ABSTRACT
Introduction
Despite the availability of several commercial rapid diagnostic tests (RDTs) developed to detect typhoid fever, the cost-effectiveness in resource-limited settings is unclear. This review aimed to explore the literature on typhoid economic evaluations in order to assess the cost-effectiveness of using typhoid RDTs in resource-limited settings.
Areas covered
A systematic review was conducted focusing on the identification of economic evaluations of typhoid RDTs to diagnose patients with suspected typhoid fever. Two studies were identified and included for narrative synthesis. Whilst highlighting a gap in the published literature, this review shows the use of typhoid RDTs to potentially be cost-effective in resource-limited settings. Factors that appeared as significant in determining cost-effectiveness related to test characteristics (such as sensitivity, specificity, and cost) and the prevalence of typhoid fever and should factor into any future evaluations.
Expert opinion
Concerted effort is needed in resource-limited settings with regard to medical device regulation to ensure that clinically effective and cost-effective typhoid RDTs are widely available and introduced into clinical practice. Typhoid modeling (with respect to typhoid testing and treatment strategies) represents an understudied area and further work is needed.
Article highlights
Despite the availability of several commercial RDTs for typhoid fever, the cost-effectiveness in resource-limited settings is unclear and understudied.
There are currently two published economic evaluation studies on the cost-effectiveness of typhoid RDTs.
There is potential for the application of typhoid RDTs to be cost-effective in resource-limited settings.
Significant factors in determining cost-effectiveness related to test characteristics (such as sensitivity, specificity, and cost) and the prevalence of typhoid fever. These should be prominent in future evaluations and furthermore incorporate and explore the impact of test result adherence on cost-effectiveness.
Typhoid modeling (with respect to typhoid test-and-treat strategies) represents an understudied area and further work is needed.
Concerted effort is needed in resource-limited settings with regard to medical device regulation to ensure that clinically effective and cost-effective typhoid RDTs are available and introduced into clinical practice.
2. Introduction
Typhoid fever is an acute bacterial infection caused by the bacteria Salmonella typhi and paratyphi [Citation1]. Transmission occurs via the ingestion of food or water contaminated with feces from infected individuals [Citation2]. The World Health Organization (WHO) estimates the global typhoid fever burden at 11–20 million cases annually, resulting in 128,000–161,000 deaths per year [Citation3]. The case fatality rate of typhoid fever is 10–30% in the absence of treatment but drops to 1–4% with appropriate antibiotics [Citation3]. Globally, typhoid fever remains an important cause of disease [Citation4], especially in resource-limited countries [Citation5]. Poor hygiene practices [Citation6] and inadequate diagnostic laboratory capacity [Citation2] have been noted as reasons for the continually high rates of infections in resource-limited settings.
The current gold-standard method used for diagnosis is blood culture. However, this lacks sensitivity (40–75%) [Citation7], meaning that the disease is often missed in patients resulting in lost opportunities for treatment. This in turn can lead to increased morbidity and mortality. Furthermore, blood culture requires trained laboratory personnel, equipment, supplies, and electricity, which may not be routinely available in many primary health-care facilities in resource-limited countries. In addition, it can take 2–3 days before test results are returned, leading to delays in diagnosis and possible complications such as perforation of the intestine and death if treatment is postponed or patients are lost to follow-up [Citation8]. Low-cost diagnostic tests, such as the Widal test, are still widely used; however, the Widal test is limited by both a lack of sensitivity (44–77%) and may be geographically specific/unspecific (50–92%) depending on the epidemiology of other infections [Citation9,Citation10], making it an unreliable indicator for typhoid fever. Furthermore, typhoid fever clinically resembles other febrile conditions including malaria and is therefore easily misdiagnosed without laboratory confirmation [Citation11], causing over-prescription of antimalarial therapies associated with a huge economic impact [Citation12]. Therefore, the availability of a test that is accurate, simple, and affordable with a quick time-to-result for the diagnosis of typhoid fever in resource-limited settings has an obvious attraction. Over the past decade, there have been over 20 commercially available RDTs and their prototypes (including TUBEX, Typhidot, Typhidot‐M, Test‐it Typhoid, and other tests) [Citation11]. However, despite the availability of commercial RDTs for typhoid fever [Citation11], the cost-effectiveness in resource-limited settings is unclear.
