ABSTRACT
Background
Fracture nonunions impact on morbidity and health care costs and are associated with substantial pain, reduced mobility, prolonged morbidity, and a lower quality of life. CMF OrthoLogic 1000 (OL1000) is a bone growth stimulator used to promote fracture healing potentially reducing the need for surgical intervention. A cost analysis comparing CMF OL1000 versus surgical care for patients with nonunion tibial fractures was conducted.
Methods
A Markov model was developed to compare the difference in costs between CMF OL1000 versus surgical care within the English National Health Service over a 2-year time horizon. The effectiveness of CMF OL1000 was based on recently published registry data. Cost data were derived from published sources and national cost databases. Sensitivity and scenario analyses were conducted.
Results
The use of CMF OL1000 is estimated to lead to cost-savings of £1,104 per patient, a reduction in average healing time of 2.1 months and a relative risk of infection of 0.19 compared to immediate surgical intervention (standard of care). The results of the model are robust to most changes in input parameters and scenarios considered.
Conclusions
This early analysis shows cost-savings for CMF OL1000 compared with surgical intervention for individuals with nonunion tibial fractures.
1. Introduction
Fracture nonunions, although representative of a smaller proportion of fracture care outcomes, have devastating impacts on morbidity and health care costs. The National Institute of Health and Care Excellence (NICE) defines fracture nonunion as fractures that have not reached bony union within 6 to 9 months after the initial fracture treatment [Citation1]. Rates of nonunion vary by the bone fractured. A population-based study estimated the incidence of nonunion to be 13 per 1,000 per annum for pelvis and femur fractures, 30 per 1000 per annum for the humerus fractures, and 55 per 1000 per annum for the tibia and fibula fractures [Citation2]. Tibia fractures are at higher risk of nonunion given the lack of soft tissue envelope and resulting limitation of blood supply.
Fracture nonunions are associated with substantial pain, reduced mobility, prolonged morbidity, and a lower quality of life [Citation3]. Fracture nonunions often require surgical intervention to promote fracture healing. Types of procedures used to promote fracture healing include internal fixation or external fixation and bone grafting. Additional surgeries are burdensome for fracture patients as they often lead to prolonged disability and an increased loss of function and productivity. In addition, there is a high cost to the health care system due to the need for surgical intervention and extended patient care and follow-up.
To reduce the need for surgical intervention, noninvasive bone healing devices known as bone growth stimulators may be used to treat fracture nonunions. EXOGEN, a device that uses low-intensity pulsed ultrasound (LIPUS) at the fracture nonunion site, has demonstrated cost-savings when compared with invasive nonunion surgery for tibial fractures [Citation4]. While EXOGEN uses LIPUS to facilitate fracture healing, other bone growth stimulators use electrical currents or magnetic stimulation to achieve this outcome. For example, the CMF OrthoLogic 1000 (OL1000) device is a portable, battery-powered medical device that provides local magnetic field treatment through very low-energy combined static and dynamic magnetic fields. Broad uptake in bone growth stimulators has been slow due to compliance factors [Citation5,Citation6]. These include i) issues caused by the requisite use of a gel medium for device signals to transmit, and ii) long durations of wear time (20 minutes to beyond 2 hours) [Citation4,Citation7]. Due to the slow uptake of bone growth stimulators, surgical intervention represents standard of care (and the relevant comparator) within the health system [Citation8]. CMF OL1000 does not require a gel medium for the device to work and requires a comparatively shorter wear time (30 minutes).
Treatment with CMF OL1000 can either result in the fracture healing, or a fracture that does not heal and requires surgery to promote fracture healing. Following surgical intervention, patients may experience fracture healing or continued nonunion. They may also develop an infection. Additionally, patients may require multiple surgeries to treat their nonunion and/or infection [Citation9].
Evidence for the cost-effectiveness of devices using electrical currents or magnetic stimulation to achieve bony union is understood to be limited. To address this gap in the literature, a cost analysis comparing CMF OL1000 versus surgical intervention (standard of care) as treatment options for patients suffering from a nonunion following a tibial shaft fracture was conducted.
