinib. Finally, a HAQ rebound effect was applied upon treatment discontinuation, assuming that the treatment effect is lost whenever active treatment is terminated, in line with clinical practice.
2.3.7. Mortality
Mortality was estimated in the model by applying hazard ratios (HR) stratified by baseline HAQ score bands to country specific life tables, which was in line with a published review of methodologies for modeling mortality in RA [Citation18,Citation36].
2.3.8. Safety
The SLR of previously conducted economic models in RA found that adverse events (AEs) were not commonly included or did not significantly impact analysis results. In line with these findings, AEs are assumed not to be a key driver of the model. The aforementioned internal clinical SLR found that AEs were not systematically reported in RCTs of comparators, and that differences in clinical definitions and rescue time across studies raised concerns about inclusion of safety outcomes and AE related costs within the model. This was further validated by clinical specialists and health economists in a consultation workshop conducted prior to model development. The baricitinib cost-effectiveness model therefore does not consider serious AEs and their management costs in the base case analysis. However, the model does include the functionality to conduct a scenario analysis including severe AEs, based on data from the head-to-head trial with adalimumab, RA-BEAM.
2.3.9. Resource use and unit costs
The model considers drug acquisition costs alongside administration and monitoring costs associated with each treatment. In line with previous economic models in RA [Citation18] and consistent with the SLR conducted to inform the economic model, the same monitoring tests and frequency of monitoring visits/tests were assumed to apply to both csDMARDs and bDMARDs. In the model, no administration costs were applied to treatments administered subcutaneously as patients are assumed to self-administer these. Hospitalization costs were calculated by applying the cost of an inpatient day to the average number of hospital days per year per HAQ band, as derived from the published literature [Citation37].
2.4. Model settings
Given the increasing focus on including a societal perspective in economic modeling, the baricitinib model was developed with functionality to consider both a health care payer perspective and a societal perspective (using either a human capital or friction cost approach), the latter of which additionally considers costs attributable to productivity losses as a result of RA-related absenteeism. Similarly, the time horizon is fully customizable but is set to lifetime for the base case given the chronic, lifelong nature of the condition.
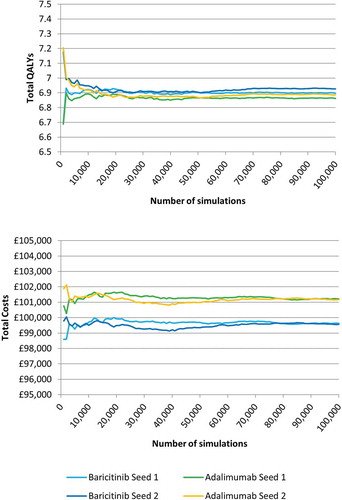
In order to determine the appropriate number of patients to be simulated for the generation of stable results, an exploratory analysis was conducted to assess convergence. Two sets of comparisons were run using different seeds for the ‘random walk’ in the model so that each random number generator would provide a different set of random numbers in each case (seed 1 and seed 2). Outcomes were recorded up to a maximum of 100,000 patients and results plotted in separate graphs for quality adjusted life years (QALY) and costs. The two generated convergence graphs for total QALYs and total costs are presented in . Based on visual inspection of the generated curves, it was deemed appropriate to run analyses with 50,000 simulated patients per arm for 1st order uncertainty analyses.
2.5. Uncertainty
The model includes functionality to run 1st order 2nd order uncertainty analyses. For 2nd order uncertainty analysis, model input parameters are randomly and simultaneously varied. The model uses predefined ranges around the mean values either based on standard errors sourced from primary sources or by applying a user defined variation (set to 10% at default) to generate random inputs that follow appropriate sampling distributions. In 2nd order uncertainty analysis, values from these distributions are repeatedly sampled to recalculate the cost-effectiveness ratio for each iteration. For all costs used in the model, a normal approximation of a gamma distribution is applied (since older versions of Excel generate errors when sampling using the in-built gamma function). Uncertainty around mean values of ACR/EULAR response applied in the model is captured by the CODA output from the NMA. For pain-to-HAQ values, a beta distribution is used to sample appropriate values in 2nd order uncertainty analysis, whereas for mortality rates per HAQ band the values remain the same since confidence intervals around the mean values overlapped in the source data [Citation18] and monotonicity should be preserved.
