ABSTRACT
Background
Pharmacoeconomic evaluation is important for breast-cancer medications due to their high costs. To our knowledge, no systematic literature reviews of pharmacoeconomic studies for breast-cancer medication use are present in developing-countries.
Objectives
To systematically review the existing cost-effectiveness evaluations of breast-cancer medication in developing-countries.
Methodology
A systematic literature search was performed in PubMed, EMBASE, SCOPUS, and EconLit. Two researchers determined the final articles, extracted data, and evaluated their quality using the Quality of Health-Economic Studies (QHES) tool. The interclass-correlation-coefficient (ICC) was calculated to assess interrater-reliability. Data were summarized descriptively.
Results
Fourteen pharmacoeconomic studies published from 2009 to 2019 were included. Thirteen used patient-life-years as their effectiveness unit, of which 10 used quality-adjusted life-years. Most of the evaluations focused on trastuzumab as a single agent or on regimens containing trastuzumab (n = 10). The conclusion of cost-effectiveness analysis varied among the studies. All the studies were of high quality (QHES score >75). Interrater reliability between the two reviewers was high (ICC = 0.76).
Conclusion
In many studies included in the review, the use of breast-cancer drugs in developing countries was not cost-effective. Yet, more pharmacoeconomic evaluations for the use of recently approved agents in different disease stages are needed in developing countries.
1. Background
Cancer is one of the most common and dangerous non-communicable diseases worldwide, with an incidence of 18.1 million new cases and 9.6 million deaths in 2018 [Citation1]. Since 2015, which recorded an incidence of 17.5 million new cases and 8.7 million deaths, the burden of cancer has an increasing trend globally [Citation2]. Breast and lung cancer are the two most prevalent types of cancer worldwide, accounting for 2.1 million of the total number of cancer cases in 2018 alone [Citation1]. Breast cancer is considered the primary cause of woman mortality worldwide, accounting for 15% of total mortality among women [Citation1]. For example, in the United Kingdom alone, breast cancer accounted for 11,563 deaths among women in 2016 [Citation3]. The prevalence of breast cancer in developed countries is higher than that in developing countries, also known as low- and middle-income countries (LMICs); nonetheless, the prevalence of breast cancer has recently been growing in LMICs as well [Citation4]. Therefore, many highly effective strategies to prevent, diagnose, and treat breast cancer have been utilized in both developed countries and LMICs to decrease the breast cancer disease burden.
Although these strategies provide clinical benefits, they raise expenditures and health-care costs. According to the National Institute of Health, the average cost of a year of patient treatment was approximately 100,000 USD in 2014, but the costs could reach up to 400,000 USD in some settings [Citation5]. Drug therapy was found to be the major driver behind the high overall cost [Citation6]. For instance, in the United States of America (USA), two different analyses have shown that pharmacotherapy ranked as the first and second contributors to the overall breast cancer treatment cost burden [Citation7,Citation8]. Since health care resources are scarce, the clinical decision regarding the use of cancer treatment should be based on the value of the monetary costs of these treatments, which is known as ‘cost-effectiveness’ [Citation9]. Therefore, pharmacoeconomic evaluations, especially cost-effectiveness analyses (CEAs), are needed for breast cancer medications to inform decision-making among responsible authorities and practitioners.
Pharmacoeconomics should be a decision guiding tool worldwide, but there should be a special emphasis on pharmacoeconomics in LMICs due to the limited resources in these countries; pharmacoeconomic evaluations such CEAs would help these countries avoid unwanted financial outcomes. In fact, systematic review of CEAs represents a powerful tool for decision-makers since these reviews combine different CEAs, which saves time by providing the decision-makers with trustworthy combined evidence [Citation10]. There are some published systematic reviews regarding general pharmacoeconomic evaluations, or CEAs specifically, of different classes of breast cancer medications in developed countries [Citation11–14]. Nevertheless, to date, none have been published regarding the use of breast cancer medications in LMICs. The available systematic reviews in LMICs address the different strategies of breast cancer control in general that does not only address pharmacotherapeutic treatment strategies as their research problem; nevertheless, they address preventative and early diagnosis strategies, such as screening methods [Citation15,Citation16]. Although screening is important for the overall control of breast cancer, there should be systematic reviews of CEAs for pharmacotherapies, as they are the major drivers for the overall cost burden of breast cancer as mentioned earlier [Citation6].
The differences among countries in global economies, the structures of the health systems, and populations make adapting the results from developed countries to developing countries misleading. Therefore, there is a need for a high-quality systematic review regarding the cost-effectiveness of breast cancer medications in LMICs, which is the main goal of this research. The research questions are whether pharmacotherapeutic agents for breast cancer are used cost-effectively in LMICs and whether the presented CEAs are of high quality. The specific aims of this review are to systematically review the CEAs of breast cancer medication use in LMICs, evaluate the quality of the presented CEAs, and investigate the gaps in the available CEAs to guide future search.