2. Methods
2.1. Model overview
An economic Markov state transition model was developed to compare the difference in costs between CMF OL1000 versus surgical care in patients with tibial shaft nonunions.
2.2. Clinical outcomes
In the absence of comparative data for CMF OL1000 and surgical care, clinical effectiveness data for both groups were obtained from separate sources. The tibial shaft fracture healing data for outcomes following treatment with CMF OL1000 were obtained from registry data published in 2016 [Citation9]. The reported registry data included rates of fracture healing and time to healing for tibial fracture nonunions in monthly increments from the date of the initial fracture [Citation9]. Following the NICE definition of nonunion, fracture healing times between 6 to 9 months from the initial fracture were aggregated to estimate an average time to healing for nonunion tibial fractures. These rates were then converted into a transition probability of successful treatment of the tibial shaft nonunion. For the surgical care group, the transition probability of successful treatment was extracted from published literature [Citation10]. Since the baseline characteristics of patients were not available from the registry data, statistical matching of the treatment and comparator groups was not possible. summarizes the clinical estimations that were used in this analysis.
Table 1. Clinical estimations
2.2.1. Determination of costs
NHS reference costs and Personal Social Services Research Unit costs (Supplementary Material A) were primarily used to estimate the total NHS cost implications for both treatments (). The perspective of the model was the payer (English NHS). Costs and effects in the model were discounted at 3.5% per annum in line with the NICE reference case [Citation11]. A time horizon of two years was used to capture the expected benefits of CMF OL1000 and surgical intervention as it was assumed based on clinician input that all patients would achieve fracture union by two years. Costs were valued in 2018/19 prices.
Table 2. Cost inputs
2.3. Markov modeling
A Markov model with 1,000 simulated patients was developed to estimate the expected changes in costs and to capture movements between health states over time for both treatment groups. Model cycles were monthly to capture the granularity in the registry data for CMF OL1000 and the potential time for movement between health states. The structure of the model is presented in . The primary outcome of the model was the incremental total cost per patient. This was determined by calculating the total costs (device and health state costs) associated with CMF OL1000 and standard of care and calculating the difference. The model therefore used a total of 24 cycles, for a total time horizon of 2 years.
Figure 1. Markov model of all health states associated with tibial fracture nonunion.
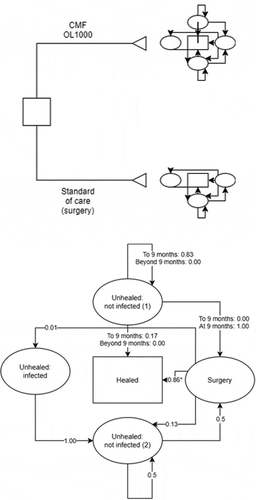
All patients enter the model in the unhealed: not infected (1) health state.
Following treatment with CMF OL1000, the health states a patient may enter are:
The fracture heals: Healed
The fracture does not heal and requires surgery to promote fracture healing: Surgery
Following surgical intervention, the fracture heals: Healed
Following surgical intervention there is continued nonunion: Unhealed, not infected 2
Following surgical intervention there is continued nonunion and an infection: Unhealed, infected
Patients may require multiple surgeries to treat their nonunion and/or infection: Unhealed, not infected 2
These health states can be either progressive or regressive, with potential movements in and out of surgery.
The first health state: unhealed: not infected (1) represents individuals who have a nonunion fracture that does not have a diagnosed deep infection. A proportion of these individuals do, however, have an unexpected (or undiagnosed) deep infection. Such deep infections are not diagnosed until patients undergo surgery at which point the infection is treated. Superficial infections do not prevent treatment with CMF OL1000, and outcomes associated with superficial infections are not included in the model.
The cost of this additional surgical treatment to remove a deep infection, as reported in , was applied to 5% of the initial cohort of patients in both arms of the model [Citation12]. All individuals begin in this health state, as this state represents all people eligible for treatment with CMF OL1000. Each cycle, individuals either heal from CMF OL1000 or standard of care (transition to healed) or remain in unhealed: not infected (1) for up to 9 cycles (maximum continuous use of CMF OL1000 is 270 days as per the instructions for use of the device). After a maximum of 9 cycles within unhealed: not infected (1), patients transition to surgery and CMF OL1000 can no longer be administered. This is applied within the model using time-dependent transition probabilities as presented in .
