ABSTRACT
Objective
In the absence of head-to-head comparisons, the objective of this study was to conduct a network meta-analysis (NMA) to indirectly compare the relative efficacy and safety of rimegepant, ubrogepant, and lasmiditan for the acute treatment of migraine.
Methods
A systematic literature review was conducted to identify randomized controlled trials (RCTs) of rimegepant, ubrogepant, and lasmiditan in adults with acute migraine. Outcomes included sustained pain freedom and -relief 2–48 hours post-dose, and adverse events. No RCTs were identified that directly compared these interventions. Therefore, a fixed-effects Bayesian NMA was conducted by identifying a connected (via comparison to placebo) network of RCTs.
Results
Five RCTs were identified as follows: rimegepant study 303 (n = 1,466), ubrogepant ACHIEVE I and II (n = 1,672 and n = 1,686, respectively), and lasmiditan SAMURAI and SPARTAN (n = 2,231 and n = 3,005, respectively). Efficacy outcomes (pain freedom and relief at 2, 24, 48 hours) tended to be highest for lasmiditan 200 mg and rimegepant followed lower doses of lasmiditan and all doses of ubrogepant. However, lasmiditan 200 mg was also associated with higher rates of adverse events, particularly somnolence and dizziness.
Conclusions
Lasmiditan, rimegepant, and ubrogepant all performed significantly better than placebo with respect to pain freedom and pain relief. Efficacy results were similar for rimegepant and lasmiditan with rimegepant having higher rates of pain freedom and relief than lower doses of lasmiditan, while somnolence and dizziness outcomes were lower for rimegepant than higher doses of lasmiditan.
1. Introduction
Migraine is a common, chronic disease with a substantial functional, social, and financial burden [Citation1–5]. People with migraine utilize more healthcare resources, are less productive and more likely to experience work loss, have increased odds of filing disability claims, and have a lower quality of life during and between migraines (interictal) compared to people without migraine [Citation6–8]. The likelihood of migraine having a detrimental effect on family members, relationships, careers, and financial security increase with increased migraine frequency [Citation2]. It is therefore important to manage migraine effectively [Citation9].
Management approaches include education to make lifestyle changes and avoid or manage triggers, cognitive behavioral therapy, and acute and preventive pharmacological treatments that are able to address migraine pain and its consequences [Citation1,Citation10,Citation11]. The goals of acute treatment for migraine are to rapidly achieve and maintain freedom from pain and other symptoms, to restore functional ability, and to minimize the use of rescue medications, repeat doses, adverse effects, and other resources [Citation1]. It is challenging to optimize treatment for individuals with migraine due to variations in the characteristics, frequency, and severity of migraines between people, as well as within the same person over time [Citation1]. The choice of treatment depends on migraine characteristics such as severity, symptoms, and accompanying disability, as well as patient preference and characteristics such as pregnancy, response to previous treatments, and comorbidities and concomitant treatments that could result in contraindications [Citation1]. Although treatment is individualized, first-line treatments for mild to moderate migraine include oral non-steroidal anti-inflammatory drugs (NSAIDs), acetaminophen, or combination analgesics containing caffeine [Citation1,Citation12]. Treatments for moderate to severe migraine, or for people with an inadequate response to mild or moderate migraine treatments, include migraine-specific treatments such as 5-hydroxytryptamine (5-HT)1B/1D receptor agonists (triptans) or dihydroergotamine (DHE) [Citation1,Citation12]. Triptans have a better efficacy and adverse effect profile than DHE, and triptans are recommended as a first-line therapy for the acute treatment of moderate to severe migraine attacks, whereas DHE is recommended as a second-line therapy [Citation1,Citation12,Citation13].