2.1. Aim
The aim of this systematic review was to assess the cost-effectiveness of using RDTs to diagnose typhoid fever in patients with suspected typhoid fever in resource-limited settings. Specifically.
To report the results of existing economic evaluations of typhoid RDTs in terms of cost-effectiveness.
To gain insights, through the studies identified, into the relevant factors that influence the cost-effectiveness of the use of typhoid RDTs.
3. Methods
The systematic review protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO), in accordance with PRISMA guidelines (PROSPERO CRD42021253072). In May 2021, we searched CAB Abstracts (Ovid), Cochrane library (Wiley), Embase (Ovid); Global Health; Global Index Medicus (WHO), MEDLINE (Ovid), Web of Science core collection, International HTA database (INAHTA), and NHS EED for studies that conducted economic evaluations of diagnostic tests for typhoid.
The searches were developed using the concepts: typhoid and generic testing terms, as well as a second approach searching specifically for named typhoid diagnostic tests and peer reviewed by a senior information specialist using the PRESS checklist [Citation13]. The search strategies were customized for each database using a combination of database subject headings and index terms including these key terms (enteric fever or typhoid or paratyphoid) AND (diagnostic tests, Widal or TUBEX or Typhifast) AND the Academic Unit of Health Economics Information Specialists economic evaluation strategies [Citation14]. See search strategies in the Appendix. The searches were not limited by date or language. Searching was not limited by language to make it more inclusive.
3.1. Inclusion criteria
Studies were required to meet all the inclusion criteria outlined in in order to be included in this review. All published literature that relates to the cost-effectiveness of typhoid RDTs were considered for inclusion. Only studies with full texts available in English were included in the final set of studies because of a lack of expertise to translate non-English language studies.
Table 1. Inclusion criteria
We excluded studies not published as a full-text manuscript or conference paper (e.g. all abstracts, review, editorials, and opinion pieces were excluded); however; any relevant reviews were flagged, and their citations checked for relevant studies not already captured in the review. The reference lists of the included studies were also screened. Studies that did not diagnose typhoid in patients were excluded.
3.2. Data management and screening
Records were stored and deduplicated in Endnote software and reviewed using the web app, Rayyan [Citation15].
A two-stage screening of titles and abstracts, followed by full-text review, was undertaken independently (i.e. blinded to the other reviewer’s decisions) by two reviewers (SF and GS). During the initial title and abstract screening stage, citations considered relevant by either reviewer were included in the full-text review. At the full-text review stage, disagreements between reviewers as to article inclusion (as outlined in ) were resolved via discussion with a third reviewer (NK).
3.3. Data extraction
A data extraction form was developed and piloted in Microsoft Excel 2016. For each of the included studies, data extraction was conducted to answer the following questions:
Is the use of typhoid RDTs for the diagnosis of typhoid fever cost-effective in resource-limited settings?
What are the relevant factors that influence the cost-effectiveness of the use of typhoid RDTs in resource-limited settings?
Data extraction included key items related to design of study, study population, type of economic evaluation, form of economic evaluation, perspective, location, currency and price year, intervention(s), comparator(s), methodology, measurement of health outcomes, time horizon and discount rate, base case findings and results of sensitivity and scenario analysis. Data extraction was undertaken independently and in duplicate (SF and GS) with any disagreements between reviewers resolved by a third reviewer (NK).
3.4. Quality assessment
The methodological quality of each included study was assessed independently and in duplicate (SF and GS) using a modeling quality checklist modified from Philips et al. [Citation16]. Each item on the checklist was rated using the categories ‘yes,’ ‘no,’ ‘unclear,’ and ‘not applicable’ allowed by the extent of reporting.
4. Results
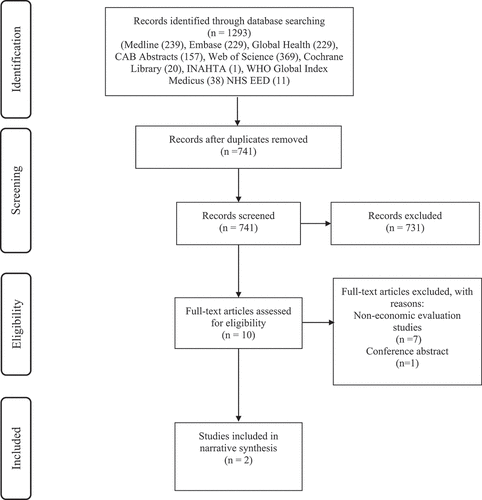
Database searches identified 1293 records. Once duplicates were removed, there were 741 records eligible for title and abstract screening. Ten studies were potentially eligible for inclusion after title and abstract screening. Following the full-text screening, two studies were included in this review. The PRISMA diagram below () illustrates the results of the screening process with reasons for exclusion noted.