2. Methodology
2.1. Literature search
A literature search was conducted by systematically searching the following databases: PubMed, EMBASE, SCOPUS, and EconLit. In addition, Google, Google Scholar, dissertations, and published abstracts from conferences were searched, and the references of the eligible articles were screened to identify any non-indexed published literature or gray literature. Our search strategy was based on key terms that correspond to the predesigned main domains of cost-effectiveness (e.g., ‘cost-effectiveness analysis,’ ‘cost-effectiveness evaluation,’ ‘cost-utility,’ ‘economic evaluations,’ ‘pharmacoeconomics’), breast cancer (e.g., ‘breast neoplasms’), medications (e.g., ‘treatments,’ ‘drug therapy’), and developing countries (e.g., ‘middle- and low-income countries,’ ‘poor resource settings’). The main search domains were connected using the Boolean operator ‘AND,’ and the keywords of the same domain were connected using the Boolean operator ‘OR.’ The search strategy was adapted for use in every database in combination with database-specific filters such as original text, English language, full text, MESH filters, and exclusion of the results from some countries by name (developed countries) where available. The search process covered all results before May 2019. The protocol for this review was registered on PROSPERO on 20 May 2019 [available at: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019128806].
2.2. Eligibility criteria
Studies are included if they were full-text studies, written in English language, addressed the areas of cost-effectiveness or cost-utility with pharmacoeconomic evaluations, related to breast cancer drug therapy, and conducted in developing countries, or countries with developing economies as listed by the United Nations classification 2020 [Citation17]. However, studies were excluded if they were non-pharmacoeconomic evaluations, not related to evaluating medications, not related to breast cancer, and non-primary studies such as reviews and editorials, conducted in countries with economies in transition, or developed economies as listed by the United Nations classification [Citation17].
2.3. Data extraction
Data from the included studies were extracted by two independent researchers using a predesigned data extraction tool. The extracted data included the following: authors, publication date, country, quality of the publication journal, main outcomes, results, and characteristics of the patients and the disease. In addition, additional pharmacoeconomic related data were extracted, such as the study perspective, the type of model/approach, the primary clinical effectiveness unit, if discounting analyses were executed, if a sensitivity analysis was executed, and the type of sensitivity analysis, if applicable. All the extracted data were reported descriptively using tables and figures following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria [Citation18], and the quality of the reported data as well as the quality of the whole review were ensured by following a tool developed to assess the methodological quality of systematic reviews (AMSTAR) checklist [Citation19].
2.4. Quality assessment
The quality of the cost-effectiveness studies was independently assessed by two reviewers using the Quality of Health Economic Studies (QHES) instrument [Citation20]. This instrument is composed of 16 items scored out of a total of 100 points, with the score of each item was weighted from 1 to 9. The score of each item was fully weighted if it was fully or mostly satisfied in the study, or the item was given a zero if it was not satisfied. As in previous health economic evaluations that used the same tool, a study was assumed to be high quality if the total score was higher than 75 points, moderate if the score was 51 to 75 points, and low if the score was 50 points or lower. The interclass correlation coefficient (ICC) was calculated to test the agreement between the two reviewers. Major discrepancies between the two reviewers were resolved by discussion until a consensus was reached, and then, the quality scores were averaged between the two reviewers.
2.5. Statistical analysis
Descriptive statistics such as frequencies, means, and standard deviations were calculated. In addition, as mentioned earlier, the ICC between the raters was calculated to estimate interrater reliability. All statistical analyses were performed using SPSS 25 software.
3. Results
3.1. Literature search
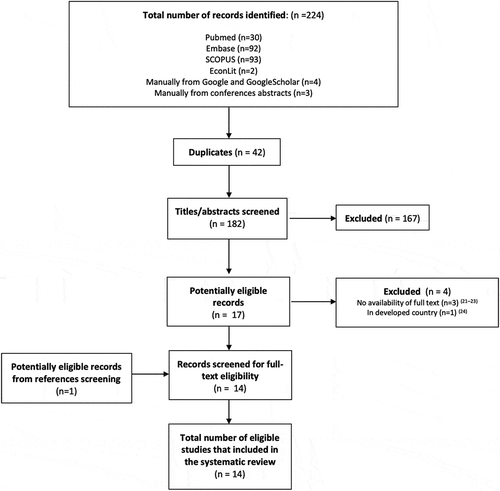
The literature search identified a total of 224 abstract citations – 30 from PubMed, 92 from EMBASE, 93 from SCOPUS, two from EconLit, four manually added abstracts from Google and Google Scholar, and three from abstracts presented at conferences (. After removing the 42 duplicates, 182 titles and abstracts were screened for eligibility, of which, only 17 abstracts were found potentially eligible. The eligible abstracts were moved to the next step for full-text eligibility screening. Four of these abstracts were excluded; three were excluded since a full-text version was unavailable [Citation21–23], and one was excluded because the study was conducted in developed countries [Citation24]. Thirteen records were eligible after the full-text eligibility screening process [Citation25–37]. After screening the references of these 13 articles, there was one more eligible study to be included in this review [Citation38]. A total of 14 articles were included for the final review (25–38).