Several novel treatments have been developed and recently approved by the US Food and Drug Administration (FDA) for the acute treatment of migraine [Citation14–21]. These novel treatments include the calcitonin gene-related peptide (CGRP) receptor antagonists rimegepant [Citation16] and ubrogepant [Citation15,Citation18] and the selective 5HT1F-receptor agonist lasmiditan [Citation14,Citation17,Citation19]. CGRP receptor antagonists block CGRP receptor and reduces inflammation, vasodilation, and pain [Citation13]. Due to the more specific mechanism of action of CGRP receptor antagonists on the trigeminovascular system, these treatments have fewer adverse effects compared to older treatments [Citation20,Citation22]. Of particular note, first-generation gepants, which were first introduced more than 15 years ago, were not utilized due to liver toxicity, while the second-generation gepants rimegepant and ubrogepant are not associated with liver safety concerns [Citation23]. Lasmiditan is a highly selective agonist that binds to the 5-HT1F receptor with high affinity, and activation of this receptor blocks trigeminal neuron activation which inhibits ‘the acute migraine pathway;’ [Citation13,Citation14]; lasmiditan has been shown to be efficacious in reducing migraine pain relief and pain freedom [Citation24] and reductions in disability following long-term treatment [Citation25]. However, because lasmiditan crosses the blood–brain barrier, there is the potential for greater adverse effects than CGRP receptor antagonists [Citation17,Citation26]. The safety and efficacy of these treatments have been demonstrated in randomized, double-blind, placebo controlled trials (NCT03461757 [Citation27,Citation28], NCT02828020 [Citation29,Citation30] and NCT02867709 [Citation31,Citation32], and NCT02439320 [Citation33,Citation34] and NCT02605174 [Citation35,Citation36]), but they have not been compared head-to-head [Citation37]. When direct comparisons are not available, indirect comparisons via a network meta-analysis (NMA) can assist in generating evidence for decision-making [Citation38]. The objective of this study was to conduct an NMA study to indirectly compare the relative efficacy and safety of rimegepant, ubrogepant, and lasmiditan for the acute treatment of migraine. Novel treatments may be able to fill an unmet need for patients that are not well managed with historic treatment options, and a comparison of the efficacy and safety of novel treatments can help to guide decision-making for patients and clinicians to select the optimal treatment for patients’ needs. Ideally, head-to-head trial data would be available for all pairwise comparisons, but in the absence of this, NMA can be used to estimate relative efficacy and safety between treatments that have not been compared head to head.
2. Methods
2.1. Data sources, searches, and study selection
A systematic literature review (SLR) was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [Citation39] and a prespecified protocol to identify, among others, relevant RCTs for comparing the efficacy and safety of rimegepant, ubrogepant, and lasmiditan. Of note, this search was part of a broader search to identify a range of studies related to the burden of migraine in addition to RCTs of efficacy and safety, and as such a sensitive search strategy was employed, resulting in a large number of hits in addition to those relevant to the NMA described here. Outcome measures were not included as indexing terms in the search strategy but were incorporated into the selection criteria which were based on patient population, interventions/comparators, outcome measures, and study design selection (PICOS). Embase and Medline were searched from database inception to present on 22 July 2019 using a combination of free-text words, indexing terms (e.g. MeSH) and their relationship using Boolean operators (e.g. ‘and,’ ‘or,’ ‘not’). The search was limited to articles in English. Additional sources were identified through hand searches. The focus of the SLR was broader than the NMA, and tables of the search terms and PICOS criteria for the full SLR can be found in the online resources/supplementary material. The PICOS criteria that were used for the NMA are listed in . In brief, the population of interest was adults 18 years and older with acute migraine who are ineligible for or refractory to first-line treatments such as NSAIDS and triptans; the interventions that were compared were rimegepant orally disintegrating tablets (ODT), oral ubrogepant, and oral lasmiditan; outcomes included sustained pain freedom and sustained pain relief 2–48 hours post-dose, freedom from most bothersome symptoms, and safety outcomes including adverse events such as somnolence, paresthesia, dizziness, and nausea; and only RCTs providing direct or indirect evidence between treatments of interest were considered. Outcomes were chosen because they are frequently used as primary endpoints in clinical trials. Pain freedom was defined as having no pain 2 hours after treatment and sustained pain freedom meant maintaining being pain free without relapse or the use of rescue medications for up to 24- or 48-hours post-dose. Pain relief is defined as improvement from moderate to severe pain before treatment, to mild or no pain after a specific interval.
