ABSTRACT
Background
To translate and linguistically validate the Assessment of Quality of Life 8-dimensions (AQoL-8D) for use in the Netherlands and to compare the psychometric properties of AQoL-8D with the EuroQol 5-dimensions 5-levels (EQ-5D-5L) in two patient samples.
Methods
AQoL-8D was translated from English into Dutch. The translated AQoL-8D was then administered alongside the EQ-5D-5L at baseline and follow-up of two Dutch randomized controlled trials among patients with epilepsy and schizophrenia. These data were subjected to a post-hoc analysis assessing the psychometric properties of AQol-8D vis-à-vis EQ-5D-5L in terms of known-groups construct validity, responsiveness, and floor/ceiling effects.
Results
In total, 103 epilepsy patients and 99 schizophrenia patients were included in this study. In both datasets, the two instruments discriminated between known-groups, but in schizophrenia, AQoL-8D showed higher responsiveness than EQ-5D-5L, while both instruments showed equal responsiveness in epilepsy. Ceiling effects were only found for EQ-5D-5L in both epilepsy (26.6%) and schizophrenia (6.1%).
Conclusion
Our results have shown that, among other things, AQoL-8D presents better ability to discriminate between known-groups and shows no ceiling effect. Based on our results, we would recommend the use of AQoL-8D in addition to EQ-5D-5L in trials assessing patient’s quality of life in patients with epilepsy or schizophrenia.
1. Introduction
The quality-adjusted life year (QALY) is a generic, not a disease-specific, outcome measure, which is often used in health-economic evaluations as it permits the comparison of the effects of interventions across diseases, including mental ill-health [Citation1]. The QALY is generally computed using preference-based instruments that describe health states and weight these health states by the time spent in that state. These preference-based measurement instruments are referred to as health-related patient-reported outcome measures (PROMs) or utility instruments [Citation2–4].
The EuroQoL 5-dimension (EQ-5D) is one of the most widely used utility instruments as a result of its brevity, simplicity, and user-friendliness [Citation5,Citation6]. However, previous studies have shown that these characteristics also act as its major drawback, especially in populations with more complex changes in well-being, such as patients with brain diseases that highly impact their mental health (i.e. schizophrenia and epilepsy) [Citation4,Citation7–12]. In addition, both epilepsy and schizophrenia have major impact on daily life and tend to run a chronic course. The previous 5-dimension and 3-level version of the EuroQoL (EQ-5D-3L) could describe 35 = 243 health states, which seemed to limit its ability to fully discriminate different levels among individuals, resulting in lower sensitivity to detect important clinical changes over time when compared with other utility instruments [Citation13]. In addition, studies have reported a ceiling effect in both general population and patient samples [Citation14,Citation15]. To increase its sensitivity, the EuroQoL group introduced a new five-level version (EQ-5D-5L), which describes more health states (55 = 3125), resulting in improved sensitivity and a greater power to discriminate between healthy and ill populations and reduced ceiling effects [Citation16,Citation17].
In complex mental health disorders that affect people in multiple ways, such as schizophrenia, studies have suggested that a single utility instrument is insufficient to capture the complete picture of the condition and treatment effects [Citation18–20]. In addition, Mulhern et al. [Citation10] suggested that the EQ-5D-3L is only related to some condition-specific domains of schizophrenia (e.g. blunted effects stemming from negative symptoms), rather than for the whole range of domains (e.g. social withdrawal, delusional thinking, and disorganized speech).
In epilepsy, both seizures and antiepileptic drugs impact patient’s HRQoL in multiple ways (e.g. mental health, cognitive, and social functioning) [Citation10]. It has been argued that the EQ-5D-3L may not capture all the HRQoL dimensions relevant to patients with epilepsyin particular, dimensions relevant to mental health [Citation21,Citation22]. In addition, lower responsiveness and high ceiling effect from the EQ-5D-5L were shown in a study with patients with epilepsy [Citation23].
Brazier [Citation4] argued that generic instruments may be inadequate for certain mental health conditions, therefore suggesting a need for utility instruments that are more tailored to mental health. Albeit still a generic instrument, a recent study by Campbell et al. recommended the use of the Assessment of Quality of Life 8 Dimension (AQoL-8D) over EQ-5D-5L given that the psychosocial aspects of patients were assumed to be captured more thoroughly by the AQoL-8D [Citation24]. The AQoL-8D has been developed by the Centre for Health Economics, Monash University (Melbourne) [Citation25–27]. AQoL-8D consists of eight domains (independent living, happiness, mental health, coping, relationships, self-worth, pain, and senses) assessed by 35 items. Five out of eight dimensions of the AQoL-8D encompass psychosocial domains of health. Hence, it may be reasonable to assume that the AQoL-8D is more sensitive to conditions where psychosocial aspects play a more prominent role in patient’s HRQoL [Citation24]. The AQoL-8D was designed with a clear focus on the use in economic evaluations and, more specifically, to improve the sensitivity to change in the psychosocial dimensions, which was lacking in previous utility instruments [Citation28].