4.1. Characteristics of included studies
The identified studies included in the review were published between 2018 and 2020. One study conducted an early economic evaluation [Citation17] and the other, traditional economic evaluation conducted at the late phase of product development [Citation18]. The patient population considered in one study was febrile children who attended a health-care center [Citation18], and the other study considered individuals presenting with symptoms suggestive of typhoid. In both studies, decision tree models were used for cost-effectiveness modeling based on hypothetical cohorts. illustrates the characteristics of the included studies.
Table 2. Characteristics of the included studies
4.2. Quality assessment of included studies
The methodological quality of the included studies as rated using the categories ‘yes,’ ‘no,’ and ‘not applicable’ found that 64% of the checklist items were categorized as ‘yes,’ 19% as ‘no,’ and 17% as ‘not applicable.’ No item on the checklist was categorized as ‘unclear.’ Both included studies clearly stated the decision problem and specified the objectives of their model that were consistent, in our opinion, with the stated decision problem. However, in one study, the primary decision-maker was not explicitly stated [Citation18], and the other did not specify the sources of the information used to develop the model structure [Citation17].
Both studies provided a clear definition of options under evaluation but neither evaluated all feasible and practical options. Furthermore, neither study provided any justification for the exclusion of feasible options, and it was noted that heterogeneity was not dealt with by running the model separately for different subgroups. See Appendix for details of the quality assessment.
4.3 Summary of the results of existing economic evaluations of typhoid RDTs in terms of cost-effectiveness
One study conducted a cost-effectiveness analysis [Citation18] and the other study conducted a cost-utility analysis [Citation17]. The primary measurement of health outcomes in the study that conducted a cost-effectiveness analysis was treatment success at 7 days; therefore, the incremental cost-effectiveness ratio (ICER) was reported as cost per additional treatment success [Citation18]. The measurement of health outcomes in the study that conducted a cost-utility analysis was the QALY; therefore, the incremental cost-effectiveness ratio (ICER) was reported as cost per QALY gained [Citation17]. In both studies, no discounting was applied to future costs and outcomes because of the short time horizon used.
Different assumptions were used across the two studies. For example, different assumptions were made about the time horizon. One study used a time horizon of 7 days [Citation18] and the other study used a time horizon of 180 days. A time horizon of 7 days was chosen because the authors consider treatment success at day 7 to be a useful measure of diagnosis since it represents the effect of correct diagnosis and subsequent treatment choice which is commonly used to assess efficacy. A time horizon of 180 days was chosen in the other study because the authors considered it to be a more appropriate timeframe over which patients would benefit from the impact of both testing and treatment for typhoid fever [Citation17]. Increasing the time horizon to 180 days also allows other feasible options with a longer treatment time (compared to the 34 days considered in the analysis) to be added to the model. In terms of the patient population modeled, one study assumed patients presenting with suspected typhoid who subsequently do not have typhoid then had malaria because this was the most probable differential diagnosis [Citation17]. In the other study, a febrile child was considered to only have typhoid with malaria excluded [Citation18]. It is worth highlighting that despite the different assumptions used, both studies made the assumption of no co-infections and health workers perfectly adhering to test results and treatment protocols.
In terms of cost-effectiveness in the base case results for both studies, the use of typhoid RDT was cost-effective compared to the comparators they were evaluated against. In one study, the base case results showed that immunoglobulin M lateral flow assay (IgMFA) is more expensive but also more effective than presumptive clinical diagnosis without an RDT. The IgMFA detected 5.87 more true positives than clinical diagnosis (38.45 versus 43.17). The incremental cost of the IgMFA was estimated at US$5700 resulting in an ICER to detect one additional correct diagnosis of typhoid fever as US$972. In the same study, the ICER for one additional treatment success (their primary health outcome, IgMFA had 3.61 more treatment successes versus clinical diagnosis) was estimated to be US$1579. In the other study, the base case results show that, at certain price and accuracy pairs for the hypothetical test (HT-test), the HT-test has the potential to be more effective compared with the Widal test in terms of QALYs gained and cost-effectiveness.