In addition, the model was programmed to allow for the exploration of additional scenario analyses by changing key parameters or modeling assumptions, such as the inclusion of serious AEs, different patient populations, different treatment sequences and the mapping algorithm used to generate EQ-5D values from HAQ scores or the HAQ trajectory on palliative care.
2.6. Validation
Model validation was carried out in three distinctive steps. First, the conceptual framework of the model was assessed by two leading rheumatologists and two health economists experienced in health economic modeling within the therapeutic area of RA. All experts unanimously responded that the conceptual model was appropriate based on a critical review and discussion of how the natural history of the disease was modeled in the conceptual framework, and how amenable the framework was to economic evaluation, including in terms of comparators, perspective and included costs. While no structural changes to the draft model framework were recommended by the experts, the requirement for a different modeling methodology was pointed out in settings where clinical treatment goals are focused on a treat-to-target approach (see Discussion).
The fully programmed model was then subjected to formal quality control, validation and testing by an external party, which identified a small number of minor programming outages for rectification and found the modeling assumptions to be reasonable and well justified.
Finally, the model was appraised using the Assessment of the Validation Status of Health-Economic decision models (AdViSHE) validation-assessment tool [Citation38] to ensure all critical considerations for the economic model had been adequately addressed (see supplementary material).
3. Discussion
The aim of this study was to develop a cost-effectiveness model in a transparent and flexible way that would allow the assessment of the economic value of baricitinib for the treatment of moderately-to-severely active RA patients. A discrete event simulation model approach was selected based on a SLR of published economic evaluations in RA, which found that DES is commonly recommended as the modeling approach of choice and has been adopted for key economic models in this disease area. Contrary to cohort-based models, DES models better capture patient heterogeneity as well as the treatment pathways of RA patients.
The model developed for baricitinib provides a robust model design and structure informed by best modeling practices in RA as well as an internal SLR on published models. It adopts a comprehensive approach for assessing the cost-effectiveness of RA treatments by incorporating functionalities that allow testing of numerous assumptions related to key parameters. Using a DES approach, treatment pathways of individual patients are approximated by using treatment sequences that mirror real world clinical practice.
The modeling framework builds on previous economic evaluations in RA but differs from previous studies in a number of ways. Most notably, it integrates different modeling approaches to afford maximum flexibility to test alternative settings. While use of either ACR or EULAR response as clinical efficacy and physical function parameters is consistent with prior models, the present modeling framework allows switching between these alternative response measures. This not only provides flexibility to align with different country settings but also permits an assessment of the impact of using different response measures on modeled outcomes and cost-effectiveness results. Similarly, the framework integrates linear and non-linear modeling of HAQ progression on palliative care. Several HAQ to EQ-5D mapping algorithms are programmed into the model, including a novel algorithm based on QOL data from the RA-BEAM study.
The developed cost-effectiveness model has a number of limitations, most of which stem from a lack of published evidence. Since clinical efficacy and physical function data are usually reported from short-term studies, the model either relies on assumptions of constant treatment benefit (e.g. HAQ on treatment) or extrapolation of outcomes over the model time horizon (e.g. all-cause discontinuation). This is, however, consistent with other published economic models in RA given the dearth of publicly available, long-term follow-up data. The same discontinuation rate is applied to all included treatments since treatment-specific discontinuation data was deemed inappropriate due to potential confounding; discontinuation is not stratified by ACR/EULAR response category due to a lack of published data. In order to calculate generic QALYs, the disease-specific measure of physical function, HAQ, was mapped to the EQ-5D measure. However, a series of mapping algorithms were included in the model to allow testing their impact on cost-effectiveness results. Long-term discontinuation was estimated irrespective of the ACR/EULAR response level due to lack of published data. Similarly, stratification of long-term discontinuation by treatment is not implemented in the model as this approach was deemed inappropriate due to risk of confounding bias. Additional research with long-term follow-up is warranted to further inform and validate the constructed model.