The AQoL-8D has recently been translated into Dutch and was administered alongside the EQ-5D-5L into two randomized controlled trials (RCTs); 1) a study investigating the effects of a multi-component self-management intervention for adults with epilepsy (ZMILE-study) [Citation29]; and 2) a study examining the effects of cognitive behavior therapy focusing on social activation in adults with recent onset schizophrenia (SOFIA-study) [Citation30,Citation31]. Those patient-reported outcome data allowed us to address both aims of the current study:
To linguistically validate the newly translated Dutch version of the AQoL-8D for use in the Netherlands.
To compare the psychometric properties (i.e. discriminative validity and responsiveness) between the AQoL-8D and EQ-5D-5L in patients with epilepsy and schizophrenia.
2. Methods
2.1. Patients and setting
2.1.1. Multi-component self-management intervention in epilepsy (ZMILE-trial)
Both EQ-5D-5L and AQoL-8D were administered to patients in two randomized controlled trials in the Netherlands.
The epilepsy trial compared care as usual (CAU) with a multi-component intervention comprising a self-management program and e-health interventions [Citation29]. Questionnaires including EQ-5D-5L and AQoL-8D were administered at baseline, 3, 6, 9, and 12-month follow-up (FU). A total of 102 eligible patients with epilepsy were included, of which 52 and 50 were randomly allocated to the intervention or the control condition, respectively. For more information, see Supplementary Material Table S1.
2.1.2 Cognitive behavioural therapy in schizophrenia (SOFIA-trial)
The schizophrenia trial compared cognitive behavior therapy focusing on social activation (CBTsa) to CAU in patients with recent onset schizophrenia. Questionnaires were administered at baseline, directly after the intervention, and 6 months post-treatment. A total of 99 patients with schizophrenia were included, of which 49 and 50 were randomly allocated to CBTsa or the CAU condition, respectively. See Supplementary Material Table S2.
The schizophrenia trial was approved by the ethics committee of the Academic Medical Center of Amsterdam University Medical Center, The Netherlands. The ZMILE trial was approved by the Ethics Committee of Maastricht University/University Hospital Maastricht, The Netherlands.
2.2. Utility instruments
Both EQ-5D-5L and AQoL-8D are generic quality of life instruments with multiple domains. The EQ-5D-5L covers five domains of health (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression), each of which consists of one question with five possible response levels ranging from (1) ‘no problem’ to (5) ‘extreme problems.’ This results in a total of 3125 possible health states, and it takes about one minute to complete [Citation24,Citation32]. The EQ-5D-5L is paired with a visual analog scale (VAS), which assesses patient’s self-rated health on a scale ranging from 0 (‘worst imaginable health’) to 100 (‘perfect health’).
The AQoL-8D consists of eight dimensions measured by 35 items with four to six levels each. Five out of eight dimensions assess the psychosocial domains of health: happiness, coping, self-worth, relationships, and mental health. Consequently, the AQoL-8D describes billions of possible health states and it takes 5 minutes to complete [Citation33].
Value sets for the EQ-5D-5L are generated using the time trade-off (TTO) valuation technique, for example, to create the Dutch EQ-5D-5L tariff [Citation34]. Based on this value set, the descriptive health states can be converted into a single summary index score (utilities) ranging from −0.446 (worse than death) to 1.00 (perfect health) [Citation35]. For the generation of individual utility values from AQoL-8D, the Australian utility algorithm is available on the AQoL group’s website (www.aqol.com.au), which was constructed using the best-worst scaling methodology [Citation27].
2.3. Translation of AQoL-8D
The translation of AQoL-8D was a collaborative project between Maastricht University and Trimbos Institute, the Netherlands institute for mental health and addiction. The original Australian-English version was translated in three steps. First, an independent professional translator performed the forward translation. Second, a backward translation was performed by another independent professional translator and was compared with the original. Finally, differences between the translations were solved in a consensus meeting with experts (n = 2). The final version of the Dutch AQoL-8D is given in Supplementary Material S3.
2.4. Data analysis
The COSMIN (Consensus-based Standards for the selection of Health Measurement Instruments) guidelines [Citation36] for evaluating methodological quality of utility instruments were consulted for definitions and recommendations. Following those guidelines, a selection of psychometric properties was made (i.e. construct validity, responsiveness, and floor/ceiling effect). Furthermore, EQ-5D-5L was evaluated separately for its multi-attribute and VAS-components.
2.4.1. Descriptive statistics
First, mean and median utility scores of the EQ-5D-5L (utility), EQ-5D-5L (VAS) and the AQoL-8D were obtained from the epilepsy and schizophrenia studies along with their standard deviation (SD) and interquartile range (IQR), as well as their observed minimum and maximum scores.
