ABSTRACT
Background
Ceftazidime-Avibactam (CAZ-AVI) is a new antimicrobial against carbapenem-resistant Klebsiella pneumoniae. The aim of the study is to examine the cost-effectiveness of CAZ-AVI compared to colistin-meropenem (COL+MEM) in Colombia.
Methods
A decision tree model was developed from health-care system perspective assuming a 30-day time horizon. The clinical course was simulated based on treatment response between 48 and 72 hours, and the duration of the treatment was 7–14 days. Cost inputs were extracted from a published Colombian manual tariffs and official databases, expressed in 2019 dollars (USD).
Results
In the base case analysis, CAZ-AVI was associated with reduced mortality, length of hospital stay and fewer add-on antibiotics, resulting in an increase of 1.76 QALYs per patient versus COL+MEM and incremental costs associated in CAZ-AVI were $2,521 higher per patient compared to COL+MEM ($755 versus $3,276). The incremental costs were partially increased due to the lower mortality rate observed with CAZ-AVI. The incremental cost-effectiveness ratio was estimated to be $3,317 per QALY. In the probabilistic sensitivity analysis, with a willingness to pay above $2,438, CAZ-AVI has higher probability of being cost-effective.
Conclusion
CAZ-AVI demonstrates cost-effectiveness as a treatment for Carbapenem-resistant Klepsiella pneumoniae infections by reducing the number of deaths and increasing QALYs.
Expert commentary
Previous studies and surveillance programs from Colombia have reported prevalence of pathogens and the antimicrobial susceptibility of infections caused by multidrug-resistant Gram-negative bacteria. The health authorities have to consider and plan adequate surveillance systems in order to predict the resistance type and in choose the optimal antibiotics when infections occur.
1. Introduction
Carbapenems-resistant Enterobacterales (CRE), mainly Klebsiella pneumoniae, are a serious threat to public health, being associated with high mortality rates and poor clinical outcomes [Citation1]. This may be due to a delay in treatment time, limitations in available treatment options, and the fact that patients with CRE infections tend to be critically ill [Citation2].
The growth in resistance rates of Gram-negative bacteria has limited the benefit in few effective and well-tolerated treatment options [Citation3,Citation4]. Currently, there are new antibiotics that have showed improved pharmacokinetic and clinical efficacy compared to available treatments, they have become as alternatives to manage these bacteria; however, their efficacy is uncertain due to not focused program of development in the drug-resistant pathogens [Citation5,Citation6]. Recently, ceftazidime/avibactam (CAZ-AVI) which has activity against gram-negative bacteria such Enterobacteriacea and this combination with avibactam protects to infective agents producers of Extended spectrum beta-lactamase, K. pnemoniae carbapenemases, and AmpC [Citation7]. It was launched worldwide which demonstrated to be effective and safety in patients with gram-negative infections such as complicated urinary infections [Citation8,Citation9], complicated intra-abdominal infection [Citation10] and hospital associated pneumonia including ventilator-associated pneumonia [Citation11]. For CRE, CAZ-AVI has demonstrated important results through observational studies in terms of clinical and microbiological results [Citation12], and also in reduction of mortality, failure renal, and length of stay compared to colistin [Citation13].
Currently in Colombia colistin, polymyxin, tigecycline, fosfomycin are available, in combination with carbapenems between the most common treatments to tackle CRE infection. However, the polymyxins are associated with high rates of nephrotoxicity, low efficacy, uncertainty on dosing and resistance [Citation14–16]. Recently, CAZ-AVI is accessible for infectologists as alternative for gram negative infection including CRE.
Considering that it is a new treatment option in the country, it reflects the widespread interest in the evidence of economic value of this treatment along with the clinical evidence. Therefore, the aim of the study to assess the cost-effectiveness of CAZ-AVI compared to colistin in combination with meropenem (COL + MEM) in the treatment of Carbapenem-resistant Klepsiella pneumoniae infections.
2. Materials and methods
Clinical management of Carbapenem-resistant Klepsiella pneumoniae infections is complex, especially when the evidence in this population is limited. In order to unify the evidence of economic value of treatments together with clinical efficacy, a simplified decision tree was developed; this was validated with clinicians with extensive clinical experience in the management of these infections and critically ill patients, in this way, ensure that the economic model developed reflects the clinical practice of the country. The structure, temporary horizon and assumptions used were validated, which are detailed below.