In addition to the above specific limitations relating to the implementation of the modeling framework, more recent proposals for alternative cost-effectiveness evaluation methodologies in RA should be noted. Alemao et al. recently presented an RA conceptual model proposing, among other things, use of composite measures of disease activity to evaluate treatment response as well as disease progression, utility mapping and mortality assessment based on disease activity, and incorporation of extra-articular outcomes and of subgroups based on prognostic factors [Citation39]. While the baricitinib modeling framework is consistent with the overarching model structure of the conceptual model suggested by Alemao et al, the consensus suggestions highlight areas for future extension of the baricitinib model.
Similarly, an alternative modeling methodology has been suggested for jurisdictions where treatment goals are focused on up-titration to a pre-specified disease activity target (either remission or low disease activity) and maintaining such targets over time (‘treat-to-target’) [Citation40–Citation43]. While this approach is challenging to incorporate in our modeling framework, a recent study has presented an economic evaluation of baricitinib adopting this alternative methodology [Citation44].
In conclusion, an economic model was built to international best practices in DES modeling to assess the cost-effectiveness of baricitinib for the treatment of moderately-to-severely active RA patients. The model was developed considering modeling requirements capturing the natural history of the disease, real world treatment patterns, as well as clinical evidence from the baricitinib trials. It provides a robust framework for future cost-effectiveness analyses in specific country settings.
Article Highlights
A comprehensive modelling framework was developed to allow robust assessment of the cost-effectiveness of baricitinib, an orally administered, selective and reversible Janus kinase (JAK) inhibitor, for the treatment of moderate-to-severe rheumatoid arthritis (RA) in comparison with other available treatments.
The framework was informed by findings from a systematic literature review of published economic evaluations in RA, international guidance and best practices in health economic modelling, and validated by a panel of clinical specialists and health economists.
A discrete event simulation model was programmed considering the natural history of the disease and clinical evidence from the baricitinib trials, and providing flexibility to align the analysis with different real world treatment pathways.
The economic model developed provides a framework for future analyses assessing the cost-effectiveness of baricitinib for the treatment of RA in specific country settings.
Declaration of interest
M Schlueter, P Rouse and A Pitcher are full-time employees and served as paid consultants to Eli Lilly for this study. W Fakhouri, PL Graham-Clarke and C Nicolay are employees of Eli Lilly and Company and shareholders of Eli Lilly and Company. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
One peer reviewer has received speaking fees from Bristol-Myers, Pfizer, Takeda, and Eli Lilly. Another peer reviewer is an employee of Bristol-Myers Squibb.
Supplemental Material
Download MS Word (225.5 KB)Supplementary material
The supplementary data for this article can be accessed here.
Additional information
Funding
References
- Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016 Oct 22;388(10055):2023–2038.
- Gibofsky A. Epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis: a synopsis. Am J Manag Care. 2014;20(7 Suppl):S128–35.
- Nomen nescio. The global burden of rheumatoid arthritis will increase. Pharmaceutical Technology [Internet]. 2017 May 26. Available from: https://www.pharmaceutical-technology.com/research-reports/researchreportthe-global-burden-of-rheumatoid-arthritis-will-increase-5825875/.
- Van Onna M, Boonen A. The challenging interplay between rheumatoid arthritis, ageing and comorbidities. BMC Musculoskelet Disord. 2016;17(1). DOI:10.1186/s12891-016-1038-3
- National Rheumatoid Arthritis Society. The economic burden of rheumatoid arthritis. United Kingdom: National Rheumatoid Arthritis Society; 2010.