3.2. Studies characteristics and synthesis of cost-effectiveness evaluations
Although we did not apply time limits in our search, all 12 included articles were published between 2009 and 2020. The articles were published in various journals of different qualities ranging from Q1 to Q4 as identified by the Scimago journal rankings. The included analyses were from the following countries: three from Taiwan [Citation25,Citation35,Citation38], two from Colombia [Citation26,Citation33], two from Brazil and Latin American countries [Citation30,Citation31], two from Iran [Citation32,Citation34], two from China [Citation36,Citation37], one from eleven sub-Saharan African countries [Citation27], one from Philippines [Citation28], and one from India [Citation29]. The general characteristics of each study are summarized in . Most of the studies addressed the cost-effectiveness on the use of trastuzumab as a single agent or in combination with chemotherapeutic regimens and their comparators (n = 10) [Citation26–29,Citation31–34,Citation36,Citation38]. Most of the studies focused on the breast cancer population with positive human epidermal growth factor receptor 2 (HER2+) expression, n = 11 [Citation26–29,Citation31–36,Citation38]. The detailed clinical settings (disease type/treatment settings), comparators, and conclusions of each of the included studies are summarized in .
Table 1. General characteristics of the included articles of CEAs
Table 2. Objectives, comparators, population characteristics, and main conclusions of the included CEAs
The included CEAs were also categorized in terms of the following pharmacoeconomic characteristics: perspective, analysis approach (model), the primary unit of effectiveness, whether a discounting analysis was conducted, sensitivity analysis and type of analysis, and the time horizon. Nine CEAs were conducted from the health-care payer perspective [Citation25,Citation26,Citation28Citation30–32,Citation34,Citation35,Citation37], of which, only two combined both the health-care payer and societal perspectives [Citation28,Citation35]. Whereas three of the included CEAs were conducted from the third-party payer perspective [Citation33,Citation36,Citation38], and two were solely conducted from the societal perspective [Citation27,Citation29]. All the CEAs used the Markov model as their pharmacoeconomic approach, except for one CEA that used net benefit regression to conduct the pharmacoeconomic analysis [Citation25]. All the studies, except for two [Citation35,Citation38], calculated patient gained life years (LYs) as an effectiveness unit. In addition, 10 of the included studies used quality-adjusted life years (QALYs) as the primary unit for effectiveness in their analyses [Citation26–29,Citation31,Citation32,Citation34,Citation35,Citation37,Citation38]. All the studies, except one [Citation25], conducted discounting analyses for the costs and outcomes. All the included CEAs incorporated a sensitivity analysis in their pharmacoeconomic analysis, and the majority of the CEAs used both deterministic and probabilistic sensitivity analyses (n = 9) [Citation26,Citation28,Citation30–33,Citation35,Citation37,Citation38]. Most of the CEAs were conducted based on a life-time horizon (n = 7) [Citation26–32,Citation36,Citation37]. summarizes all of the pharmacoeconomic parameters for each included CEA.
Table 3. Pharmacoeconomic characteristics and the synthesis of quality assessment results (by the reviewers)
3.3. Quality assessment results
The overall quality of all the included CEAs was high (score >75 using the QHES scoring system), with an average of 91.42 ± 5.25. The overall QHES score of each study is also indicated in . The calculated ICC to measure interrater reliability was 0.76. Both reviewers agreed that most of the items of the QHES tool were well fulfilled by the included CEAs. For example, the methodology of data abstraction was clear in all of the included CEAs except for four [Citation25,Citation31,Citation33,Citation36]. In addition, there was appropriate discounting and a sufficient time horizon for all the studies except for one [Citation25]. The methodology of cost abstraction was also clear in all but three of the studies [Citation25,Citation27,Citation32]. The direction and magnitude of bias were discussed in all the studies except for two [Citation29,Citation30]. However, the item related to the estimations from subgroup analysis (item 4) was not fulfilled in any of the included CEAs, except for two [Citation29,Citation33]. Other than these items, the other items were fulfilled in more than 90% of the included studies.
4. Discussion
This review focused on published pharmacoeconomic analyses of the cost-effectiveness of the use of breast cancer medications in LMICs. A total of 224 bibliographic records were found, of which, 14 studies were included and fully reviewed based on our inclusion and exclusion criteria. The 14 studies were published between 2009 and 2020 in three different regions, including a total of 23 countries: Iran, China, Taiwan, Philippines, India, and seven countries from Latin America (Argentina, Bolivia, Brazil, Chile, Colombia, Peru, Uruguay), 11 countries of Sub-Saharan Africa (Congo, Ethiopia, Guinea, Kenya, Namibia, Nigeria, Rwanda, Uganda, Zambia, Zimbabwe, and South Africa). The articles were published in various journals that are more related to cancer and biomedicine than to pharmacoeconomics. This can be justified by the fact that the field of pharmacoeconomics is important for decision-making in biomedicine, especially for diseases such as cancer, whose medications are extremely expensive.