Table 1. PICOS Criteria
2.2. Screening, data extraction, and quality assessment
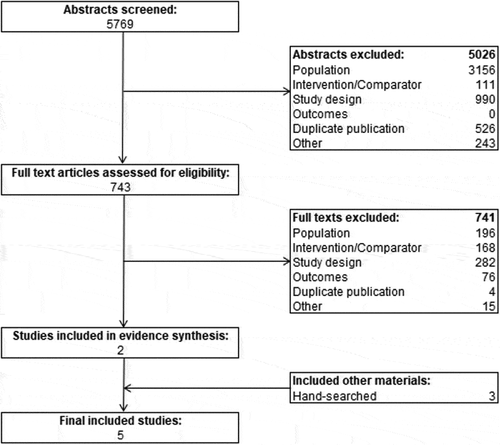
Two reviewers independently reviewed the titles and abstracts of the identified studies against the PICOS criteria to determine relevancy, and after exclusions reviewed the full text of retained articles. A third reviewer resolved any conflicts between the two reviewers. Study quality assessment was performed by one reviewer using the Cochrane Risk of Bias Tool (see supplementary materials) [Citation40]. Articles that passed the full-text review were used for data extraction, and the total number of papers that were excluded, as well as the reason for their exclusion was recorded. Study selection for the NMA is illustrated in and based on PRISMA [Citation39].
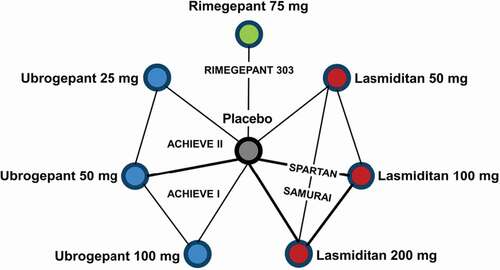
From the SLR, a connected network of trials was identified to compare the treatments indirectly (). All trials were placebo-controlled, which allowed for all comparators of interest to be connected through placebo.
2.3. Model specifications, fitting, and data analysis
All NMA models were fit using a Bayesian framework. These methods are in accordance with the guidelines set forth by the NICE Decision Support Unit (DSU) [Citation41]. All analyses were conducted using R (V3.6.1) and JAGS (V4.3.0). Binomial likelihood models incorporating a logit link were used for all outcomes. No additional regression adjustments such as baseline risk or the adjustment for specific baseline characteristics were made. As the network of evidence was sparse in terms of the number of included trials, fixed-effect models were decided upon a priori as there was insufficient evidence to effectively estimate heterogeneity between studies using a random-effects model.
2.4. Interpretation of results
Results are expressed in terms of risk differences (95% credible interval [CrI]) of competing interventions, i.e. the incremental proportion of respondents achieving the outcome of interest across treatments. Results are presented using the surface under the cumulative ranking curve (SUCRA), to provide clearer interpretation on the presence of multiple interventions and outcomes [Citation42]. A higher numerical value (closer to 100) indicates a higher likelihood that the intervention is ranked at the top (best = 1) [Citation42]. For each intervention, the entire risk-benefit profile must be considered. That is, some interventions may rank higher with regard to efficacy, but this must be balanced with possible lower rankings with regard to safety, and vice versa [Citation42].
3. Results
3.1. Search results
The search strategy yielded 5,769 abstracts for review. After 526 duplicates were removed, 5,243 abstracts were reviewed for inclusion, of which 743 underwent full-text review. Four additional duplicates were found and removed during full-text review and further 737 articles were excluded after applying the NMA PICOS criteria. Three additional articles were found via hand searches. These included full-text articles of the included studies, which were only available in abstract form (i.e. not yet indexed) at the time of search strategy execution [Citation28,Citation30,Citation32].
3.2. Study characteristics
In total, five randomized placebo-controlled trials contributed data to the network and were included in the NMA. These were Study 303 for rimegepant (n = 1,466) [Citation28], ACHIEVE I (n = 1,672) [Citation30] and ACHIEVE II (n = 1,686) [Citation32] for ubrogepant, and SAMURAI (n = 2,231) [Citation34] and SPARTAN (n = 3,005) [Citation36] for lasmiditan. All trials were phase III, multicenter trials from the US, with the exception of SPARTAN which was a multi-country trial from the US, UK, and Germany. All trials assessed the acute treatment of a single migraine attack over a period of 4 to 11 weeks.