Patients may undergo surgery to promote fracture healing. Patients cannot remain in this health state in a subsequent cycle and must transition to a healed or unhealed health state. In order to capture the delay to healing post-surgery (reported to be 7.2 months from surgery in aseptic nonunion tibia fractures [Citation13]) a delay of 7 months occurred in the model before the patients transition into the healed health state. This is applied using tunnel health states which are not pictured on for simplicity.
When surgery fails but the patient did not contract a deep infection, and are in a health state of watchful waiting before a subsequent surgery (unhealed: not infected (2)), patients can experience spontaneous fracture healing (assumed probability of 0.2%), transition back to surgery (assumed probability of 50%), or remain in this health state.
The unhealed: infected health state includes patients whose fracture has not healed following surgery, and who have developed a deep infection at the nonunion site following surgery. It was assumed that individuals in this health state would require additional surgery to treat the infection which was assumed successful 100% of the time. This would move them to the unhealed (not infected) health state. Individuals in the unhealed (infected) health state cannot remain in this state in a subsequent cycle.
The final health state is healed, in which patients’ fractures are healed from successful treatment from CMF OL1000 or standard of care. This is an absorbing health state; for individuals in this health state there are no subsequent transitions.
2.4. Sensitivity and scenario analyses
The robustness of the assumptions used and their impact on the model’s results were investigated using sensitivity analyses (deterministic and probabilistic) and scenario analyses.
Deterministic sensitivity analysis (DSA) is a method to investigate the sensitivity of the model results to variations in a specific input parameter or set of parameters. Deterministic, univariate sensitivity analyses were conducted around the model inputs, whereby inputs were varied independently between an upper and lower limit to determine the impact of different parameter values on the model results.
Probabilistic sensitivity analysis (PSA) is a technique used in economic modeling to determine the level of confidence in the outputs of a model based on uncertainty in the model inputs. PSA allows for the combined model parameter uncertainty to be explored; each model input has an associated distribution for which the value of the input can be drawn from. Each time the model is run using PSA, that iteration of the model results is recorded, with the results reflecting the values of each of the inputs from their random draws. This process is repeated over many iterations, with model results recorded in each iteration, until stability in mean results of the model is achieved. The PSA was conducted using 1,000 iterations, which is typically deemed to achieve stability in results. Within the PSA, upper and lower values for the distributions were determined using the upper and lower limits of the respective standard error for inputs, or ±25% of the base case value when a standard error was not available. Probabilities in the model had a beta distribution applied, and costs had a gamma distribution applied.
The first scenario analysis (Scenario 1) was conducted using costing data from the NICE External Assessment Center review report for the X-ray and physiotherapy cost inputs [Citation14]. This was conducted because the costs of these two inputs were much higher than the costs used in the base case. In the base case an X-ray is costed at £29.00 compared with £87.29 in the EAC report after inflation, and physiotherapy in the base case is costed at £58.00 compared with £286.98 in the EAC report. Both costs in the EAC report were inflated to 2018/19 pricing using PSSRU data [Citation15]. Whilst recognizing the merits of the EAC review report, these costs were not used in the base case because alternatives were identified that were judged to better reflect the current cost of these parameters. Specifically, the model results were generated using alternative cost data from the EAC review report [Citation14] for an X-ray and physiotherapy appointment.
An additional scenario analysis (Scenario 2) was undertaken using the data from the Study to Prospectively Evaluate Reamed Intramedullary Nails in Patients with Tibial Fractures (SPRINT). In this, currently unpublished data, 38 participants with either a closed or Type 1 open fracture had a nonunion surgery or a surgery to promote fracture healing. The results of this study show that 5/38 participants (13.2%) were diagnosed with an infection after surgery. In Scenario 2 the base-case infection risk value of 1.4% is replaced with 13.2%.