- Roubille C, Richer V, Starnino T, et al. Evidence-based recommendations for the management of comorbidities in rheumatoid arthritis, psoriasis, and psoriatic arthritis: expert opinion of the canadian dermatology-rheumatology comorbidity initiative. J Rheumatol. 2015 Oct;42(10):1767–1780.
- Dougados M, van der Heijde D. Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: results from the RA-BUILD study. Ann Rheum Dis. 2017 Jan;76(1):88–95.
- Cutolo M, Meroni M. Clinical utility of the oral JAK inhibitor tofacitinib in the treatment of rheumatoid arthritis. J Inflamm Res. 2013;6:129.
- Cooper NJ. Economic burden of rheumatoid arthritis: a systematic review. Rheumatology. 2000;39(1):28–33.
- Wasserman AM. Diagnosis and management of rheumatoid arthritis. Am Fam Physician. 2011 Dec 1;84(11):1245–1252.
- Smolen JS, Landew R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 2010;69(6):964–975.
- Luqmani R, Hennell S, Estrach C, et al. British society for rheumatology and british health professionals in rheumatology guideline for the management of rheumatoid arthritis (after the first 2 years). Rheumatology (Oxford). 2009 Apr;48(4):436–439.
- Einarsson JT, Willim M, Ernestam S, et al. Prevalence of sustained remission in rheumatoid arthritis: impact of criteria sets and disease duration, a nationwide study in Sweden. Rheumatology (Oxford). 2019 Feb 1;58(2):227–236.
- Fleischmann R, Mysler E, Hall S, et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet. 2017;390(10093):457–468.
- Taylor PC, Keystone EC, van der Heijde D, et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med. 2017;376(7):652–662.
- Genovese MC, Kremer J, Zamani O, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med. 2016;374(13):1243–1252.
- Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. 5th ed. Chichester: Wiley Online Library; 2008.
- Stevenson M, Archer R, Tosh J, et al. Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for the treatment of rheumatoid arthritis not previously treated with disease-modifying antirheumatic drugs and after the failure of conventional disease-modifying antirheumatic drugs only: systematic review and economic evaluation. Health Technol Assess. 2016;20(35):1–610.
- Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. 1st ed. Oxford: Oxford university press; 2006.
- Norton S, Sacker A, Dixey J, et al. Trajectories of functional limitation in early rheumatoid arthritis and their association with mortality. Rheumatology (Oxford). 2013;52(11):2016–2024.
- Malottki K, Barton P, Tsourapas A, et al. Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure of a tumour necrosis factor inhibitor: a systematic review and economic evaluation. [ Review]. Health Technol Assess. 2011;15(14):1–278.
- Institute for Clinical and Economic Review (ICER). targeted immune modulators for rheumatoid arthritis: effectiveness & value, draft evidence report. 2017 [cited 2020 Jan]. Available from: https://icer-review.org/wp-content/uploads/2016/08/NECEPAC_RA_Draft_Report_012017.pdf
- Scholz S, Mittendorf T. Modeling rheumatoid arthritis using different techniques-a review of model construction and results. Health Econ Rev. 2014;4(1):1–16.
- Vij AS, Malaviya AN, Kumar S. Characteristics of rheumatoid arthritis patients at first presentation to a specialized rheumatology department. Int J Res Med Sci. 2015;3(8):2073.
- Caro J, Briggs AH, Siebert U, et al. ISPOR-SMDM modeling good research practices task force. Modeling good research practices - overview: a report of the ISPOR-SMDM modeling good practices task force-1. Value Health. 2012;15(6):796–803.
- Karnon J, Stahl J, Brennan A, et al. Modeling using discrete event simulation: a report of the ISPOR-SMDM modeling good research practices task force-4. Value Health. 2012;15(6):821–827.