4.4 Summary of the relevant factors that influence the cost-effectiveness of the use of typhoid RDTs
Both studies performed and reported on at least some form of deterministic sensitivity analysis and reported a probabilistic sensitivity analysis.
Across both studies, factors that appeared as significant in determining cost-effectiveness related to test sensitivity, specificity, cost, and the prevalence of typhoid fever. One study reported that the relative sensitivity of IgMFA versus clinical diagnosis, the cost of IgMFA, and the prevalence of typhoid fever or multi-drug-resistant (MDR) strains were the key drivers for the ICER [Citation18]. When the sensitivity of IgMFA was changed (from 59% used in the base case) to 42% (lower 95% CI value), the ICER was estimated to be US$1776 resulting in 3.21 less cases of treatment success than versus clinical diagnosis. The ICER changes to US$285 with perfect sensitivity resulting in treatment of 20.05 more successful cases. The cost of IgMFA was US$70 higher per treatment success versus the clinical diagnosis. With the typhoid fever prevalence set at 4.5% (lower 95% CI value), the ICER was estimated to be US$2292. When using a higher prevalence of 9.0% (upper 95% CI value), the estimated ICER was US$1147. Having a prevalence of 25% for MDR strains (50% prevalence at base case), the ICER increased to $2219, whereas a much higher prevalence of MDR strains (90%), IgMFA was cost-effective ($1081 per an additional treatment success). The other study reported that, for the HT-test to perform better, in terms of QALYs gained and cost-effectiveness, than the existing test (Widal), it is necessary for it to have a high specificity (at least 70%) and should not be priced more than US$4.
5. Discussion
This review has examined previous economic evaluations focused on the cost-effectiveness of using rapid diagnostic tests for the diagnosis of typhoid fever in patients with suspected typhoid fever, with particular focus on whether the use of typhoid RDTs is cost-effective and what the relevant factors that influence the cost-effectiveness are. This review identified only two published economic evaluation studies on the cost-effectiveness of typhoid RDTs, despite the availability of several commercial RDTs for typhoid fever [Citation11].
It is noted from the review that both studies concluded that the use of typhoid RDTs is cost-effective. However, an explicit statement on the cost-effectiveness of the use of typhoid RDTs in resource-limited settings cannot be made here because of the limited number of studies identified, difference in patient population evaluated, difference in diagnostic tests evaluated, and difference in the evaluative approaches used, all of which can potentially impact on the cost-effectiveness of the use of typhoid RDTs. What this shows, though, is that the application of typhoid RDTs in resource-limited settings has the potential to be cost-effective. Although both studies concluded that the use of typhoid RDTs is cost-effective, different assumptions were made across the studies in their analysis. Plausible reason for the difference in assumptions is the potential difference in typhoid care pathways in both settings. An informative economic model requires knowledge of care pathways if it is to appropriately represent a simplification of the disease, persons not having the disease and misclassifications, and be fit for the purpose of aiding decision-making [Citation19]. In both analyses, models were built to represent the existing care pathway (current practice) in the different settings, thus the differences in model structure and assumptions used. For example, the antimicrobial given when a test was positive for typhoid fever was azithromycin for 5 days in the study by Saito et al. [Citation18], whereas ciprofloxacin for 14 days was given in the study by Frempong et al. [Citation17]. Furthermore, the comparator test was different in the studies.
In both studies, it was noted that the models were not run separately for different subgroups. Consequently, it is not possible to comment on what the cost-effectiveness would be if a different patient population to the ones used in the analysis were considered. Considering all patient subgroups give an indication of the patient group in which the application of the test will be optimal. Another issue worth highlighting is the assumption of health workers perfectly adhering to test results and treatment protocols. In reality, health workers may not perfectly adhere to test results and treatment protocols if they do not have confidence in the test result. They may completely ignore test results and prescribe treatment in all patients who have a negative test result or prescribe treatment in a proportion of patients with a negative test result or in some instances, request further testing. Non-adherence leads to these added complexities that should ideally be captured and explored if the true value of the test is to be established. Omitting these scenarios from the analysis may lead to misleading conclusions on the cost-effectiveness on the application of the test.