2.1. Model structure
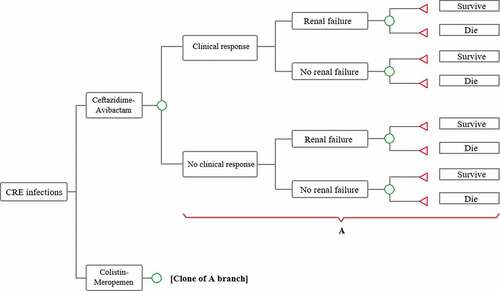
A decision tree was designed in Microsoft Office Excel version 1908. The model was based on data derived mainly from Van Duin et al. [Citation13]. This study met the inclusion criteria as source of the clinical inputs where we compared the studied alternatives and also target population, patients with infection with carbapenem-resistant Klepsiella pneumoniae, and other studies were not considered due to not meeting any criterion. This included the 30-day hospital follow-up with its hospital discharge, mortality rate, the duration of initial therapy, the percentage of patients who develop renal failure, and the likelihood of receiving additional antibiotics.
In the model, patients begin treatment with CAZ-AVI or COL + MEM. Colistin alone was excluded as a monotherapy comparator, since, in the opinion of experts, it does not reflect clinical practice where combination therapies are usually the most appropriate treatment strategies [Citation2]. Therefore, based on the results of the clinical study by Paul et al., it is assumed that the combination therapy, COL + MEM, generates similar clinical outcomes compared to colistin monotherapy [Citation17].
In these models, therapeutic failure is supposed to occur between 48 and 72 hours, which represents an extension of treatment [Citation18]. This failure has been defined as the addition of other antibiotics (tigecycline or fosfomycin). The effect of the added therapy was not included in the model. Patients who respond and do not require a second antibiotic will be discharged according to the probability of occurrence reported by Van Duin et al. [Citation13] and the treatment length will be only 10 days as was observed in the same study. Among those who do not respond, a second antibiotic is added, either after 2–3 days; after this, a new 10-day course of treatment begins. Among other treatment choices against CRE infections in the country, there are polymyxin and tigecycline, which will correspond to the treatment that will be added, both for CAZ-AVI and COL + MEM, in the proportions reported by Van Duin et al. [Citation13]. Renal failure had a 30-day rate as was reported in the observational study ().
2.2. Effectiveness
Effectiveness was expressed as quality-adjusted life years (QALY), where cohort survival is calculated after 30 days of follow-up. Follow the recommendations on the use of QALYs as a result of the economic evaluations carried out in Colombia [Citation19]. The life years saved were calculated by multiplying the percentage of people alive at the end of the follow-up by the life expectancy of this type of patients. The life expectancy reported in the National Administrative Department of Statistics (DANE) [Citation20] was adjusted to relative risk of death for survivors of 0.51, according to a study by Garcia-Hernandez and Mayrhofer [Citation21].
Regarding utilities, for patients who achieved a clinical cure it was assumed to be 0.92, according to a study by Song et al. [Citation22], and the utilities of the non-respondents was 0.61, according to the publication by Delate et al. [Citation23].
2.3. Costs
All costs are reported in dollars (USD) 2019. The exchange rate used was COP$3,282 per dollar [Citation24]. The costs of medications for treatments were obtained from the January to March 2019 reports of the Drug Price Information System (i.e. SISMED). For dose determination, the average weight of 65 kg was taken; the daily dosage was derived for the case of colistin from the study by Van Duin [Citation13]; meropenem, from the study by Paul et al. [Citation17], and for the other antibiotics included CAZ-AVI, those reported in the study by Morrill et al. [Citation2] ().
Table 1. Total treatment costs
The fees in the Mandatory Traffic Accident Service (i.e. SOAT) [Citation25] and Tariff Manual ISS 2001 [Citation26], manuals were used for hospital costs, and includes the monitoring costs and stays in ward and in the Intensive Care Unit (ICU).
The cost associated with the adverse event of treatment-related renal failure was extracted from the published literature [Citation27], and from the Electronic System for Public Procurement (i.e. SECOP) [Citation28]. To update prices, the Consumer Price Index (CPI) reported by the Central Bank was used [Citation29] ().