2.4.2. Floor/ceiling effects
Second, the percentage of respondents with the lowest possible utility score (% on-floor) and the highest possible utility score (% on-ceiling) was obtained to evaluate floor and ceiling effects. These effects may occur when a relatively large proportion of respondents score the lowest or highest possible scores. This phenomenon may potentially affect the instruments’ ability to capture extreme data and changes over time. The high floor/ceiling effect indicates that an instrument is unable to distinguish changes in patient’s scores at or near the lower/upper limit, reducing variability in data [Citation37].
2.4.3. Responsiveness
Next, responsiveness is the sensitivity of an instrument to detect clinically relevant changes over time [Citation38]. We computed the effect size (ES) and the standardized response mean (SRM) to assess the responsiveness of the EQ-5D-5L compared with AQoL-8D in patients with epilepsy and schizophrenia. The estimates of responsiveness were measured at baseline and 6-month FU for epilepsy (since the 12-month FU was only available for the intervention group) and at baseline and 9-month FU for schizophrenia.
The effect size (ES) was calculated using Cohen’s equation, by taking the average difference between the means of before and after treatment and subsequently dividing it by the pooled standard deviation (SD) of both measurements. This magnitude of change is categorized as small (0.2), medium (0.5), or large (0.8 or more), where the large effect size indicates a higher sensitivity to change [Citation39].
SRM is calculated as the average mean difference divided by the SD of the differences between the paired measurements. Interpretation of the SRM is similar to Cohen’s d, where a positive SRM suggests an increase in HRQoL. However, SRM values are generally lower than the corresponding ES values [Citation40] and used to evaluate which instrument has a greater sensitivity to detect clinically relevant changes.
2.4.4. Agreement between EQ-5D-5L and AQoL-8D
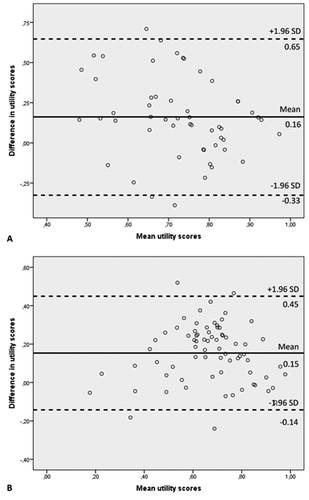
The level of agreement between the utility scores of EQ-5D-5L and AQoL-8D was assessed using the Bland-Altman plot [Citation41]. This technique allows the visualization of the relationship between two quantitative measures scored on the same scale. We plotted the distribution of mean values of the EQ-5D-5L and the AQoL-8D on the X-axis against the difference (i.e. AQoL-8D minus EQ-5D-5L) on the Y-axis and assessed the number of dots outside the 95% limits of agreement (±2 SD of the difference from the mean) [Citation10,Citation42,Citation43]. If one measure consistently reports results above or below the mean of the two measures combined, then all points will lie above or below the zero line, which represents the mean. As such, the Bland-Altman plot may reveal possible overestimation of high values and underestimation of low values of one method compared to the other. When the points are scattered all over the plot, evenly above and below the zero line, then it can be assumed that there is no systematic bias of one measure compared to the other (i.e. such differences are likely to follow a normal distribution).
2.4.5. Construct validity
Construct validity is ‘the degree to which the scores of a HR-PRO (health-related patient-reported outcome) instrument are consistent with hypotheses based on the assumption that the HR-PRO instrument validly measures the construct to be measured,’ as defined in the COSMIN checklist [Citation38]. In theory, a perfect validation process can only be achieved when outcomes of the examined instruments are compared with a ‘gold standard.’ However, there is no ‘gold standard’ for an abstract construct such as the utility score [Citation44]. Therefore, we used the ‘known-groups’ method to assess the validity of the utility instruments by hypotheses testing. This allowed us to examine whether the instruments (EQ-5D-5L [utility], EQ-5D-5L [VAS] and AQoL-8D) have the ability to discriminate between pre-specified clinical groups. As these groups are expected to differ due to known characteristics based on theory or prior research [Citation44–48].
For epilepsy, clinical different groups were determined based on A) age of the patients, divided into two age categories (patients aged between 18 and 40 years and patients aged 41 years and older; using the mid-point of the age categories as split); B) patient’s HADS depression and anxiety scores, where ‘no case’ and ‘possible case’ were categorized based on the median split of HADS scores; C) number of antiepileptic drugs (AEDs) that patients were taking, where ‘low’ and ‘high’ groups were also based on the median split of numbers (median = 4 AEDs; range: 0–14 AEDs); and D) number of seizures four weeks prior to baseline, where ‘low’ and ‘high’ numbers were again based on the median split of numbers (median = 1 seizure; range: 0–60 seizures). Patient’s age, HADS scores, number of AEDs prescribed, and number of seizures were measured using the demographic questionnaire, the Hospital Anxiety Depression Scale (HADS) [Citation49], the iMTA Medical Consumption Questionnaire (iMTA-MCQ) [Citation50], and the National Hospital Seizure Severity Scale (NHS3), respectively [Citation51].