3. Results
3.1. Base case analysis
The use of CMF OL1000 is cost saving when compared with surgical intervention in people with nonunion tibial fractures in the English NHS over a two-year time horizon (). Furthermore, the clinical outcomes are also presented in and show a breakdown in the absolute and relative differences of clinical events between CMF OL1000 and surgical care.
Table 3. Cost per patient breakdown and clinical outcomes
3.2. Deterministic sensitivity analysis
The results are shown in a ‘tornado chart’; a summary (stack) of bar graphs representing univariate sensitivity analyses for a wide range of input values, ordered according to the spread of variation of the resulting model output value (with the widest variation on top). The tornado chart for this model identified that both the monthly healing probability of CMF OL1000 (a model input) and the cost of surgery had sufficient power to change the direction of the base-case results from cost saving to cost incurring (see ).
Figure 2. Tornado diagram presenting deterministic sensitivity analysis.
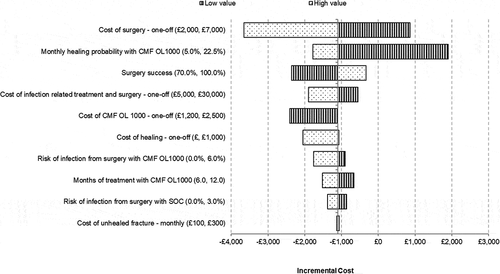
The monthly healing probability of CMF OL1000 was calculated to be 16.8%. The device was estimated to be cost saving provided that the monthly healing probability with CMF OL1000 was above 11.1% per month. The cost of surgery was identified as £4,174 and was also a key driver of the model results. The higher the cost of surgery, the greater the cost savings estimated with CMF OL1000 because people using CMF OL1000 require relatively fewer operations than patients who receive operative treatment of the tibial nonunion. Provided surgery costs were over £2,950, CMF OL1000 was estimated to be cost saving.
3.3. Probabilistic sensitivity analysis
When all of the input parameters for CMF OL1000 and standard of care were varied simultaneously, using 1,000 iterations of the simulated data, CMF OL1000 was cost saving compared with standard of care in 76% of the 1,000 simulations. The average incremental cost saving for CMF OL1000 compared with surgery was £870 per person.
3.4. Scenario analyses
In Scenario 1 (Supplementary Material B, Table B.2) the source for the cost inputs for an X-ray and physiotherapy is the NICE External Assessment Center review report [Citation14]. The results show the cost outcomes per patient increased in both arms of the model, but particularly for time spent in the unhealed: not infected health state in the CMF OL1000 arm. However, CMF OL1000 remained cost saving by £543 per patient. There was no impact on the clinical outcomes.
In Scenario 2 (Supplementary Material B, Table B.2) unpublished SPRINT data was used for the rate of infection input. The increased risk of infection in this scenario resulted in CMF OL1000 remaining cost saving. The impact on the cost outcomes is primarily shown in the increased cost per patient in the unhealed: infected health state, where the cost increased for both interventions. The cost increase was caused by the increased need for infection treatment compared to the base case (3 infections compared with 29 infections for CMF1000, and 16 infections compared with 153 infections for standard of care). As a result, the total cost saving per-patient increased from £1,104 to £2,792 with CMF OL1000. In addition, the difference in average time to fracture healing increased from 6.43 to 6.45 months with CMF1000, and increased from 8.49 to 8.62 with standard of care.
4. Discussion
The current model found that the management of tibial fracture nonunions with CMF OL1000 costs less than immediate surgical intervention. The differences in costs were driven by several factors including: the difference in treatment costs between CMF OL1000 compared with immediate surgical management (£2,500 compared with £4,174); a shorter time to healing of the nonunion for patients treated with CMF OL1000 compared with surgical intervention (6.4 months compared with 8.49 months); fewer patients requiring surgical management when treated with CMF OL1000 (221 compared with 1,162; 81% reduction); and a lower rate of infection in patients treated with CMF OL1000 (3 infections compared with 16, a relative risk of 0.19).