Table 2. Costs inputs
2.4. Sensitivity analyses
In order to assess the robustness of the study findings, univariate sensitivity analyses were performed to examine the impact of the key variables on the incremental cost-effectiveness ratio (ICER). The data of the utilities, costs, proportion of patients who developed renal failure, treatment length and failure, and dose of colistin were the variables selected for this analysis.
The probabilistic sensitivity analysis (PSA) was carried out assuming distributions for the model parameters. For clinical parameters and utilities, it was assumed that they followed Beta or Uniform distributions and, in terms of cost parameters, Gamma distribution. The results of this analysis are presented in the acceptability curve.
3. Results
3.1. Base-case scenario
For the treatment of carbapenem-resistant Klepsiella pneumoniae infections at a follow-up time of 30 days, CAZ-AVI ($8,284) incurred an incremental cost per patient of $3,317 over COL + MEM ($4,966). The general difference in costs is mainly due to the cost associated with the treatment. However, the main cost driver for both groups was hospitalization, representing 59.92% for CAZ-AVI and 82.53% for COL + MEM of the total cost, where the percentage of people discharged is higher in the case of CAZ-AVI.
Despite the higher expected cost, CAZ-AVI showed to reduce mortality and, therefore, obtain better results in effectiveness compared to COL + MEM. In a hypothetical cohort of 1,000 patients, 92 deaths are estimated in the case of CAZ-AVI and 320 in the case of COL + MEM. Hence, it is estimated that CAZ-AVI generates an additional 1.76 QALY compared to COL + MEM. The model suggests that CAZ-AVI is associated with an ICER of USD $2,584/QALY ().
Table 3. Events per 1,000 patients at the 30 days of follow-up
Table 4. Total cost per 1,000 patients at the 30 days of follow-up
3.2. Univariate sensibility analyses
This analysis indicated that the ICER was not sensitive to changes of the variables. The variables' treatment length, utility of patients with clinical cure, and life expectancy are those that generate the greatest variability in the results. When all model parameters were changed, the ICER was in the range of USD $1,817–3,380/QALY, demonstrating that CAZ-AVI is a cost-effective strategy in all the scenarios of these analyses.
3.3. Probabilistic sensitivity analysis
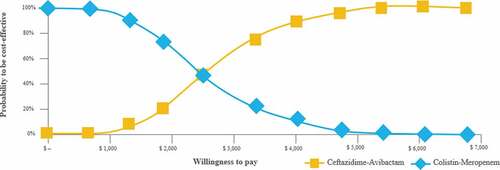
This analysis showed that CAZ-AVI is more likely to be a cost-effective strategy when the threshold is higher than USD $2,438 per QALY. When these two alternatives are compared at a threshold of one GDP per capita (USD $6,030) per QALY, CAZ-AVI would have a 99% chance of being cost-effective ().
4. Discussion
This is the first economic study conducted in the country related to the treatment of carbapenem-resistant Klebsiella pneumoniae infections with CAZ-AVI. Both in the baseline scenario and in the sensitivity analysis, CAZ-AVI was shown as a cost-effective strategy below the thresholds usually used in Colombia, from one to three times the GDP per capita.
Given the specific characteristics of Colombian healthcare system, which is based on public health insurance, where the Health Maintenance Organizations (HMOs) organize and manage the provision of the medical interventions and medicines. The results from this analysis could be used to provide information to set priorities or allocate resources.
Jointly to these results, it is necessary to strength strategies such as antimicrobial stewardship program to reduce the antimicrobial resistant but also to optimize the use of antibiotics. In Colombia, the implementation of these strategies have demonstrated reduction in the use of antibiotics such as vancomycin, third generation of cephalosporins, aminoglycosides, and carbapenems, and also decrease of rate of health care associated infection [Citation30,Citation31].
The model reflects that management with CAZ-AVI provides a better opportunity for clinical success by decreasing the percentage of people who require additional antibiotics, reducing the number of deaths, and increasing the number of QALY compared to COL + MEM. Despite the clinical benefits of CAZ-AVI treatment, no savings are generated in the management of patients, both in treatment and hospital management. The first is due to the difference in treatment prices and the second, due to number of people who life during hospital stay. Since the mortality rate is higher in the case of COL + MEM, the use of resources in these patients is lower; in the base case, the absolute difference in the mortality rate was 23%. In this analysis, with perspective and the incorporation of just direct medical costs, the patients who survived the infection stayed in hospital longer and had higher costs. Different finding of an observational study, which showed that the costs of deceased people tend to be higher than the costs of the survivors [Citation32].