Many drug therapies have been addressed in the different included articles. Trastuzumab and trastuzumab-containing regimens were addressed in most of the CEAs in this review. Trastuzumab is a targeted therapy for HER2+ patients and has a weekly procurement cost that ranges from 2,761 USD to 14,261 USD depending on the country and the setting, which is relatively higher than that of other chemotherapies and hormonal therapies [Citation39]. Therefore, this drug represents a cost-effectiveness concern in countries with developing economies. The major focus of all the included studies was to evaluate the cost-effectiveness of trastuzumab compared to that of its alternatives; one-year adjuvant trastuzumab treatment was found to be not cost-effective in all of the studies, except for two studies that were conducted in China and Taiwan [Citation36,Citation38]. Of note, both of these two CEA were conducted from the third-party payer perspective, whereas the majority of the other evaluations concerning trastuzumab were conducted from the health-care payer perspective, except for another two that were mentioned to be fully from a societal perspective, but we did not notice they fill the societal perspective analysis criteria fully; that is, did not analyze the loss of productivity and other patient personal related costs [Citation27,Citation29]. Thus, the studies had different cost abstraction methodologies and clinical effectiveness evaluations. The Taiwanese health system is based on a universal single insurer, the National Health Insurance (NHI) [Citation40]. NIH is a government-implemented social insurance started in 1995 and developing accordingly where all citizens and residents need to pay a monthly co-payment called ‘premium’ [Citation40]. However, the NHI was designed to avoid waste, so the amount of copayment may vary depending on the disease status, which may impose an obstacle for cancer expensive medications and care [Citation40]. Therefore, the nature of the NHI in Taiwan may have impacted the cost calculations for trastuzumab and led to the different conclusion from the other countries. Regarding the Chinese health system, although it also has a universal health coverage, the rates of reimbursement are still low reimbursement leading to a high out-of-pocket expenses for cancer medications from the patient side [Citation41]. Therefore, due to having out-of-pocket money paid for the cancer medications, and since the analysis is from the third-party payer perspective, with not taking into consideration the co-payment by the patient, this may be a bias that led to the different conclusion of having 1-year trastuzumab as cost-effective.
Other explanation for the different cost-effectiveness conclusion in these two studies may be that because they were done in Asian countries. That is, although the bases of breast cancer are similar worldwide, Asian women have differences in their genetic make-up of which can result in different interactions toward breast cancer etiological factors, and drug therapies [Citation42]; therefore, they ‘may’ have higher improvement toward trastuzumab at lower cost which may result in having trastuzumab as a cost-effective option. On the other hand, one of the analyses conducted in Iran concluded that although one-year adjuvant trastuzumab treatment was not cost-effective, 6-month adjuvant trastuzumab treatment was cost-effective [Citation32]. In fact, this conclusion was similar to other CEAs that were conducted in different settings worldwide. For example, a recent study presented in the European Society for Medical Oncology (ESMO) showed that 6-month adjuvant trastuzumab treatment was more cost-effective than one-year adjuvant trastuzumab treatment [Citation43]. Therefore, further studies regarding the cost-effectiveness of the 6-month adjuvant trastuzumab strategy should be conducted in LMICs.
Although trastuzumab is a well-established biological agent for HER-2 positive patients, there have been four drugs that are approved by the FDA as trastuzumab biosimilars: trastuzumab-dkst (MYL-1401O) branded Ogivri and approved in December 2017, Trastuzumab-pkrb (CT-P6) branded Herzuma and approved in December 2018, trastuzumab-dttb (SB3) branded Ontruzant and approved in January 2019, and trastuzumab-qyyp (PF-05280014) branded as Trazimera and firstly FDA approved in March 2019 [Citation44]. Although these biosimilars have approved by the FDA, they were not yet available in the market till mid-2019 due to the existence of the patency of the known commercial trastuzumab (Herceptin ®) [Citation44]. Of note, the majority of the pharmacoeconomic analyses that did not conclude the cost-effectiveness of it were either conducted before the availability of these trastuzumab biosimilars in the market, or after its availability but based on simulation from other clinical trials such as HERA trial, whose final analysis was in 2017 and based on the trastuzumab brand ‘Herceptin ®’. With the availability of these trastuzumab biosimilars in the market, there will be a proposed cost-saving of 20% to 30% of trastuzumab treatments due to the availability of new competitors [Citation44]. Therefore, future pharmacoeconomic analyses of trastuzumab in the presence of other biosimilars should be conducted since the final conclusion about its cost-effectiveness can change accordingly due to the possible change of the original cost.