Trial baseline characteristics are compared in . The trials were generally well balanced in terms of the baseline characteristics for sex (82% to 88.2% female), and age (mean age ranging from 40.2 to 42.7 years), and most bothersome symptom (photophobia), but the SAMURAI and rimegepant 303 studies had fewer white participants compared with the other trials. More participants reported a history of migraine with an aura in the lasmiditan trials (32% to 36%) compared to the rimegepant 303 (30%) and ACHIEVE II trails (24%). These data were not reported for ACHIEVE I. Participants in the lasmiditan trials, for which an inclusion criterion was a Migraine Disability Assessment (MIDAS) score ≥ 11, experienced more migraine attacks per month (mean >5) at baseline compared to ACHIEVE II and Rimegepant 303 (mean<5), while ACHIEVE I did not report on attack severity.
The raw efficacy and safety data that were used in the model and analysis are presented study and outcome in .
Table 2. Raw Efficacy and Safety Data by Study and Outcome used in the Analysis/Model
3.3. Efficacy outcomes
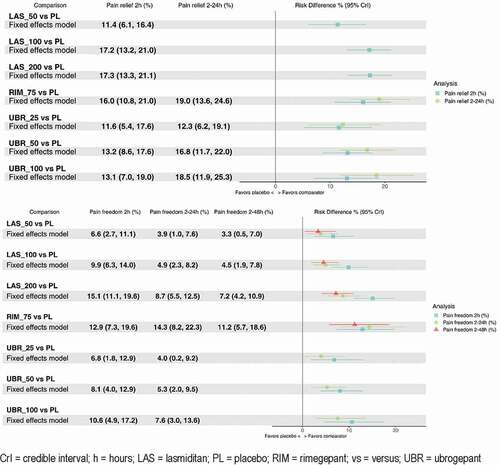
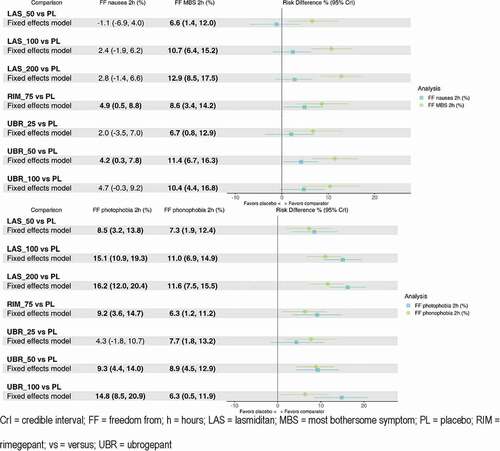
Pain relief at 24 hours was not reported in lasmiditan studies, pain freedom at 48 hours was not reported in ubrogepant studies, and pain relief data were not available for any comparison at 48 hours. Data were available for each treatment for each other outcome. All active comparators demonstrated a significant increase in pain relief and pain freedom compared to placebo at 2 hours and 2–24 hours where data were available ( and ). Rate of pain freedom at 2 hours were highest for lasmiditan 200 mg (risk difference vs. placebo 15.1% [95% CrI: 11.1, 19.6%]), followed by rimegepant. For sustained pain freedom at 2–24 hours, risk difference vs. placebo was highest for rimegepant (14.3% [8.2–22.3%]), followed by lasmiditan 200 mg (8.7% [5.5–12.5%]) and ubrogepant 100 mg (7.6% [3.0%-13.6%]). Regarding 2–24 hour pain freedom, lasmiditan 200 mg was favored compared to the two lower doses of lasmiditan; in addition, rimegepant was superior to lasmiditan 50 mg (10.4% [CrI 3.4–18.8%]) and 100 mg (9.4% [2.6–17.6%]), as well as ubrogepant 25 mg and 50 mg (). Rimegepant was favored with regard to pain freedom vs. placebo and the two lower doses of lasmiditan at 2–48 hours post-dose (ubrogepant data not available for this outcome).