Adopt a broader, social perspective that captures death-related costs and the inclusion of other social service costs, as productivity, emotional, and behavioral disorders related with the loss of a family member or friend and long-term support. Given the difference in mortality among comparators, it could infer that the COL + MEM strategy will require additional financial resources at short term compared to CAZ-AVI.
Relative to difference in terms of effectiveness and safety between colistin and polymyxin B there is a discussion, mainly due to the process pharmacokinetic process to obtain colistin from colistin methanesulfonate [Citation33]. From this uncertainty, a systematic review which compared both treatments and found that there is no statistical difference in mortality at 30 days or in-hospital. Conversely, colistin was associated to present more cases of nephrotoxicity [Citation34]. Considering this evidence available and the sensibility analysis, in which the results in the ICER were not sensitive to frequency of renal failure; therefore, it could be extrapolated to polymyxin B.
The model attempted to reflect the relevant outcomes in this type of infections for clinicians and payers, following the clinical practice of the country using the most frequent treatment and dosage for CRE as the COL-MEM. Additionally, the study adheres the recommendations to conduct economic analysis based on the guidelines established by the Health Technology Assessment Institute in Colombia (i.e. IETS) [Citation19].
The clinical variables used in the model are consistent with other studies. Shields et al. reported better results of CAZ-AVI in terms of clinical success (85% vs 40%) and 30-day mortality (8% vs 30%) compared to colistin [Citation12]. Likely, Tumbarello et al. found less mortality with CAZ-AVI compared to other regimens as salvage therapy for CRE infections (36.5% vs 55.8%) [Citation35].
There are several limitations that must be considered when interpreting the results in this study. The results of this analysis will be applicable only to patients with similar characteristics to those of the population included in this study. One of these is the lack of randomized trials that reflect the efficacy of the comparative treatments, given that weakness, the data of this analysis were based on an observational study which has limitations on the design and nature of study. It is a limitation of the new antibiotics whereby clinical trials are not available in populations with multiresistant infections [Citation6]
Another limitation is related to the long-term quality of life and survival that were extrapolated from the literature, whose extrapolation goes beyond the 30-day temporary horizon, which is the study follow-up from which efficacy and safety information was obtained. However, this was considered acceptable by clinical experts given the limited information in the Colombian population, in addition to being widely used in other economic studies with the same information limitation.
Having made these caveats, it is highlighted that the results found in this study are not far from what was found in another economic evaluation for this type of patients, where CAZ-AVI is found as a cost-effective strategy taking into account the thresholds established in the United States [Citation36].
The increase in resistance is a reality [Citation37,Citation38], given the limited treatment options, there is a need for new antibiotics in the country; in this case, CAZ-AVI is presented as a therapeutic option for hospitalized patients with carbapenem-resistant Klepsiella pneumoniae infections, which leads to good clinical results and decreased antibiotic use, showing itself as a cost-effective alternative compared to conventional therapy (COL + MEM).
5. Conclusion
The cost-effectiveness model suggests that CAZ-AVI demonstrates an increase in QALYs and reduction in the cases of deaths, renal failure, and treatment failure, resulting in a cost-effective treatment for carbapenem-resistant Klebsiella pneumoniae infections compared to colistin-meropenem.
Declaration of interest
Elkin Lemos, Natalia Castaño and Juan Manuel Reyes are paid employees of Pfizer. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Author contributions
Juan M Reyes contributed towards the literature review, data analysis, data management, and manuscript development. Fabio Varón-Vega contributed towards the data analysis, discussion and manuscript development. Elkin Lemos contributed towards the literature review, data analysis, data management and manuscript development. Natalia Castaño contributed towards the literature review, data analysis, data management, and manuscript development. All authors read and approved the final manuscript.
Additional information
Funding
References
- National Center for Emerging and Zoonotic Infectious Diseases/Centers for Disease Control and Prevention (CDC). Facility guidance for control of carbapenem-resistant enterobacteriaceae (CRE). 2015.
