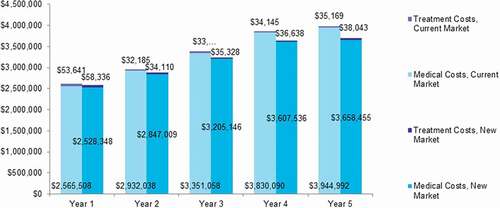ABSTRACT
Background
Opioid use disorder (OUD) is associated with high healthcare resource utilization (HCRU) and costs. reSET-O is an FDA-cleared prescription digital therapeutic that delivers neurobehavioral therapy as an adjunct to treatment-as-usual (TAU; buprenorphine, face-to-face counseling, and contingency management).
Methods
A budget impact model was developed to evaluate reSET-O as an adjunct to TAU in OUD for a 1 million-member US mixed health plan over a 5-year time horizon. Model inputs included treatment costs and medical costs of hospitalizations, partial hospitalizations, intensive care unit stays, and emergency department visits.
Results
The base-case results and the alternative scenario analysis showed the addition of reSET-O was projected to result in consistently lower total yearly costs vs TAU and no treatment. The estimated total and per member per month (PMPM) budget impact over 5 years was -$763,026 and -$0.0116, respectively. When the upper range of cost estimates was used, the total and PMPM budget impacts over 5 years were -$2,481,563 and -$0.0378, respectively. Sensitivity analysis showed results were most sensitive to the proportion of patients untreated.
Conclusion
The introduction of reSET-O in addition to TAU for OUD has the potential to reduce healthcare resource utilization and costs from 12 weeks up to 5 years.
1. Introduction
Opioid use disorder (OUD) is considered a chronic, relapsing disease. It is currently fueled by the widespread availability, low cost, and greater potency of illicit opioids such as heroin and fentanyl. An early goal of therapy is harm reduction, or the implementation of patient-centric measures to reduced exposure to, and harm from, illicit opioids [Citation1]. Although 40% to 60% of patients relapse following withdrawal from opioids, relapse rates are significantly reduced by 50% or more in patients who stabilize on an opioid agonist medication [Citation2–6]. OUD is a serious national crisis that affects public health and is responsible for a substantial social and economic burden [Citation7–9]. In the United States (US) in 2019, it was estimated that 10.3 million individuals misused opioids and 1.6 million were diagnosed with OUD [Citation10]. With the disruption to OUD treatment due to the COVID-19 pandemic and its impact on patient cognition and behavior, a recent sharp increase in opioid-related mortality has been reported [Citation11]. In the 12-month period ending January 2021, 70,456 individuals died from an opioid-related overdose, which translated to approximately 193 deaths every day, and was approximately 20,000 additional deaths, or 40% higher than the 12-month period ending January 2020 [Citation11]. In addition to lost workplace productivity, criminal activity, and premature mortality associated with OUD, the annual direct medical costs were recently estimated to be $90 billion annually, which, across the 1.6 million patients in the US, represents a per-patient per-year cost of $56,000 [Citation9].
Current standard of care for OUD includes medications for opioid use disorder (MOUDs) such as buprenorphine, used in conjunction with counseling, and behavioral therapies [Citation12,Citation13]. Buprenorphine therapy is associated with reductions in opioid overdose deaths (38% over a 12-month period), healthcare resource utilization (eg, hospitalizations are twice as likely in patients nonadherent to buprenorphine therapy), and total cost of care (to about $2,500 to $11,000 per-patient per-year) if used appropriately and without early discontinuation [Citation6,Citation14–16]. However, the retention rates of MOUDs are low overall and range widely across settings.
A systematic review reported median 1- and 3-year retention rates of 57% and 38.4%, respectively, for opioid substitution treatment [Citation17]. Other literature reported that approximately 20% of dropouts from treatment programs occurred within the first month [Citation18]. In one study, patients retained on buprenorphine for 15 to 18 months experienced significantly better outcomes compared to patients retained for 6 to 9 months, including a lower risk of ED visits, inpatient hospitalizations, and filling of opioid prescriptions [Citation19]. The combination of counseling and behavioral therapies (e.g. cognitive behavioral therapy [CBT] and contingency management [CM]) has been recognized as being useful in increasing patient engagement, improving adherence, and facilitating behavior change [Citation20]. The combination has also been shown to be superior to treatment-as-usual (TAU) in increasing retention in treatment, and the odds of testing negative for opioids and cocaine increased during the third month of treatment when delivered as a prescription digital therapeutic [Citation21].