Pertuzumab is another anti-HER2+ targeted therapy that was firstly FDA approved for the treatment of HER2+ metastatic breast cancer in combination with trastuzumab and docetaxel for patients who were not previously treated with anti-HER2 therapy or chemotherapy combination, and then expanded as an adjuvant treatment of HER2+ early breast cancer patients in combination with trastuzumab and chemotherapy at high risk of recurrence [Citation45]. One of the included analyses from Taiwan compared adjuvant pertuzumab treatment with treatment with trastuzumab and docetaxel together versus treatments with trastuzumab and docetaxel alone in HER2+ metastatic breast cancer patients found that pertuzumab was not cost-effective [Citation35]. When compared to other similar CEAs such as a one from the USA, the findings were different since in the USA they concluded that pertuzumab with trastuzumab and chemotherapeutic regimens were cost-effective [Citation46]. These differing results can be attributed to the differences in the willing-to-pay threshold (WTP) of both the analyses, the differences in the health-care systems, and the differences in the stage at which the CEA was conducted (invasive HER+ in the Taiwan analysis and early HER2+ in the USA analysis).
Endocrine therapies, such as aromatase inhibitors (AI) and tamoxifen, are used for patients with hormone receptors (HR) positive (Estrogen and/or progesterone receptors) [Citation47]. The cost-effectiveness of aromatase inhibitors versus tamoxifen was also compared in one of the included analyses conducted in Brazil [Citation28]. This study concluded that anastrozole was more cost-effective than tamoxifen, which is also consistent with the findings of a published systematic review from Canada of 18 CEAs [Citation13]. In fact, the recent guidelines offer three main strategies recommending extending endocrine therapy use up to 10 years for early and locally advanced breast cancer HR+ postmenopausal patients which are: tamoxifen alone for five to 10 years in case of present contraindications or intolerability to AIs, AI alone for 5 years followed by tamoxifen for more 5 years, or sequential therapy of tamoxifen for 2–3 years followed by AI to complete a total of 10 years of treatment with endocrine therapy [Citation48]. One of the included CEAs in our review has addressed the cost-effectiveness of these three strategies in China in HR+ postmenopausal early breast cancer women [Citation37]. Therein, AI-based strategies were both more cost-effective compared to the 5-year monotherapy of tamoxifen, with having the AI-monotherapy strategy for 5 years as the most cost-effective among the three strategies [Citation37]. Finally, a study from Colombia compared the combined regimen of lapatinib and capecitabine with the combination of trastuzumab and capecitabine or vinorelbine or a taxane in the treatment of HER2+ metastatic breast cancer patients [Citation33]. The results showed that the lapatinib and capecitabine regimens were more cost-effective than the other regimens. This is also similar to the findings from another published systematic review conducted in the USA that concluded that the combination of lapatinib and capecitabine for advanced or early-stage HER2+ breast cancer was more cost-saving than trastuzumab-containing regimens [Citation49].
Most of the evaluations were conducted from the health-care payer perspective. While the health-care payer perspective is considered the most feasible, the most commonly used in health-care settings, and most influential, this perspective does not take into account patient-related costs or productivity [Citation50]. Therefore, other perspectives that are more comprehensive, such as the societal perspective, should be addressed in the CEAs. In our review, only three included evaluations stated their perspective as a societal perspective [Citation27–29]. However, we noticed that only direct medical costs were calculated, and the other related costs to the societal perspective such as productivity and other patients related costs were not considered fully in two of them, which does not really qualify them to state that it was from the societal perspective [Citation27,Citation29]. Quality-adjusted life-years (QALYs) are important measurements of effectiveness in CEAs that take the patient quality of life into consideration [Citation51]. Eight of the included studies included combined between the patient-life years (LYs) gained and QALYs as their effectiveness measurement [Citation26–29,Citation31,Citation32,Citation34,Citation37], which enables practitioners to consider the patients’ quality of life. Whereas four of the evaluations used only the LYs in their outcome measurement [Citation25,Citation30,Citation33,Citation36], and two evaluations used only QALYs as their outcome measurement [Citation35,Citation38] as indicated in .
Overall, there was good agreement between the two reviewers for the quality scores, as indicated by the ICC (i.e. 0.76). All the included CEAs were categorized as high quality based on the QHES scores, which means that the studies filled most of the pharmacoeconomic quality-related parameters. For instance, all the studies conducted sensitivity analyses and discounting analyses. In only a few CEAs, the methodology of cost abstraction and data abstraction were not clear, which may be because these publications were performed in local settings, so there might have been an assumption that the health-care settings were already familiar with these parameters. All the studies were performed with an appropriate time horizon starting from 5 years, which is also used to estimate disease-free survival in breast cancer in some clinical studies [Citation52]; only one study was conducted over a time horizon of 3 years [Citation25], which may be assumed non-sufficient.