Table 3. Results of fixed-effect models for pain relief and pain freedom at various intervals
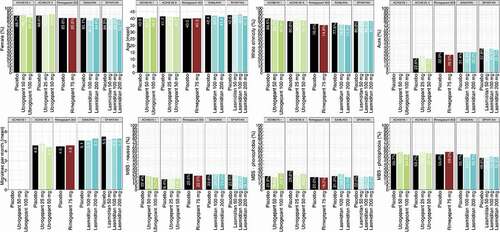
In terms of the ‘freedom from’ outcomes (), all active comparators were favored over placebo for all outcomes (although not all estimates were statistically significant), except for lasmiditan 50 mg which had a worse outcome for freedom from nausea 2 hours post-dose compared to placebo. The lowest doses of ubrogepant (25 mg) and lasmiditan (50 mg) performed worse than the higher doses of those treatments with regard to freedom from MBS at 2 hours, while the highest doses of ubrogepant (100 mg) and lasmiditan (100 mg and 200 mg) performed the best degree of freedom with regard to photophobia at 2 hours. Rimegepant was comparable to all other treatments for all other outcomes except for the high doses of lasmiditan for freedom from photophobia, where it was shown to be inferior.
3.4. Safety outcomes
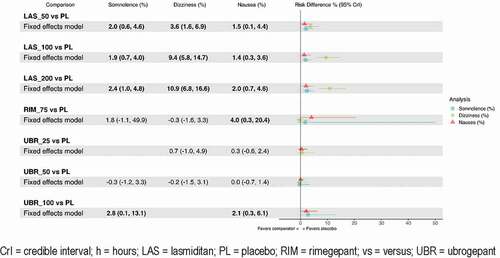
Data were available for all treatments for each specific adverse event outcome examined. Ubrogepant 25 mg and 50 mg had comparable results regarding somnolence and dizziness compared to placebo for all adverse event outcomes. Rimegepant was comparable to placebo regarding dizziness and somnolence. Placebo was superior to all other active comparators for all other adverse event outcomes (). The higher doses of lasmiditan had the worst dizziness outcomes, ubrogepant 100 mg fared the worst on somnolence, and rimegepant had the worst nausea outcome.
When estimates are very uncertain, raw rankings can be biased. To get around this limitation, the SUCRA captures this uncertainty by taking into account the full area under the ranking curves [Citation42]. The rankings and SUCRA percentages of the interventions by study and outcomes are presented in . Therefore, although lasmiditan 200 mg ranks highest on most efficacy outcomes, it must be balanced with the fact that it ranked lowest or second lowest with regard to safety outcomes of somnolence, dizziness, and nausea. Rimegepant ranked high on both efficacy and safety outcomes, except for nausea. Ubrogepant 50 mg fared better on safety outcomes compared to rimegepant, but worse when it came to efficacy outcomes.
Table 4. Surface under the cumulative ranking curve (SUCRA)
4. Discussion
This NMA compared the safety and efficacy of rimegepant, ubrogepant, and lasmiditan for the acute treatment of migraine, and found significant differences between these treatments. All active comparators were superior to placebo with regard to pain freedom and pain relief 2 to 24 hours post-dose. Rimegepant and all doses of ubrogepant were comparable for 2–24 hour pain relief, but rimegepant was superior to the lower doses of lasmiditan and ubrogepant for 2 to 24 hour pain freedom. Lasmiditan was superior to rimegepant for freedom from photophobia after 2 hours, while rimegepant was superior to all doses of lasmiditan regarding dizziness. A recent NMA of these three treatments by the Institute for Clinical and Economic Review (ICER) presented similar findings [Citation37]. In that analysis, rimegepant, ubrogepant, and lasmiditan were all found to be associated with improved function and decreased migraine symptoms compared to placebo, for a subset of the outcomes considered here [Citation37]. These findings are similar to the results of the SUCRA rankings in the current study. Cost-effectiveness was not considered in the current study and the cost-effectiveness of the respective treatments may ultimately play a role in decision making.