For schizophrenia, clinical different groups were determined based on A) age of the patients, divided into two categories: 1) patients aged between 18 and 24 years and 2) patients aged between 25 and 44 years (using the midpoint of the age categories as split); B) whether patients were taking anti-psychotic medications, where groups were defined as simply ‘yes’ and ‘no’; and C) negative, positive, and general Positive and Negative Syndrome Scale (PANNS) score, where ‘low’ and ‘high’ score were based on the median split PANNS score.
In epilepsy, it was hypothesized that 1) patients in younger age groups will show lower QoL; 2) patients suffering from epilepsy-related depression and anxiety will show lower QoL; 3) patients taking a high number of prescribed AEDs will show lower QoL; and 4) patients with a high number of seizures will show lower QoL. In schizophrenia, it was hypothesized that 1) patients in younger age groups will show lower QoL; 2) patients taking antipsychotic medications will show lower QoL; and 3) patients with higher symptom severity will show lower QoL.
To evaluate the constructed validity by testing the differences between groups, independent sample t-tests were used for the EQ-5D-5L (utility), EQ-5D-5L (VAS), and AQoL-8D scores among the groups of patients who differed in their pre-determined characteristics. Baseline data from both studies were used for this purpose given the completeness at baseline.
For all statistical analyses, p-values < 0.05 were considered to be statistically significant. All analyses were conducted using STATA 13.0 (Stata Corp. LP, Station, TX, USA) and SPSS 23 (IBM Corp. SPSS Statistics, Armonk, NY, USA).
3. Results
The AQoL-8D was translated to Dutch and back-translated by two different professional translators. There were no major discrepancies, and the minor ones were solved by an expert panel (see Supplementary 3 for the resulting questionnaire).
3.1. Descriptive statistics
Baseline characteristics of both studies are shown in Tables S1 and S2. In , the mean scores (SD; minimum and maximum scores achieved) and floor/ceiling ratios are presented for each instrument (EQ-5D-5L [utility], EQ-5D-5L [VAS], and AQoL-8D) in each trial (epilepsy and schizophrenia). The mean scores found in the epilepsy trial were for EQ-5D-5L (utility): 0.82 (0.16; 0.12–1.0); EQ-5D-5L (VAS): 74.74 (16.11; 30.0–100.0), and AQoL-8D: 0.64 (0.2; 0.24–0.95). From the schizophrenia trial, we found for EQ-5D-5L (utility): 0.75 (0.18; 0.15–1.0); EQ-5D-5L (VAS): 68.21 (17.34; 5.0–100.0), and for AQoL-8D: 0.59 (0.18; 0.2–0.99).
Table 1. Summary statistics of EQ-5D-5L, EQ-VAS, and AQoL-8D utility valuations, percent achieving worst and best health states, effect size (ES), and standardized response mean (SRM) for responsiveness
3.1.1. Ceiling and floor effects
In both epilepsy and schizophrenia trials, ceiling effects were found in EQ-5D-5L, which were 26.3% (EQ-5D-5L [utility]) and 3.9% (EQ-5D-5L [VAS]) in epilepsy and 6.1% (EQ-5D-5L [utility]) and 1.0% (EQ-5D-5L [VAS]) in schizophrenia. No floor/ceiling effect was found in AQoL-8D from either epilepsy or schizophrenia trial.
3.1.2. Responsiveness
As shown in , all ESs are negative, indicating that the effect is larger for the FU scores than for the baseline scores. Although, in epilepsy, AQoL-8D showed the largest ES compared to those from the EQ-5D-5L (utility) and EQ-5D-5L (VAS), the value indicates a small effect size. In schizophrenia, both the EQ-5D-5L (utility) and AQoL-8D showed −0.59, indicating a medium ES, indicating that there is a relatively noticeable difference between the two instruments. The SRM scores presented the same magnitudes of changes as the ES.
3.1.3. Agreement between EQ-5D-5L and AQoL-8D
,b) show the Bland-Altman plots comparing EQ-5D-5L and AQoL-8D utility scores for the epilepsy and schizophrenia trials, respectively. The line in the middle, also called the zero line, indicates the mean differences, which were 0.16 for epilepsy and 0.15 for schizophrenia. The two outer lines are the limits of agreement, which are the bounds at ±1.96 SD of the difference away from the mean. These are −0.33 and 0.65 for epilepsy and −0.14 and 0.45 for schizophrenia. Both Bland-Altman plots show a rather equal distribution of points above and below the zero line, which suggests that there is no consistent bias of one instrument versus the other.
3.1.4. Construct validity
present the t-test results for assessing the construct validity. The tests were performed on the EQ-5D-5L (utility), EQ-5D-5L (VAS), and AQoL-8D baseline utility scores from the epilepsy and schizophrenia trials, respectively. For epilepsy, , statistically significant differences were found between ‘HADS cases’ groups for all instruments. For the ‘number of AEDs’ groups, statistical significance was only found in EQ-5D-5L (utility) at baseline. Furthermore, no other statistically significant difference was found between the groups of ‘age categories’ and ‘number of seizures.’ This was not in line with our hypothesis.