Our review has several strengths. First, to our knowledge, this is the first review addressing the cost-effectiveness of pharmacotherapeutic agents in LMICs. Second, this review was conducted with high-quality studies by following the PRISMA reporting guidelines and was internally appraised with the AMSTAR-2 checklist for systematic review appraisal. However, like any other systematic review, ours has some limitations. First, we only searched for CEAs published in English, so we may have missed some acceptable CEAs in LMICs that were written in other languages. Second, we could not access the full text for some of the articles, so we missed these CEAs. Despite these drawbacks, our systematic review included most of the published literature, and this enabled us to draw conclusions regarding the cost-effectiveness of medications in LMICs and the quality of the included studies.
5. Conclusion
Pharmacoeconomics, particularly CEAs, has become an important decision-making tool. The findings of this review confirm the presence of high-quality cost-effectiveness evaluations regarding breast cancer medication use in developing countries. The conclusion of cost-effectiveness for a pharmacotherapeutic agent or regimen varies among different settings. Some of the drugs that were proven to be cost-effective in developed countries were not cost-effective in developing countries. Therefore, CEAs should be setting-specific rather than adapted from different settings. Although the present CEAs were high quality, they were limited to only certain agents. As a result, more high-quality research is needed to evaluate different classes of anti-cancer medications, such as biological therapies, newer agents of chemotherapy and targeted therapies, and newer classes of medications such as the CDK4/6 inhibitors in breast cancer different stages.
6. Expert Opinion
Pharmacoeconomics represent a great decision-making tool in healthcare intervention. Since health resources are scarce, and since the developing countries are more in need for resources, there should be a special consideration for pharmacoeconomics and health-economics to assure the cost-effectiveness of the provided health intervention. Therefore, cancer medications, including breast cancer medications especially in developing countries, should be questioned and determined by cost-effectiveness evaluations. The present systematic review has presented an inclusive insight about the cost-effectiveness of breast cancer medications in developing countries. However, there are some data gaps that should benefit future research regarding pharmacoeconomic evaluations for breast cancer
medications in developing countries, including:
More pharmacoeconomic evaluations are needed for more breast cancer regiments
More pharmacoeconomic evaluations are needed for more recent drugs
More pharmacoeconomic evaluations are needed for different disease states of breast cancer
Declarations of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewers disclosure
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018 Nov 1;68(6):394–424.
- Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015. JAMA Oncol. 2017 Apr 1 [cited 2019 Apr 29] ;3(4):524. Available from: http://oncology.jamanetwork.com/article.aspx?doi=10.1001/jamaoncol.2016.5688
- Breast cancer statistics. Cancer research UK . cited 2019 Apr 29. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer
- Comparing more and less developed countries. World cancer research fund . cited 2019 Apr 29. Available from: https://www.wcrf.org/dietandcancer/cancer-trends/comparing-more-and-less-developed-countries
- Addressing Cancer Drug Costs and Value - National Cancer Institute . cited 2019 Apr 29. Available from: https://www.cancer.gov/news-events/cancer-currents-blog/2018/presidents-cancer-panel-drug-prices
- The High Cost of Cancer Treatment- AARP . cited 2019 Apr 30. Available from: https://www.aarp.org/money/credit-loans-debt/info-2018/the-high-cost-of-cancer-treatment.html
- Barron JJ, Quimbo R, Nikam PT, et al. Assessing the economic burden of breast cancer in a US managed care population. Breast Cancer Res Treat. 2008 May 3 [cited 2019 Apr 30];109(2):367–377. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17674201
- Campbell JD, Ramsey SD. The Costs of Treating Breast Cancer in the US. Pharmacoeconomics. 2009 [cited 2019 Apr 30];27(3):199–209. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19354340
- Pallis A, Tsiantou V, Simou E, et al. Pharmacoeconomic considerations in the treatment of breast cancer. Clinicoecon Outcomes Res. 2010 [cited 2019 Apr 29];2:47–61. Available from http://www.ncbi.nlm.nih.gov/pubmed/21935314
- Schlosser R. The role of systematic reviews in evidence-based practice, research, and development. Tech Br . 2006 cited 2019 Apr 30;(15). Available from: https://ktdrr.org/ktlibrary/articles_pubs/ncddrwork/focus/focus15/Focus15.pdf
- Foster TS, Miller JD, Boye ME, et al. The economic burden of metastatic breast cancer: a systematic review of literature from developed countries. Cancer Treat Rev. 2011 Apr [cited 2019 Apr 30];37(6):405–415. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21477928
- Blank PR, Dedes KJ, Szucs TD. Cost effectiveness of cytotoxic and targeted therapy for metastatic breast cancer. Pharmacoeconomics. 2010 Aug;28(8):629–647.