- Morrill HJ, Pogue JM, Kaye KS, et al. Treatment options for carbapenem-resistant enterobacteriaceae infections. Open Forum Infect Dis. 2015;2(2):ofv050. .
- Kanj SS, Kanafani ZA. Current concepts in antimicrobial therapy against resistant gram-negative organisms: extended-spectrum β-lactamase–producing enterobacteriaceae, carbapenem-resistant enterobacteriaceae, and multidrug-resistant pseudomonas aeruginosa. Mayo Clin Proc. 2011 Mar;86(3):250–259.
- Raman G, Avendano E, Berger S, et al. Appropriate initial antibiotic therapy in hospitalized patients with gram-negative infections: systematic review and meta-analysis. BMC Infect Dis. 2015;15(1):395. .
- Karaiskos I, Lagou S, Pontikis K, et al. The “old” and the “new” antibiotics for MDR gram-negative pathogens: for whom, when, and how. Front Public Health. 2019;7:151.
- Karaiskos I, Galani I, Souli M, et al. β-lactam-β-lactamase inhibitor combinations: expectations for the treatment of carbapenem-resistant Gram-negative pathogens. Expert Opin Drug Metab Toxicol. 2019;15(2):133–149.
- de Souza Mendes C. Pipeline of known chemical classes of antibiotics. Antibiotics 2013;2(4):500–534.
- Carmeli Y, Armstrong J, Laud PJ, et al. Ceftazidime-avibactam or best available therapy in patients with ceftazidime-resistant Enterobacteriaceae and Pseudomonas aeruginosa complicated urinary tract infections or complicated intra-abdominal infections (REPRISE): a randomised, pathogen-directed. Lancet Infect Dis. 2016 Jun;16(6):661–673. .
- Wagenlehner FM, Sobel JD, Newell P, et al. Ceftazidime-avibactam versus doripenem for the treatment of complicated urinary tract infections, including acute pyelonephritis: RECAPTURE, a phase 3 randomized trial program. Clin Infect Dis. 2016 Sep;63(6):754–762. .
- Mazuski JE, Gasink LB, Armstrong J, et al. Efficacy and safety of ceftazidime-avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infection: results from a randomized, controlled, double-blind, phase 3 program. Clin Infect Dis. 2016;62(11):1380–1389. .
- Torres A, Zhong N, Pachl J, et al. Ceftazidime-avibactam versus meropenem in nosocomial pneumonia, including ventilator-associated pneumonia (REPROVE): a randomised, double-blind, phase 3 non-inferiority trial. Lancet Infect Dis. 2018 Mar;18(3):285–295. .
- Shields RK, Potoski BA, Haidar G, et al. Clinical outcomes, drug toxicity, and emergence of ceftazidime-avibactam resistance among patients treated for carbapenem-resistant enterobacteriaceae infections. Clin Infect Dis. 2016 Dec;63(12):1615–1618.
- van Duin D, Lok JJ, Earley M, et al. Colistin versus ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant enterobacteriaceae. Clin Infect Dis. 2018;66(2):163–171. .
- Yao X, Doi Y, Zeng L, et al. Carbapenem-resistant and colistin-resistant Escherichia coli co-producing NDM-9 and MCR-1. Lancet Infect Dis. 2016 Mar;16(3):288–289.
- Liu -Y-Y, Wang Y, Walsh TR, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016 Feb;16(2):161–168.
- Rojas LJ, Salim M, Cober E, et al. Colistin resistance in carbapenem-resistant klebsiella pneumoniae: laboratory detection and impact on mortality. Clin Infect Dis. 2017;64(6):711–718.
- Paul M, Daikos GL, Durante-Mangoni E, et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis. 2018 Apr;18(4):391–400.
- Bassetti M, Montero JG, Paiva JA. When antibiotic treatment fails. Intensive Care Med. 2018;44(1):73–75. Available from: http://link.springer.com/10.1007/s00134-017-4962-2.
- Colombian Health Technology Assessment Institute. Guidelines for the economic evaluation of healthcare technologies in Colombia. Bogotá D.C.: IETS; 2014 [ cited 2019 Mar 22]. Available from: http://www.iets.org.co/manuales/Manuales/Manual%20Evaluacion%20economica.pdf.