Table 2. Construct validity for clinical groups in the epilepsy study
Table 3. Construct validity for clinical groups in the schizophrenia study
As for schizophrenia, , statistically significant differences were found between ‘PANNS positive’ groups in AQoL-8D and between ‘PANNS general’ groups in both EQ-5D-5L (utility) and in AQoL-8D. No other statistically significant difference was found between the ‘age categories’ groups, ‘use of AP medication’ groups, and in the ‘PANNS negative’ groups. This is in line with our hypothesis, indicating that higher symptom severity is correlated with lower QoL.
4. Discussion
This study provides a translated and linguistically validated Dutch version of the AQoL-8D in order for it to be used clinical practice and health-economic evaluations.
Moreover, this study compared two utility instruments (EQ-5D-5L and AQoL-8D) for the assessment of preference-based HRQoL in patients with epilepsy and in patients with schizophrenia. The results of our study suggest that AQoL-8D is more responsive (i.e. not suffering from a ceiling effect and higher ES and SRM when measuring change over time) than the EQ-5D-5L, especially in epilepsy. When looking at their discriminative ability, AQoL-8D performed slightly better in schizophrenia and the EQ-5D-5L slightly better in epilepsy. Although the distribution of means was in line with our expectations, not all between groups differences were statistically significant.
Next to the statistically significant difference found between ‘the number of AEDs’ groups by the EQ-5D-5L in epilepsy, further statistically significant differences were found in psychosocial comparisons only, both AQol-8D and EQ-5D-5L (utility and VAS) showed statistically significant difference between the ‘HADS’ groups in epilepsy. In schizophrenia, AQoL-8D showed statistically significant differences in two out of the three PANNS categories and EQ-5D-5L in one of the three categories only. This seems to be in line with what we previously mentioned that AQoL-8D was mainly designed to increase sensitivity to the psychosocial elements of health.
The medium pre-post effect sizes of EQ-5D-5L (utility) and AQoL-8D from the schizophrenia trial indicate that both measures are responsive at detecting changes over time, whereas effect size values from the epilepsy trial indicate that AQoL-8D has a greater responsiveness than the EQ-5D-5L, even though both are considered small effect sizes. Moreover, EQ-5D-5L showed ceiling effects in both studies, while AQoL-8D did not show any ceiling or floor effects. In a study of Wijnen et al. [Citation23], who compared the EQ-5D-5L with a condition-specific epilepsy instrument (i.e. in patients with epilepsy), the EQ-5D-5L also expressed higher ceiling effects than its comparator, which is in line with our findings [Citation23]. This indicates the potential of EQ-5D-5L to be less sensitive to changes over time (especially in the extreme ends of the scale) compared to a condition-specific instrument (i.e. see Wijnen et al.) but also compared to another generic quality of life instrument (i.e. AQoL-8D). Albeit previous studies have shown lower ceiling effects in EQ-5D-5L compared with EQ-5D-3L [Citation52,Citation53], our study results suggest that high ceiling effects remain a limitation of the EQ-5D instrument, especially in epilepsy and to a smaller extent in schizophrenia. This suggests that EQ-5D-5L and AQoL-8D could not be regarded as interchangeable, neither in epilepsy nor in schizophrenia.
However, looking at the Bland-Altman plots, agreement was high between both EQ-5D-5L and AQoL-8D [Citation33,Citation54–56], indicating that each instrument rated patients in the same direction (i.e. higher scores on the EQ-5D-5L indicated higher scores on the AQoL-8D and vice versa). This emphasizes that, although both instruments have different psychometric properties, they do tend to measure the concept of health-related quality of life in the same way.
Our findings may support the idea that AQoL-8D is slightly more adequate than EQ-5D-5L in measuring HRQoL in mental disorders such as schizophrenia. Previous studies already demonstrated the robustness, general sensitivity, and applicability of the AQoL-8D in the general population [Citation57]. Although the AQoL-8D performed slightly better on several aspects, mainly regarding responsiveness, it did not necessarily outclass the EQ-5D-5L in all aspects. Previous studies, not necessarily focusing on psychosocial problems, demonstrated similar results. For example, Hawthorne et al. [Citation26] concluded that there was some evidence indicating that the AQoL-8D has greater sensitivity to a change in health states than other utility instruments. However, it was also stated that no single utility instrument can claim to be the ‘gold standard’ [Citation26]. A study in elderly demonstrated that the AQoL-8D appeared to have more favorable construct validity compared to the EQ-5D-3L, but the EQ-5D-3L was easier to administer, had a higher completion rate, and appeared more sensitive to change [Citation58]. This supports the idea that researchers should select an instrument sensitive to the health states they are investigating [Citation26], and that the AQoL-8D appears to be superior in terms of responsiveness, in particular in the psychosocial domains. Hence, in the absence of clear guidance, we recommend using both. However, it is important to emphasize that, when measuring HRQoL, a trade-off between comprehensiveness versus brevity is a serious clinical challenge. For example, the AQoL-8D is lengthier, which results in a wider distribution of possible scores at the costs of increased patient burden. Hence, especially in vulnerable patient groups, one may still consider using the EQ-5D-5L only for reasons of feasibility.