The cost of the test, the relative accuracy of the test, and the prevalence of typhoid fever were found to be the significant factors that influence cost-effectiveness. A test price of US$3.58 was used in the study by Saito et al. [Citation18], and Frempong et al. [Citation17] estimated that the test should not be priced more than US$4. This gives an indication of the potential price range for a typhoid RDT to be cost-effective in a resource-limited setting. Although it must be highlighted that other factors such as the willingness-to-pay threshold used might influence the maximum price that can be charged for the test in any setting. This information might be useful for test developers who might consider developing new typhoid RDTs whether it will be feasible to produce and sell the test around this price range and still make a profit.
In the study by Frempong et al. [Citation17], specificity was found to be key to the cost-effectiveness argument, whereas sensitivity was found to be key to the cost-effectiveness argument in the study by Saito et al. [Citation18]. In the study by Frempong et al. [Citation17], this was explained by the prevalence of typhoid fever and what happens to false positives and false negatives following the test–treat pathway for a suspected typhoid case. In the models, false-positive results are worse for patients than false-negative test results because of the extra testing and delays in receiving effective treatment that patients undergo before they are eventually cured. Thus, it should be recommended that a high specificity is required in order to avoid these unnecessary testing and treatment strategies associated with these pathways. The prevalence of the disease in the population is a key driver for cost-effectiveness, as shown in the study by Saito et al. [Citation18]. They showed that at a prevalence of 4.5% for typhoid fever (the lower 95% CI value), the ICER was estimated to be US$2292, whilst at the upper CI value of 9.0%, the estimation was US$1147. The prevalence of disease differs for different patient groups hence the need to consider different subgroups in order to establish the patient group in which the application of the test will be optimal. Running the model for a different population (in this case, adults instead of children) might have a different impact on cost-effectiveness. The two studies identified highlight that typhoid modeling (with respect to typhoid testing and treatment) represents an area that has been relatively understudied and further work is needed.
Despite the availability of several commercial RDTs for typhoid fever [Citation11], surprisingly, only two economic evaluations were identified. This might be explained by considering the wider context of medical device regulation in resource-limited settings. Regulation of medical devices in resource-limited settings is weak and is particularly problematic with diagnostic tests [Citation20]. Health technology assessments are rare because there is a lack of proper systems and structures in place to ensure an adequate evaluation of the clinical and cost-effectiveness of tests before they are introduced in clinical practice (this citation refers to a retracted paper) [Citation21]. Although there is a proliferation of typhoid RDTs, only two economic evaluations were identified. As little regulatory controls exist, these may be sold with little or no evidence of their effectiveness and cost-effectiveness. Concerted efforts are needed to ensure that clinically effective and cost-effective typhoid RDTs are introduced into clinical practice.
Another plausible explanation for the dearth of cost-effectiveness evidence might be because cost-effectiveness studies can often be undertaken by industry or industry sponsored and manufacturers may not readily make available the results of their analyses.
6. Expert opinion
Whereas pharmaceutical products are widely regulated, less attention has been placed on the regulation of other medical devices, particularly diagnostic tests. However, medical devices, like pharmaceuticals, also carry a degree of risk and could cause problems in specific circumstances when treatment is based on test results. This highlights the need for governments to put in place policies to address all elements related to medical devices, ranging from access to high-quality, affordable products, through to their safe and appropriate use and disposal. Although most countries in resource-limited settings have a legal mandate to regulate medical devices, there is limited capacity to implement and enforce that regulation. The two economic evaluations identified in this study might be a clear indication of the lack of medical device regulation in resource-limited settings (particularly, with respect to typhoid fever) which results in less incentive to undertake such studies prior to implementation of such tests and highlight the need for more work to be done in this area. The two studies identified also highlight that typhoid modeling (with respect to typhoid testing and treatment) represents a relatively understudied area and further work is needed.
6.1. Five-year view
Given the disease burden of typhoid fever, there remains the need to develop rapid diagnostic tests for resource-limited settings. The validation and evaluation of such tests should include a demonstration of the value of such tests (clinical effectiveness and cost-effectiveness) before their introduction into routine clinical practice. It is our hope that an increased effort in generating evidence in resource-limited settings as would be required by medical device regulators will help to ensure that clinically effective and cost-effective typhoid RDTs are available and introduced into clinical practice.
Author contributions
All study authors meet the criteria for authorship as outlined by the journal policy and agree for the final version of the manuscript to be published.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
S N Frempong and G S Sagoo are supported by the National Institute for Health Research Leeds In Vitro Diagnostics Co-operative. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care.