Limitations of this study include limited events that hindered the precise estimates of safety outcomes, as well as a lack of long-term efficacy and safety data. While lasmiditan and ubrogepant were both assessed in independent confirmatory trials, rimegepant was not, although no methodological or bias-related concerns have been raised regarding the single rimegepant trial. Long-term safety data has been collected in 52-week open-label studies of rimegepant [Citation43], ubrogepant [Citation44], and lasmiditan [Citation25]. A limitation of indirect comparisons via NMA is that it creates more uncertainty than when treatments are compared directly [Citation37]. However, this NMA was based on a connected network of acute migraine RCTs that were well-balanced with regard to subject characteristics, and this improves the validity of conducting an indirect comparison. However, external validity may have been compromised due to the homogenous populations that were selected for the RCTs that were included in this NMA, as is usual for studies that are used for regulatory purposes [Citation45]. The internal validity of the analyses is contingent on three factors: 1) the appropriate identification of the studies that make up the evidence network, 2) the quality of the individual RCTs, and 3) the extent of confounding bias due to similarity and consistency violations. In this instance, RCTs were identified via SLR, and both quality-assessment of included trials and comparison of baseline characteristics implied appropriateness of NMA conduct.
SUCRA rankings have several limitations, including that they do not take differences in effect magnitude between interventions into account, and its inability to capture the possibility that the differences between interventions are due to chance [Citation42]. However, the results have also been reported in terms of risk difference in this paper. Another strength is that NMAs are able to combine evidence, whether direct or indirect, and ‘increase the precision of effect estimates’ to show the relative value or advantages of interventions that have not been compared directly in head-to-head trials [Citation42]. Future research could compare the interventions directly, and cost-effectiveness analysis may be able to improve decision-making for healthcare providers, payers, and patients. The unpredictability, severity, and frequency of migraines have a tremendously negative effect on patients’ quality of life, their careers, income, and families, and there is currently a substantial unmet need for safe and effective treatments [Citation37]. Therefore, it is important to compare available treatments to determine which treatments may offer a net benefit. The current NMA provides insight into the comparative efficacy and safety of rimegepant, ubrogepant, and lasmiditan. All three of these acute migraine treatments offer convenience because they are orally administered, but rimegepant offer benefits in terms of efficacy compared to lower doses of the comparators, and safety benefits compared to higher doses of the comparators.
5. Conclusions
All active comparators were more effective compared to placebo, but placebo had fewer adverse events in general. Active comparators were comparable, but rimegepant was more efficacious in giving subjects sustained freedom from pain compared to lower doses of lasmiditan and ubrogepant. Subjects experienced increased dizziness with higher doses of lasmiditan. Rimegepant 75 mg ODT may provide efficacy benefits versus lower doses of comparators and safety benefits versus higher doses of active comparators for the acute treatment of migraine.
6. Clinical implications
The relative efficacy of newer acute treatments for migraine is uncertain because most randomized trials compare these interventions to placebo.
In the absence of direct comparison trials, randomized placebo-controlled trials of rimegepant, ubrogepant, and lasmiditan were included in a network meta-analysis to indirectly compare the relative efficacy and safety of these interventions for the acute treatment of migraine.
All active comparators were superior to placebo with regard to pain freedom and pain relief 2 to 24 hours post-dose.
Rimegepant and all doses of ubrogepant were comparable for 2–24 hours pain relief, but rimegepant was superior to the lower doses of lasmiditan and ubrogepant for 2-to-24-hour pain freedom.
Rimegepant 75 mg ODT showed safety benefits compared to the higher doses of the active comparators, except for nausea.
Article Highlights
Systematic literature review and network meta analysis methods were used to estimate relative efficacy and safety across three novel acute treatments for migraine.
The evidence base comprised a connected network based on five placebo-controlled Phase III trials (Study 303 for rimegepant, ACHIEVE I and II for ubrogepant, and SAMURAI and SPARTIN for lasmiditan.
All treatments had superior efficacy to placebo.
Rimegepant and lasmiditan were associated with similar outcomes, although the greatest efficacy for lasmiditan was observed at higher doses, which were also associated with an increased risk of safety endpoints (specifically, somnolence and dizziness).
Declaration of interest
KJ, EP, AD, and PD are employees of Broadstreet HEOR, which received funds from Biohaven for this work. LH, AT, RC, VC, and GL are employed by and own stock/stock options in Biohaven Pharmaceuticals. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewers disclosure
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Author contribution statement
KJ, EP, AD, PD, LH, and GL contributed significantly to the conception and design of the work, the analysis, interpretation, and drafting of the most recently submitted version. AT, RC, VC, and JM contributed significantly to the conception of the work, the interpretation, and drafting of the most recently submitted version.