Other benefits of CMF OL1000 include an expected improvement in patient compliance and convenience compared to other bone growth stimulators and surgical intervention. Compliance with CMF OL1000 was not be modeled because effectiveness data for CMF OL1000 is from observational (registry) data; compliance implicitly modifies the average clinical effectiveness of CMF OL1000 and should not be double counted.
By using CMF OL1000, patients reduce their risk of infection incurred from surgery as they are likely to be healed using CMF OL1000 before surgery is required. The estimated cost of treating one deep infection in the English NHS is £15,202. This includes the cost of medical treatment of the infection as well as additional surgery required for the nonunion fracture. The unhealed and infected health state is therefore the most expensive health state within the Markov model, and one which is often avoided if a patient begins treatment using CMF OL1000.
The results of this study provide similar results to previous investigations on the cost-analyses of bone growth stimulator use for fracture nonunion management. A Markov model comparing EXOGEN’s base-case analyses found EXOGEN (LIPUS) ultrasound device to be cost saving for both delayed union (cost saving of £684 relative to control) and for nonunion (cost saving of £2,310 relative to surgery) [Citation4]. While other options have demonstrated similar findings, the short daily application period of CMF OL1000 may improve compliance rates and convenience for patients.
The analysis is strengthened by our use of Markov modeling with multiple sensitivity analyses conducted. Additionally, this model used the available literature to incorporate all potential associated costs from fracture nonunion management.
As with any model, multiple assumptions were made. It was assumed that all individuals in the standard of care arm received surgical treatment rather than watchful waiting. This assumption is conservative for the incremental clinical benefit of CMF OL1000, as the success rate for surgery is much higher than the success rate for watchful waiting. The use of surgery as standard of care is also a logical assumption that has been clinically validated. We also assumed that patients in both treatment groups would have a 5% rate of undiagnosed infection on entry to the model, which was based on trial data [Citation12]. The cost of surgery has been extracted from a previous single technology appraisal in the area of nonunion tibial fractures [Citation1]. This cost has been accepted by NICE and validated by the External Assessment Center. We do not consider it likely that the cost of surgery will be significantly less than the cost which was previously presented. Finally, several parameters from published literature [Citation9] were considered when calculating the monthly healing probability for CMF OL1000 of 16.8%. To reduce the monthly healing probability of CMF OL1000 to 7.5%, at which point the direction of the results change from cost saving to cost incurring, would be a substantial underestimation of the current observational study literature surrounding the efficacy of CMF OL1000.
There are two limitations that we consider particularly important. The first is that the registry data used to populate the economic model are from American patients [Citation9]. The generalizability of the results to the English NHS are therefore uncertain as the clinical care pathways and baseline characteristics of American and English patients may differ. However, the sensitivity analysis that we have conducted has quantified the impact of some of this uncertainty and clinical input has suggested that the patients included within the registry are likely to be similar to those receiving treatment in the English NHS. Secondly, the results of the economic model represent a naïve comparison. A systematic review of the clinical evidence was not undertaken to populate the economic model with the most relevant evidence for both arms, and both arms of the model are unmatched on patient characteristics. Because potential confounding factors between study participants in the treatment and control arm were not identified or controlled for via randomization, the magnitude of the cost savings and clinical success rate for CMF OL1000 may be biased. However, clinical input recommends that the patients informing the data on CMF OL1000 and surgery are likely to be similar. Additionally, the cost data were obtained from UK sources and may not be generalizable to other jurisdictions with different health care and reimbursement systems.
5. Conclusion
The results of this study indicate a £1,104 cost saving per person when using CMF OL1000 compared with undergoing surgical intervention for individuals with nonunion tibial fractures (that are not deeply infected). A reduction in costs is caused by a lower incremental treatment cost for CMF OL1000, fewer patients requiring surgery, shorter average incremental time to healing and an incremental reduction in the incidence of deep infections arising from surgical intervention. The results of the model are robust to most changes in input parameters and scenarios considered.
Author contributions
Joel Russell and Michelle Green designed and built the model with input from all other authors. All authors contributed to the drafting of the paper which was reviewed and signed off by Mohit Bhandari.