The findings of this study must be viewed in light of the following limitations. First, we were unable to assess the content validity, criterion validity, and test-retest reliability due to limitations of our data. Thus, our evaluation of the comparative validity of AQoL-8D vis-à-vis EQ-5D-5L does not cover the full spectrum of validity assessments. We do believe, however, that the most important topics have been covered. Second, it was not possible to make comparisons to disease-specific quality of life instruments (e.g. the Quality of Life in Epilepsy instrument; QOLIE-31p [Citation59]). Such a comparison may have contributed to the interpretation of our results as it has previously been shown that condition-specific instruments are more sensitive to change [Citation23]. Third, both clinical trials were not designed to detect statistically significant differences between utilities as they were powered on different primary clinical outcomes, let alone subgroup analyses. Hence, it is reasonable to assume that we did not have sufficient power to detect differences between different clinical groups. Fourth, clinical groups were arbitrarily constructed based on expert opinion. Hence, it was assumed that these groups would differ but were not scientifically proven beforehand. Finally, preference-based HRoL was measured subjectively by self-reporting of patients and is therefore heavily dependent on biases such as social desirability and cognitive status or mood of the patient when completing questionnaires. However, as both questionnaires were completed simultaneously in both studies, these biases are likely to be similar between questionnaires and hence has limited impact on the psychometric properties.
5. Conclusions
In conclusion, despite the limitations, we expect this study to support clinical researchers and health economists in their choice for a utility instrument for use in future research, especially in trials that are in the context of epilepsy and schizophrenia. Based on our results, we would recommend the use of AQoL-8D in addition to EQ-5D-5L in trials assessing patients with epilepsy or patients with schizophrenia regarding the impact of the psychosocial aspects of those diseases on patient’s HRQoL. We would like to emphasize the importance of valid utility instruments and that their applicability may vary depending on study-specific goals and population. In line with research done previously in Australia, we recommend gaining detailed evidence regarding whether AQoL-8D is a better alternative for EQ-5D-5L and to establish the generalizability of our findings for the different clinical different group comparisons.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
One Peer Reviewer has received manuscript or speaker’s fees from Astellas, Dainippon Sumitomo Pharma, Eisai, Eli Lilly, Elsevier Japan, Janssen Pharmaceuticals, Kyowa Yakuhin, Lundbeck, Meiji Seika Pharma, Mitsubishi Tanabe Pharma, Merck Sharp and Dohme, Nihon Medi-Physics, Novartis, Otsuka Pharmaceutical, Shionogi, Shire, Tsumura, Wiley Japan, and Yoshitomi Yakuhin, and research grants from Eisai, Mochida Pharmaceutical, Meiji Seika Pharma, and Shionogi. Peer reviewers in this manuscript have no other relevant financial relationships or otherwise to disclose.
Author contributions
Overall project coordination: SMAA, MH, HJMM. Writing of manuscript: HC, BW. Critical appraisal of manuscript: FS, LAM. All authors have read and approved the manuscript.
Supplemental Material
Download MS Word (5.3 MB)Acknowledgments
We would like to thank Reina de Kinderen for her role in the translation of the AQoL-8D to the Dutch version and Angelo Lezzi (Monash University) from the AQoL-group for his support, which facilitated the translation.
Data availability statement
Due to privacy regulations, data will not be made publicly available.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Howren MB. Quality-Adjusted Life Years (QALYs). In: Gellman MD, Turner JR, editors. Encyclopedia of behavioral medicine. New York (NY): Springer; 2013. p. 1605–1606.
- Lorgelly PK, Doble B, Rowen D, et al. Condition-specific or generic preference-based measures in oncology? A comparison of the EORTC-8D and the EQ-5D-3L. Qual Life Res. 2017;26(5):1163–1176.
- Halling Hastrup L, Nordentoft M, Hjorthoj C, et al. Does the EQ-5D measure quality of life in schizophrenia? J Ment Health Policy Econ. 2011;14(4):187–196.
- Brazier J. Is the EQ-5D fit for purpose in mental health? Br J Psychiatry. 2010;197(5):348–349.
- (ISPOR), I. S. f. P. a. O. R. Pharmacoeconomics guidelines around the World; 2021.
- Lamers LM, Bouwmans CA, van Straten A, et al. Comparison of EQ-5D and SF-6D utilities in mental health patients. Health Econ. 2006;15(11):1229–1236.
- Van de Willige G, Wiersma D, Nienhuis FJ, et al. Changes in quality of life in chronic psychiatric patients: a comparison between EuroQol (EQ-5D) and WHOQoL. Qual Life Res. 2005;14(2):441–451.