All authors agreed to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Supplemental Material
Download Zip (108 KB)Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- American Headache Society. AHS consensus statement: the American headache society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59:1–18.
- Buse D, Fanning K, Reed M, et al. Life with migraine: effects on relationships, career, and finances from the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study. Headache. 2019;59(8):1286–1299.
- Messali A, Sanderson J, Blumenfeld A, et al. Direct and indirect costs of chronic and episodic migraine in the United States: a web-based survey. Headache. 2016;56(2):306–322.
- Raval A, Shah A. National trends in direct health care expenditures among US adults with migraine: 2004 to 2013. J Pain. 2017;18(1):96–107.
- Ford J, Ye W, Nichols R, et al. Treatment patterns and predictors of costs among patients with migraine: evidence from the United States medical expenditure panel survey. J Med Econ. 2017;22(9):849–858.
- Blumefeld A, Varon S, Wilcox T, et al. Disability, HRQoL and resource use among chronic and episodic migraineurs: results from the International Burden of Migraine Study (IBMS). Cephalalgia. 2011;31(3):301–315.
- Bonafede M, Sapra S, Shah N, et al. Direct and Indirect healthcare resource utilization and costs among migraine patients in the United States. Headache. 2018;58(5):700–714.
- Buse D, Rupnow M, Lipton R. Assessing and managing all aspects of migraine: migraine attacks, migraine-related functional impairment, common comorbidities, and quality of life. Mayo Clin Proc. 2009;84(5):422–435.
- Lombard L, Farrar M, Ye W, et al. A global real-world assessment of the impact on health-related quality of life and work productivity of migraine in patients with insufficient versus good esponse to triptan medication. J Headache Pain. 2020;21(1):41.
- Marmura M, Silberstein S, Schwedt T. The acute treatment of migraine in adults: the american headache society evidence assessment of migraine pharmacotherapies. Headache. 2015;55(1):3–20.
- Silberstein S, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults. Report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78(17):1337–1345.
- Mayans L, Walling A. Acute migraine headache: treatment strategies. Am Fam Physician. 2018;97(4):243–251.
- Peters G. Migraine overview and summary of current and merging treatment options. Am J Manag Care. 2019;25(2 Suppl):S23–S34.
- Eli Lilly and Company. REYVOW (lasmiditan) tablets Prescribing Information. Indianapolis, IN.: U.S. Food and Drug Administration; 2019. (Ed.^(Eds).
- Allergan USA. UBRELVY (ubrogepant) tablets Prescribing Information. Madison, NJ.: U.S. Food and Drug Administration; 2019. (Ed.^(Eds).
- Biohaven Pharmaceuticals. NURTEC ODT (rimegepant) orally disintegrating tablets Prescribing Information. New Haven, CT: U.S. Food and Drug Administration; (Ed.^(Eds), 2020.
- Clemow DB, Johnson KW, Hochstetler HM, et al. Lasmiditan mechanism of action - review of a selective 5-HT(1F) agonist. J Headache Pain. 2020;21(1): 71–71.
- Curto M, Capi M, Cipolla F, et al. Ubrogepant for the treatment of migraine. Expert Opin Pharmacother. 2020;21(7):755–759.
- Curto M, Cipolla F, Cisale GY, et al. Profiling lasmiditan as a treatment option for migraine. Expert Opin Pharmacother. 2020;21(2):147–153.
- De Matteis E, Guglielmetti M, Ornello R, et al. Targeting CGRP for migraine treatment: mechanisms, antibodies, small molecules, perspectives. Expert Rev Neurother. 2020;20(6):627–641.
- Do TP, Guo S, Ashina M. Therapeutic novelties in migraine: new drugs, new hope? J Headache Pain. 2019;20(1):37.
- Edvinsson L, Haanes K, Warfvinge K, et al. CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol. 2018;14(6):338–350.
- Tepper D. Gepants. Headache. 2020;60(5):1037–1039.
- Hou M, Xing H, Li C, et al. Short-term efficacy and safety of lasmiditan, a novel 5-HT(1F) receptor agonist, for the acute treatment of migraine: a systematic review and meta-analysis. J Headache Pain. 2020;21(1):66.