Declaration of interest
Joel Russell’s employer received funding from DJO Global for this study. Sheila Sprague reports employment from Global Research Solutions, outside the submitted work. Sam Harper's employer received funding from DJO Global for this study. Michelle Green’s employer received funding from DJO Global for this study. Mohit Bhandari reports grants and personal fees from Sanofi Aventis, personal fees from Pendopharm, grants from DJ Orthopaedics, grants and personal fees from Ferring Pharmaceuticals, grants from Anika, Flexion, Acumed outside the submitted work.
Reviewers disclosure
A reviewer on this manuscript has disclosed working for an HEOR consulting firm. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Supplemental Material
Download Zip (29.1 KB)Supplementary material
Supplemental data for this article can be accessed here.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- National Institute for Health and Care Excellence (NICE). EXOGEN ultrasound bone healing system for long bone fractures with non-union or delayed healing. 2013 [2020 Apr 6]. Available from: https://www.nice.org.uk/guidance/mtg12
- Mills LA, Simpson AHR. The relative incidence of fracture non-union in the Scottish population (5.17 million): a 5-year epidemiological study. BMJ Open. 2013;3(2):e002276.
- Bell A, Templeman D, Weinlein JC. Nonunion of the Femur and Tibia: an update. Orthop Clin North Am. 2016 Apr;47(2):365–375. PubMed PMID: 26772945; eng.
- Higgins A, Glover M, Yang Y, et al. EXOGEN ultrasound bone healing system for long bone fractures with non-union or delayed healing: a NICE medical technology guidance. Appl Health Econ Health Policy. 2014 Oct;12(5):477–484. PubMed PMID: 25060830; PubMed Central PMCID: PMCPMC4175405. eng.
- Uchiyama Y, Tateiwa T, Nakajima D, et al. An examination of the factors related to a reduction in the use of Low-Intensity Pulsed Ultrasound (LIPUS). J Orthop Trauma. 2017 2017/07/;31(7):S3. PubMed PMID: 28632663; eng.
- Zura R, Della Rocca GJ, Mehta S, et al. Treatment of chronic (>1 year) fracture nonunion: heal rate in a cohort of 767 patients treated with low-intensity pulsed ultrasound (LIPUS). Injury. 2015 Oct;46(10):2036–2041. PubMed PMID: 26052056; eng.
- Kesani AK, Gandhi A, Lin SS. Electrical bone stimulation devices in foot and ankle surgery: types of devices, scientific basis, and clinical indications for their use. Foot Ankle Int. 2006 2006/02/01;272:148–156.
- Poolman RW, Agoritsas T, Siemieniuk RAC, et al. Low intensity pulsed ultrasound (LIPUS) for bone healing: a clinical practice guideline. BMJ. 2017;356:j576.
- Phillips M, Baumhauer J, Sprague S, et al. Use of combined magnetic field treatment for fracture nonunion. J Long Term Eff Med Implants. 2016; 26(3):277–284.
- Gebauer D, Mayr E, Orthner E, et al. Low-intensity pulsed ultrasound: effects on nonunions. Ultrasound Med Biol. 2005;31(10):1391–1402.
- National Institute for Health and Care Excellence (NICE). Medical technologies evaluation programme methods guide 2017 [2020 Apr 6]. Available from: https://www.nice.org.uk/process/pmg33/chapter/introduction
- Mills L, Tsang J, Hopper G, et al. The multifactorial aetiology of fracture nonunion and the importance of searching for latent infection. Bone Joint Res. 2016 Oct 5;5(10):512–519. PubMed PMID: 27784669; PubMed Central PMCID: PMCPMC5108351. eng.
- Tsang ST, Mills LA, Frantzias J, et al. Exchange nailing for nonunion of diaphyseal fractures of the tibia: our results and an analysis of the risk factors for failure. Bone Joint J. 2016 Apr;98-b(4):534–541. PubMed PMID: 27037437; eng.
- Cedar Healthcare Technology Research Centre. Review report of MTG12: EXOGEN ultrasound bone healing system for delayed-union and non-union. 2019.
- Personal Social Services Research Unit (PSSRU). Unit costs of health and social care 2019. 2019 [2020 Apr 6]. Available from: https://www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2019/