- Brazier J, Roberts J, Tsuchiya A, et al. A comparison of the EQ-5D and SF-6D across seven patient groups. Health Econ. 2004;13(9):873-884.
- Brazier J, Connell J, Papaioannou D, et al. A systematic review, psychometric analysis and qualitative assessment of generic preference-based measures of health in mental health populations and the estimation of mapping functions from widely used specific measures. Health Technol Assess. 2014;18(34):vii–viii, xiii–xxv, 1–188.
- Mulhern B, Pink J, Rowen D, et al. Comparing generic and condition-specific preference-based measures in epilepsy: EQ-5D-3L and NEWQOL-6D. Value Health. 2017;20(4):687–693.
- Mulhern BM, Barkham C, Knapp M, et al. Using preference based measures in mental health conditions: the psychometric validity of the EQ-5D and SF-6D. The University of Sheffield; 2013. (Health Economics and Decision Science (HEDS) Discussion Paper).
- Papaioannou D, Brazier J, Parry G. How valid and responsive are generic health status measures, such as EQ-5D and SF-36, in schizophrenia? A systematic review. Value Health. 2011;14(6):907–920.
- Goranitis I, Coast J, Day E, et al. Measuring health and broader well-being benefits in the context of opiate dependence: the psychometric performance of the ICECAP-A and the EQ-5D-5L. Value Health. 2016;19(6):820–828.
- Brooks R. The EuroQol group after 25 years. 2013.
- Bharmal M, Thomas J III. Comparing the EQ‐5D and the SF‐6D descriptive systems to assess their ceiling effects in the US general population. Value Health. 2006;9(4):262–271.
- van Hout B, Janssen MF, Feng YS, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health. 2012;15(5):708–715.
- Janssen M, Pickard AS, Golicki D, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res. 2013;22(7):1717–1727.
- Millier A, Clay E, Charaf I, et al. Patient reported outcomes instruments in schizophrenia: a review of psychometric properties. Open J Med Psychol. 2014;3(2):16.
- Karow A, Wittmann L, Schottle D, et al. The assessment of quality of life in clinical practice in patients with schizophrenia. Dialogues Clin Neurosci. 2014;16(2):185–195.
- Adams J, Crowley CLR,S, Nand V, et al. Valuation of schizophrenia-related health states by the general population using the AQoL, time trade-off and visual analogue scales. ISPOR 10th Annual International Meeting; Washington (DC); 2005.
- Mukuria C, Young T, Keetharuth A, et al. Sensitivity and responsiveness of the EQ-5D-3L in patients with uncontrolled focal seizures: an analysis of Phase III trials of adjunctive brivaracetam. Qual Life Res. 2017;26(3):749–759.
- Selai C, Elstner K, Trimble M. Quality of life pre and post epilepsy surgery. Epilepsy Res. 1999;38(1):67–74.
- Wijnen BFM, Mosweu I, Majoie M, et al. A comparison of the responsiveness of EQ-5D-5L and the QOLIE-31P and mapping of QOLIE-31P to EQ-5D-5L in epilepsy. Eur J Health Econ. 2018;19(6):861-870.
- Campbell JA, Palmer AJ, Venn A, et al. A head-to-head comparison of the EQ-5D-5L and AQoL-8D multi-attribute utility instruments in patients who have previously undergone bariatric surgery. Patient. 2016;9(4):311–322.
- Hawthorne G, Richardson J, Osborne R. The Assessment of Quality of Life (AQoL) instrument: a psychometric measure of health-related quality of life. Qual Life Res. 1999;8(3):209–224.
- Hawthorne G, Richardson J, Day NA. A comparison of the Assessment of Quality of Life (AQoL) with four other generic utility instruments. Ann Med. 2001;33(5):358–370.
- Richardson J, Sinha K, Iezzi A, et al. Modelling utility weights for the Assessment of Quality of Life (AQoL)-8D. Qual Life Res. 2014;23(8):2395–2404.
- Richardson J, Iezzi A, Khan MA, et al. Validity and reliability of the Assessment of Quality of Life (AQoL)-8D multi-attribute utility instrument. Patient. 2014;7(1):85–96.
- Wijnen B, Leenen LA, de Kinderen RJ, et al. (Cost-) effectiveness of a multi-component intervention for adults with epilepsy: study protocol of a Dutch randomized controlled trial. Value Health. 2014;17(7):A582–583.
- Wijnen BF, Pos K, Velthorst E, et al. Economic evaluation of brief cognitive behavioural therapy for social activation in recent-onset psychosis. PloS one. 2018;13(11):e0206236.
- Pos K, Franke N, Smit F, et al. Cognitive behavioral therapy for social activation in recent-onset psychosis: randomized controlled trial. J Consult Clin Psychol. 2019;87(2):151.
- Devlin N, Shah KK, Feng Y, et al. Valuing health-related quality of life: an EQ-5D-5L value set for England. 2016.