Funding
This paper was not funded.
References
- World Health Organization. Background document: the diagnosis, treatment and prevention of typhoid fever: 2003 https://www.glowm.com/pdf/WHO-diagnosis%20treatment%20prevention%20of%20typhoid%20fever-2003-CustomLicense.pdf.
- Wain J, Hendriksen RS, Mikoleit ML, et al. Typhoid fever. Lancet. 2015;385:1136–1145.
- World Health Organization. Typhoid vaccines: WHO position paper – March 2018. Wkly Epidemiol Rec. 2018;93(13):153–172.
- Crump JA, Sjölund-Karlsson M, Gordon MA, et al. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive Salmonella infections. Clin Microbiol Rev. 2015;28(4):901–937.
- Keddy KH, Sooka A, Letsoalo ME, et al. Sensitivity and specificity of typhoid fever rapid antibody tests for laboratory diagnosis at two sub-Saharan African sites. Bull World Health Organ. 2011;89:640–647.
- Arora P, Thorlund K, Brenner DR, et al. Comparative accuracy of typhoid diagnostic tools: a Bayesian latent-class network analysis. PLoS Negl Trop Dis. 2019 May 8;13(5):e0007303.
- Tilahun GT, Makinde OD, Malonza D. Modelling and optimal control of typhoid fever disease with cost-effective strategies. Comput Math Methods Med. 2017;2017:1–16.
- Huang W, Gaydos CA, Barnes MR, et al. Comparative effectiveness of a rapid point-of-care test for detection of Chlamydia trachomatis among women in a clinical setting. Sex Transm Infect. 2013;89(2):108–114.
- Parry CM, Wijedoru L, Arjyal A, et al. The utility of diagnostic tests for enteric fever in endemic locations. Expert Rev Antiinfect Ther. 2011;9(6):711–725.
- Maude RR, de Jong HK, Wijedoru L, et al. CMCH Typhoid Study Group. The diagnostic accuracy of three rapid diagnostic tests for typhoid fever at Chittagong Medical College Hospital, Chittagong, Bangladesh. Trop Med Int Health. 2015;20(10):1376–1384.
- Wijedoru L, Mallett S, Parry CM. Rapid diagnostic tests for typhoid and paratyphoid (enteric) fever. Cochrane Database Syst Rev. 2017;26(5):1–146.
- Menon VB, Ramesh M, Pereira P, et al. Inappropriate prescribing of antimalarial therapy for the treatment of undifferentiated febrile illness among the elderly. J Pharm Pract Res. 2018 Dec;48(6):530–536.
- McGowan J, Sampson M, Salzwedel DM, et al. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J Clin Epidemiol. 2016;75:40–46.
- Academic Unit of Health Economics, University of Leeds. 2018. Economic Evaluation search strategies [cited 2021 Apr 5]. https://informationspecialists.leeds.ac.uk/wpcontent/uploads/sites/71/2019/05/AUHE-Search-Strategies-costs.pdf.
- Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210.
- Philips Z, Bojke L, Sculpher M, et al. Good practice guidelines for decision-analytic modelling in health technology assessment. Pharmacoeconomics. 2006 Apr 1;24(4):355–371.
- Frempong SN, Sutton AJ, Davenport C, et al. Early economic evaluation to identify the necessary test characteristics of a new typhoid test to be cost effective in Ghana. PharmacoEconomics-open. 2020 Mar;4(1):143–157.
- Saito MK, Parry CM, Yeung S. Modelling the cost-effectiveness of a rapid diagnostic test (IgMFA) for uncomplicated typhoid fever in Cambodia. PLoS Negl Trop Dis. 2018;12(11):e0006961.
- Briggs AH, Claxton K, Sculpher MJ. Decision modelling for health economic evaluation. Handbooks in health economic evaluation. UK: Oxford University Press; 2006.
- McNerney R, Sollis K, Peeling R. Improving access to new diagnostics through harmonised regulation: priorities for action. Afr J Lab Med. 2014. DOI:https://doi.org/10.4102/ajlm.v3i1.123
- Odame EA. RETRACTED: Systematic review of economic evaluation literature in Ghana: is health technology assessment the future? Value Health Reg Issues. 2013 Sep 1;2(2):279–283.