- DANE. Projection of the Colombian census 2005–2020; 2007 [cited 2018 Apr 16]. 225. Available from: https://www.dane.gov.co/files/investigaciones/poblacion/proyepobla06_20/8Tablasvida1985_2020.pdf.
- Garcia-Hernandez A, Mayrhofer T. Quality-of-life-adjusted hazard of death: a formulation of the quality-adjusted life-years model of use in benefit-risk assessment. Value Health. 2014 Mar 1;17(2):275–279.
- Song Y, Tai JHY, Bartsch SM, et al. The potential economic value of a Staphylococcus aureus vaccine among hemodialysis patients. Vaccine. 2012 May 21;30(24):3675–3682. Available from: https://www.sciencedirect.com/science/article/pii/S0264410X12004070?via%3Dihub.
- Delate T, Coons SJ. The use of 2 health-related quality-of-life measures in a sample of persons infected with human immunodeficiency virus. Clin Infect Dis. 2001 Feb 1;32(3):e47–52. Available from: https://academic.oup.com/cid/article-lookup/doi/10.1086/318492.
- Central Bank of Colombia. Market representative exchange rate [Internet]; 2019. [cited 2018 Apr 19]. Available from: http://www.banrep.gov.co/es/-estadisticas.
- National Social Security Council for Health. SOAT; 2019. Tariff Manual. 2019.
- Colombian Social Security Institute. Resolution 1896 de 2001. Tariff manual ISS; 2001. Available from: https://lexsaludcolombia.files.wordpress.com/2010/10/tarifas-iss-2001.pdf.
- Tribiño G, Maldonado C, Segura O, et al. Direct costs and clinical aspects of adverse drug reactions in patients admitted to a level 3 hospital internal medicine ward. redalyc.org; 2006. Available from: https://www.redalyc.org/html/843/84326105/.
- Online Procurement System SECOP II [Internet]; 2019. Available from: https://www.colombiacompra.gov.co/sites/cce_public/files/cce_infografias/aviso_papeleria.pdf.
- Statistical Department of Colombia DANE. Gross domestic product –GDP- per capita. [cited 2018 Apr 19]. Available from: http://www.banrep.gov.co/es/pib.
- Alvarez C, Gómez C, Rodriguez T, et al. Impact of a program for the prudent use of antibiotics in a tertiary care hospital in Bogotá, Colombia, Colombia. Rev Médica Sanitas. 2017;20(2):75–82.
- Pallares CJ, Cataño JC. Impact of the rational use of antimicrobials in a tertiary clinic in Colombia. Rev Chil Infectología. 2017 Jun;34(3):205–211.
- Raitano M, Gabriele S, Granaglia E, et al. The impact of death-related costs on health-care expenditure: a survey [Internet]; 2006 [cited 2019 Jun 5]. Available from: http://aei.pitt.edu/9487/2/9487.pdf.
- Nation RL, Velkov T, Li J. Colistin and polymyxin B: peas in a pod, or chalk and cheese? Clin Infect Dis. 2014 Jul;59(1):88–94.
- Vardakas KZ, Falagas ME. Colistin versus polymyxin B for the treatment of patients with multidrug-resistant Gram-negative infections: a systematic review and meta-analysis. Int J Antimicrob Agents. 2017 Feb;49(2):233–238. .
- Tumbarello M, Trecarichi EM, Corona A, et al. Efficacy of ceftazidime-avibactam salvage therapy in patients with infections caused by klebsiella pneumoniae carbapenemase–producing K. pneumoniae. Clin Infect Dis. 2019 Jan;68(3):355–364. .
- Sfeir M, Satlin M, Calfee DP, et al. Cost-effectiveness of ceftazidime–avibactam compared with colistin for treatment of carbapenem-resistant enterobacteriaceae bacteremia and pneumonia. Open Forum Infect Dis. 2018 Nov 26;5(suppl_1):S539–40. Available from: https://academic.oup.com/ofid/article/5/suppl_1/S539/5207575.
- Office of Health Economics. The bacterial challenge: time to react; 2011 [cited 2018 Jul 17]. Available from: www.ecdc.europa.eu
- Sharma P, Towse A. New drugs to tackle antimicrobial resistance: analysis of EU policy options; 2010 [cited 2018 Jul 17]. Available from: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=2640028.