- John-Baptiste AA, Wu W, Rochon P, et al. A systematic review and methodological evaluation of published cost-effectiveness analyses of aromatase inhibitors versus tamoxifen in early stage breast cancer. Miller TW, editor. PLoS One. 2013 May 6 [cited 2019 May 1];8(5):e62614. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23671612
- Chan AL, Leung HW, Lu C-L, et al. Cost-effectiveness of trastuzumab as adjuvant therapy for early breast cancer: a systematic review. Ann Pharmacother. 2009 Feb 3 [cited 2019 Apr 30];43(2):296–303. Available from: http://journals.sagepub.com/doi/10.1345/aph.1L504
- Zelle SG, Baltussen RM. Economic analyses of breast cancer control in low- and middle-income countries: a systematic review. Syst Rev. 2013 Dec 8 [cited 2019 Apr 30];2(1):20. Available from: https://systematicreviewsjournal.biomedcentral.com/articles/10.1186/2046-4053-2-20
- da Costa Vieira RA, Biller G, Uemura G, et al. Breast cancer screening in developing countries. Clinics (Sao Paulo) . 2017Apr [cited 2019 Apr 30];72(4):244–253. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28492725
- United Nations, New York. World economic situation and prospects 2020. Available from: https://www.un.org/development/desa/dpad/wp-content/uploads/sites/45/WESP2020_Annex.pdf
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009 Jul 21;6(7):e1000097.
- AMSTAR - Assessing the Methodological Quality of Systematic Reviews . cited 2019 Apr 27. Available from: http://amstar.ca/Amstar_Checklist.php
- Ofman JJ, Mshs MD. Examining the value and quality of health economic analyses: implications of utilizing the QHES. JMCP J Manag Care Pharm . cited 2019 Apr 30;9(1). Available from: www.amcp.org
- Abou Taleb A. Impact of sequencing of treatment lines to enhance patient’s outcomes & resource utilization for metastatic breast cancer. Eur J Cancer. 2018 Apr 1 [cited 2019 Apr 30];92:S110. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0959804918305549
- Nguyen TTC, Nguyen TTT. Cost-utility analysis of trastuzumab in treatment of metastatic her2-positive breast cancer in Vietnam. Value Heal. 2014 Nov 1 [cited 2019 Apr 30];17(7):A643. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1098301514042533
- TTC N, TTT N. Cost-effectiveness analysis of 1-year adjuvant trastuzumab therapy of early-stage her2-positive breast cancer. Value Heal. 2014 Nov 1 [cited 2019 Apr 30];17(7):A735. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1098301514020373
- Garrison LP, Lubeck D, Lalla D, et al. Cost-effectiveness analysis of trastuzumab in the adjuvant setting for treatment of HER2-positive breast cancer. Cancer. 2007 Aug 1;110(3):489–498.
- Shih YCT, Pan IW, Tsai YW. Information technology facilitates cost-effectiveness analysis in developing countries an observational study of breast cancer chemotherapy in Taiwan. Pharmacoeconomics. 2009;27(11):947–961.
- Buendía JA, Vallejos C, Pichón-Rivière A. An economic evaluation of trastuzumab as adjuvant treatment of early HER2-positive breast cancer patients in Colombia TT - Evaluación económica del trastuzumab como tratamiento adyuvante en cáncer de mama HER2- positivo en Colombia. Biomédica. 2013;33(3):411–417. Available from: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0120-41572013000300010&lang=pt%0Ahttp://www.scielo.org.co/pdf/bio/v33n3/v33n3a10.pdf
- Gershon N, Berchenko Y, Hall PS, et al. Cost effectiveness and affordability of trastuzumab in sub-Saharan Africa for early stage HER2-positive breast cancer. Cost Eff Resour Alloc. 2019;17(1):5. Published 2019 Feb 28.
- Genuino AJ, Chaikledkaew U, Guerrero AM, et al. Cost-utility analysis of adjuvant trastuzumab therapy for HER2-positive early-stage breast cancer in the Philippines. BMC Health Services Research. 2019;19(1). DOI:10.1186/s12913-019-4715-8
- Gupta N, Verma RK, Gupta S, et al. Cost effectiveness of trastuzumab for management of breast cancer in India. JCO Glob Oncol. 2020Feb;(6):205–216. doi:10.1200/JGO.19.00293
- Fonseca M, Araújo GTB, Saad ED. Cost-effectiveness of anastrozole, in comparison with tamoxifen, in the adjuvant treatment of early breast cancer in Brazil. Rev Assoc Med Bras. 2009;55(4):410–415.
- Pichon-Riviere A, Garay OU, Augustovski F, et al. Implications of global pricing policies on access to innovative drugs: the case of trastuzumab in seven Latin American countries. Int J Technol Assess Health Care. 2015;31(1–2):2–11.
- Ansaripour A, Uyl-de Groot CA, Redekop WK. Adjuvant trastuzumab therapy for early HER2-positive breast cancer in iran: a cost-effectiveness and scenario analysis for an optimal treatment strategy. Pharmacoeconomics. 2018;36(1):91–103.