- Campbell JA, Hensher M, Neil A, et al. An exploratory study of long-term publicly waitlisted bariatric surgery patients’ quality of life before and 1 year after bariatric surgery, and considerations for healthcare planners. PharmacoEcon Open. 2018;2(1):63–76.
- Lamers LM, McDonnell J, Stalmeier PF, et al. The Dutch tariff: results and arguments for an effective design for national EQ-5D valuation studies. Health Econ. 2006;15(10):1121–1132.
- Versteegh MM, Vermeulen KM, Evers SM, et al. Dutch tariff for the five-level version of EQ-5D. Value Health. 2016;19(4):343–352.
- Mokkink LB, Terwee CB, Patrick DL, et al. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol. 2010;63(7):737–745.
- Šimkovic M, Träuble B. Robustness of statistical methods when measure is affected by ceiling and/or floor effect. PloS One. 2019;14:8.
- Mokkink LB, Terwee CB, Patrick DL, et al. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Qual Life Res. 2010;19(4):539–549.
- Chen H, Cohen P, Chen S. How big is a big odds ratio? Interpreting the magnitudes of odds ratios in epidemiological studies. Commun Stat Simul Comput. 2010;39(4):860–864.
- Liang MH. Evaluating measurement responsiveness. J Rheumatol. 1995;22(6):1191–1192.
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Int J Nurs Stud. 2010;47(8):931–936.
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307-310.
- Giavarina D. Understanding Bland Altman analysis. Biochem Med (Zagreb). 2015;25(2):141–151.
- Dritsaki M, Petrou S, Williams M, et al. An empirical evaluation of the SF-12, SF-6D, EQ-5D and Michigan hand outcome questionnaire in patients with rheumatoid arthritis of the hand. Health Qual Life Outcomes. 2017;15(1):20.
- Matza LS, Boye KS, Yurgin N. Validation of two generic patient-reported outcome measures in patients with type 2 diabetes. Health Qual Life Outcomes. 2007;5:47.
- Marra CA, Woolcott JC, Kopec JA, et al. A comparison of generic, indirect utility measures (the HUI2, HUI3, SF-6D, and the EQ-5D) and disease-specific instruments (the RAQoL and the HAQ) in rheumatoid arthritis. Soc Sci Med. 2005;60(7):1571–1582.
- Hofman CS, Lutomski JE, Boter H, et al.; Consortium, T.-M. R. Examining the construct and known-group validity of a composite endpoint for The Older Persons and Informal Caregivers Survey Minimum Data Set (TOPICS-MDS); A large-scale data sharing initiative. PLoS One. 2017;12(3):e0173081.
- Davidson M. Known-groups validity. In: Michalos AC, editor. Encyclopedia of quality of life and well-being research. Dordrecht (The Netherlands): Springer; 2014. p. 3481–3482.
- Snaith RP. The hospital anxiety and depression scale. Health Qual Life Outcomes. 2003;1:29.
- Bouwmans C, Krol M, Severens H, et al. The iMTA productivity cost questionnaire: a standardized instrument for measuring and valuing health-related productivity losses. Value Health. 2015;18(6):753–758.
- O’Donoghue MF, Duncan JS, Sander JW. The national hospital seizure severity scale: a further development of the Chalfont seizure severity scale. Epilepsia. 1996;37(6):563–571.
- Ferreira LN, Ferreira PL, Ribeiro FP, et al. Comparing the performance of the EQ-5D-3L and the EQ-5D-5L in young Portuguese adults. Health Qual Life Outcomes. 2016;14(1):89.
- Greene ME, Rader KA, Garellick G, et al. The EQ-5D-5L improves on the EQ-5D-3L for health-related quality-of-life assessment in patients undergoing total hip arthroplasty. Clin Orthop Relat Res. 2015;473(11):3383–3390.
- Nolan CM, Longworth L, Lord J, et al. The EQ-5D-5L health status questionnaire in COPD: validity, responsiveness and minimum important difference. Thorax. 2016;71(6):493–500.
- McClure NS, Sayah FA, Xie F, et al. Instrument-defined estimates of the minimally important difference for EQ-5D-5L index scores. Value Health. 2017;20(4):644–650.
- Hawthorne G. Assessing utility where short measures are required: development of the short Assessment of Quality of Life-8 (AQoL-8) instrument. Value Health. 2009;12(6):948–957.
- Hawthorne G, Korn S, Richardson JJA; Health, N. Z. J. O. P. Population norms for the AQoL derived from the 2007 Australian national survey of mental health and wellbeing. Aust N Z J Public health. 2013;37(1):7–16.
- Holland R, Smith RD, Harvey I, et al. Assessing quality of life in the elderly: a direct comparison of the EQ‐5D and AQoL. Health Econ. 2004;13(8):793–805.
- Cramer JA, Perrine K, Devinsky O, et al. Development and cross‐cultural translations of a 31‐item quality of life in epilepsy inventory. Epilepsia. 1998;39(1):81–88.