- Lipton R, Lombard L, Ruff D, et al. Trajectory of migraine-related disability following long-term treatment with lasmiditan: results of the GLADIATOR study. J Headache Pain. 2020;21(1):20.
- Reuter U, Israel H, Neeb L. The pharmacological profile and clinical prospects of the oral 5-HT1F receptor agonist lasmiditan in the acute treatment of migraine. Ther Adv Neurol Disord. 2015;8(1):46–54.
- ClinicalTrials.gov. BHV3000-303: phase 3, Double-Blind, Randomized, Placebo Controlled, Safety and Efficacy Trial of BHV-3000 (Rimegepant) Orally Disintegrating Tablet (ODT) for the Acute Treatment of Migraine. NCT03461757. (Ed.^(Eds) (2020)
- Croop R, Goadsby P, Stock D, et al. Efficacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: a randomised, phase 3, double-blind, placebo-controlled trial. Lancet. 2019;394(10200):737–745.
- ClinicalTrials.gov. Efficacy, Safety, and Tolerability Study of Oral Ubrogepant in the Acute Treatment of Migraine (ACHIEVE I). NCT02828020. (Ed.^(Eds) (2019)
- Dodick D, Lipton R, Ailani J, et al. Ubrogepant for the Treatment of Migraine. N Engl J Med. 2019;381(23):2230–2241.
- ClinicalTrials.gov. Efficacy, Safety, and Tolerability of Oral Ubrogepant in the Acute Treatment of Migraine (ACHIEVE II). NCT02867709. (Ed.^(Eds) (2019)
- Lipton R, Dodick D, Ailani J, et al. Effect of ubrogepant vs placebo on pain and the most bothersome associated symptom in the acute treatment of migraine the ACHIEVE II randomized clinical trial. JAMA. 2019;322(19):1887–1898.
- ClinicalTrials.gov. Lasmiditan compared to placebo in the acute treatment of migraine: (SAMURAI). NCT02439320. (Ed.^(Eds) (2019)
- Kuca B, Silberstein S, Wietecha L, et al. Lasmiditan is an effective acute treatment for migraine: a phase 3 randomized study. Neurology. 2018;91(24):e2222–e2232.
- ClinicalTrials.gov. Three doses of Lasmiditan (50 mg, 100 mg and 200 mg) compared to placebo in the acute treatment of migraine (SPARTAN). NCT02605174. (Ed.^(Eds) (2019)
- Goadsby P, Wietecha L, Dennehy E, et al. Phase 3 randomized, placebo-controlled, double-blind study of lasmiditan for acute treatment of migraine. Brain. 2019;142(7):1894–1904.
- Atlas S, Touchette D, Agboola F, et al. Acute Treatments for Migraine: effectiveness and Value. Institute for Clinical and Economic Review, February. (Ed.^(Eds) (2020)
- Hoaglin D, Hawkins N, Jansen J, et al. Conducting indirect-treatment-comparison and Network-Meta-Analysis studies: report of the ISPOR task force on indirect treatment comparisons good research practices: part 2. Value Health. 2011;14(4):429–437.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339(jul21 1):b2535.
- Higgins J, Altman D, Gotzche P, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(oct18 2):d5928.
- Dias S, Welton N, Sutton A, et al. NICE DSU Technical Support Document 2: A Generalised Linear Modelling Framework for Pairwise and Network Meta-Analysis of Randomised Controlled Trials. Unit, DS (Ed.^(Eds) (2011)
- Mbuagbaw L, Rochwerg B, Jaeschke R, et al. Approaches to interpreting and choosing the best treatments in network meta-analyses. Syst Rev. 2017;6(1):79.
- Croop R, Berman G, Kudrow D, et al. Long-Term safety of rimegepant 75 mg for the acute treatment of migraine (Study 201) (4829). Neurology. 2020;94 (15 Supplement).
- Ailani J, Lipton R, Hutchinson S, et al. Long-Term safety evaluation of ubrogepant for the acute treatment of migraine: phase 3, randomized, 52-week extension trial. Headache. 2020;60(1):141–152.
- Jansen J, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and Network Meta-analysis for health-care decision making: report of the ISPOR task force on indirect treatment comparisons good research practices: part 1. Value Health. 2011;14(4):417–428.