- Chicaíza-Becerra LA, García Molina M, Castañeda C, et al. ErbB2+metastatic breast cancer treatment after progression on trastuzumab: a cost-effectiveness analysis for a developing country. Rev Salud Pública. 2015;16(2):270–280.
- Aboutorabi A, Hadian M, Ghaderi H, et al. Cost-effectiveness analysis of trastuzumab in the adjuvant treatment for early breast cancer. Glob J Health Sci. 2014;7(1):98–106.
- Leung HWC, Chan ALF, Muo CH, et al. Cost-effectiveness of pertuzumab combined with trastuzumab and docetaxel as a first-line treatment for HER-2 positive metastatic breast cancer. Expert Rev Pharmacoeconomics Outcomes Res. 2018;18(2):207–213.
- Chen W, Jiang Z, Shao Z, et al. An economic evaluation of adjuvant trastuzumab therapy in HER2-positive early breast cancer. Value Heal. 2009;12(SUPPL.3):S82–4.
- Ye M, Lu J, Yang F, et al. Economic evaluation of letrozole for early breast cancer in a health resource-limited setting. Biomed Res Int. 2018;2018. DOI:10.1155/2018/9282646
- Lang HC, Chen HW, Chiou TJ, et al. The real-world cost-effectiveness of adjuvant trastuzumab in HER-2/neu-positive early breast cancer in Taiwan. J Med Econ. 2016;19(10):923–927.
- Goldstein DA, Clark J, Tu Y, et al. A global comparison of the cost of patented cancer drugs in relation to global differences in wealth. Oncotarget. 2017 Sep [cited 2019 May 1] ;8(42):71548. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29069727.
- 衛生福利部中央健康保險署. 2017-2018 National Health Insurance Annual Report. 2017;
- Goss PE, Strasser-Weippl K, Lee-Bychkovsky BL, et al. Challenges to effective cancer control in China, India, and Russia. Lancet Oncol. 2014 Apr [cited 2020 Jun 20] ;15(5):489–538. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1470204514700294.
- Bhoo-Pathy N, Yip CH, Hartman M, et al. Breast cancer research in Asia: adopt or adapt Western knowledge? Eur J Cancer. 2013;49(3):703–709.
- Hulme C, Hall P, Shinkins B, et al. PERSEPHONE: 6 versus 12 months (m) of adjuvant trastuzumab in patients (pts) with HER2 positive (+) early breast cancer (EBC): cost effectiveness analysis results. Abstr B 43rd ESMO Congr . 2018;29:viii703 (ESMO 2018) Munich, Ger.
- Miller EM, Schwartzberg LS. Biosimilars for breast cancer: a review of HER2-targeted antibodies in the United States. Ther Adv Med Oncol. 2019 Nov 14;11. DOI:10.1177/1758835919887044
- FDA grants regular approval to pertuzumab for adjuvant treatment of HER2-positive breast cancer. FDA . [ cited 2019 Nov 11]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-pertuzumab-adjuvant-treatment-her2-positive-breast-cancer
- Garrison LP, Babigumira J, Tournier C, et al. Cost-effectiveness analysis of pertuzumab with trastuzumab and chemotherapy compared to trastuzumab and chemotherapy in the adjuvant treatment of her2-positive breast cancer in the United States. Value Heal. 2019 Apr 1 [cited 2019 May 1];22(4):408–415. Available from: https://www.sciencedirect.com/science/article/pii/S1098301519300488
- Schneider RE, Barakat A, Pippen J, et al. Aromatase inhibitors in the treatment of breast cancer in post-menopausal female patients: an update. Breast Cancer Targets Ther. 2011 Oct;3(3):113–125.
- Szabatura AH, Seung AH. Early and Locally Advanced Breast Cancer. ACCP. 2018 [cited 2019 Nov 12]; Available from: www.nccn.org/professionals/
- Diaby V, Tawk R, Sanogo V, et al. A review of systematic reviews of the cost-effectiveness of hormone therapy, chemotherapy, and targeted therapy for breast cancer. Breast Cancer Res Treat. 2015 May 19;151(1):27–40.
- Chapter 1. Pharmacoeconomics: Principles, Methods, and Applications. Pharmacotherapy: A pathophysiologic approach, 8e . AccessPharmacy: McGraw-Hill Medical; [ cited 2019 May 1]. Available from : https://accesspharmacy.mhmedical.com/content.aspx?bookid=462§ionid=41100767
- Räsänen P, Roine E, Sintonen H, et al. Use of quality-adjusted life years for the estimation of effectiveness of health care: a systematic literature review. Int J Technol Assess Health Care. 2006 Apr 28 [cited 2019 May 1];22(2):235–241. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16571199
- Paik H-J, Lee SK, Ryu JM, et al. Conditional disease-free survival among patients with breast cancer. Medicine (Baltimore). 2017 Jan [cited 2019 May 1] ;96(1):e5746. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28072715.