- Eli L. Data on file: systematic literature review of clinical trials in rheumatoid arthritis. 2016.
- Hoaglin DC, Hawkins N, Jansen JP, et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR task force on indirect treatment comparisons good research practices: part 2. Value Health. 2011;14(4):429–437.
- Jansen JP, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR task force on indirect treatment comparisons good research practices: part 1. Value Health. 2011;14(4):417–428.
- Dias S, Welton NJ, Sutton AJ, et al. NICE DSU technical support document 2: a generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. Sheffield: Decision Support Unit, ScHARR, University of Sheffield; 2014.
- Carlson J, Ogale S, Dejonckheere F, et al. Economic evaluation of tocilizumab monotherapy compared to adalimumab monotherapy in the treatment of severe active rheumatoid arthritis. Value Health. 2015;18(2):173–179.
- Eli L. Data on file: patient HAQ trajectory beyond RA-BEAM study (RA-BEYOND). 2018.
- NICE. Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for rheumatoid arthritis not previously treated with DMARDs or after conventional DMARDs only have failed. TA375. 2016 [cited 2020 Jan]. Available from: https://www.nice.org.uk/guidance/ta375/resources/adalimumab-etanercept-infliximab-certolizumab-pegol-golimumab-tocilizumab-and-abatacept-for-rheumatoid-arthritis-not-previously-treated-with-dmards-or-after-conventional-dmards-only-have-failed-82602790920133
- Hernandez AM, Wailoo AJ, Ara R. Tails from the peak district: adjusted limited dependent variable mixture models of EQ-5D questionnaire health state utility values. Value Health. 2012;15(3):550–561.
- Brennan A, Bansback N, Nixon R, et al. Modelling the cost effectiveness of TNF-alpha antagonists in the management of rheumatoid arthritis: results from the British society for rheumatology biologics registry. Rheumatology. 2007;46(8):1345–1354.
- Michaud K, Vera-Llonch M, Oster G. Mortality risk by functional status and health-related quality of life in patients with rheumatoid arthritis. J Rheumatol. 2012;39(1):54–59.
- Wiles N, Cooper N, Symmons D. Resource use within the Norfolk Arthritis Register (NOAR) Cohort during the first five years of disease: report for Roche (NICE Data on File). London: NICE; 2005.
- Vemer P, Corro Ramos I, van Voorn GAK, et al. AdViSHE: a validation-assessment tool of health-economic models for decision makers and model users. PharmacoEconomics. 2015;34:349–361.
- Alemao E, Al MJ, Boonen AA, et al. Conceptual model for the health technology assessment of current and novel interventions in rheumatoid arthritis. PLoS One. 2018;13(10):e0205013.
- Wailoo A, Hock ES, Stevenson M, et al. The clinical effectiveness and cost-effectiveness of treat-to-target strategies in rheumatoid arthritis: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2017;21(71):1–258.
- Vermeer M, Kievit W, Kuper HH, et al. Treating to the target of remission in early rheumatoid arthritis is cost-effective: results of the DREAM registry. BMC Musculoskelet Disord. 2013;14:350.
- van den Hout WB, Goekoop-Ruiterman YP, Allaart CF, et al. Cost-utility analysis of treatment strategies in patients with recent-onset rheumatoid arthritis. Arthritis Rheum. 2009;61(3):291–299.
- van de Laar CJ, Oude Voshaar MAH, Vonkeman HE. Cost-effectiveness of different treat-to-target strategies in rheumatoid arthritis: results from the DREAM registry. BMC Rheumatol. 2019;3:16.
- van de Laar C, Voshaar MO, Fakhouri W, et al. OP0313 Cost-effectiveness of a JAK1/JAK2-Inhibitor vs. a biologic disease-modifying antirheumatic drug in a treat-to-target strategy for rheumatoid arthritis. BMJ Ann Rheumatic Dis. 2019;78(2):238–239.