Prescription digital therapeutics (PDTs) have the potential to facilitate and expand access to OUD treatment by delivering evidence-based neurobehavioral treatment in a convenient, familiar, and confidential form. reSET-O is a 12-week Food and Drug Administration (FDA)-cleared PDT that combines CBT with fluency training (FT) and CM through 67 on-demand text, audio, and video lessons. Lessons are designed based on the community reinforcement approach (CRA) that teaches patients to master specific skills (e.g. communication skills, problem-solving, drug refusal, etc.) to achieve their recovery goals and increase satisfaction with personal, social, and vocational aspects of their lives, among others [Citation22]; lessons are designed to replicate a 30-minute face-to-face counseling session. Following each lesson, FT in the form of a simple quiz is presented to the user in order to increase the retention and understanding of positive adaptive behaviors. Patients are recognized and rewarded via CM with merit badges or gift cards of modest value upon the completion of each lesson and quiz [Citation23–26]. In the pivotal randomized controlled trial for reSET-O (NCT00929253), its precursor (therapeutic education system [TES]) in combination with TAU (buprenorphine, counseling, and CM) demonstrated a statistically significant higher retention rate than TAU alone (80% vs 64%, respectively; odds ratio for completing the 12-week treatment was 2.30 favoring TES; P = 0.018) in patients with OUD [Citation27]. In addition, patients treated with reSET-O + TAU were twice as likely to test negative for substances (opioids and cocaine) during weeks 9 to 12 compared to TAU [Citation21].
A budget impact analysis (BIA) of a new health technology is an important part of a comprehensive economic evaluation. A BIA provides guidance for budget forecasting, planning, and estimating the impact of adoption and diffusion of a new healthcare intervention on premiums in health insurance schemes given inevitable resource constraints. A previous study estimated the 12-week incremental cost per member per month (PMPM) of adding reSET-O to a commercial US healthcare plan as $0.0012 to $0.006 based on a 10% to 50% market uptake assumption [Citation28]. In the current study, the budget impact of reSET-O in OUD up to 5 years was assessed from a US third-party payer perspective incorporated recently available real-world HCRU data, re-treatment rates, and updated cost scenarios reflecting low- and high-cost treatment episodes to better account for different payer perspectives.
2. Methods
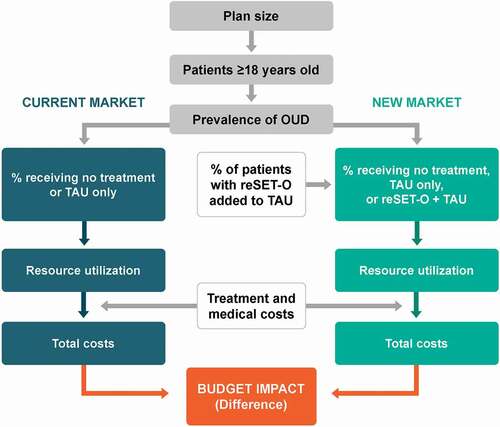
The analysis was developed following the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) guidelines for budget impact models [Citation29]. The model aimed to estimate the budget impact of reSET-O as an adjunct to TAU for OUD in which reSET-O + TAU was compared to TAU only and to no treatment. The budget impact on treatment-related costs and medical costs due to changes in treatment utilization was assessed from a US third-party payer perspective of a 1-million-member health plan that serves commercial and Medicare patients. The budget impact model structure is shown in . The differences in costs between the current and new markets for the total plan, per-treated-member per-month (PTMPM), and per-member per-month (PMPM) for each year were assessed over a 5-year time horizon. The PTMPM and PMPM budget impact results were calculated by dividing the total plan budget impact results by the treated population size and target population size, respectively, and further dividing by 12 months. Budget impact model findings are traditionally presented in this fashion, as PTMPM and PMPM.
The patient population included adults aged ≥18 years with OUD who enrolled in outpatient treatment with sublingual buprenorphine and CM under clinician supervision. The model used epidemiological inputs to determine the target population and assign treatment based on market share rates (). Market share rates prior to the introduction of reSET-O were obtained from the 2018 National Survey on Drug Use and Health [Citation30]. In the new market, utilization of reSET-O was assumed to increase 1.5% annually from 0% to 7.5% at year 5, with market share proportionally taken from all other comparators.
Table 1. Plan and market assumptions for base-case model
Untreated patients incurred no treatment cost. For treated patients, each treatment cycle was 12 weeks. Total net treatment cost to the payer over 12 weeks was calculated for each comparator as shown in . For TAU, average patient cost-share [Citation31] was subtracted from the wholesale acquisition cost (WAC) to calculate the net cost to payers. Given that reset-O delivers counseling content, patients in the reSET-O group were assumed to receive 50% fewer counseling sessions but the same frequency of urinalyses as patients in the TAU group. Average numbers of treatment cycles for the 2 treatment groups were based on an analysis of real-world claims [Citation33, Citation62]. The total per-patient annual costs were calculated by multiplying the assumed average number of treatments and total net cost per cycle of reSET-O and TAU, respectively. Treatment retention was accounted for in the calculations of total per-patient cost after the first year [Citation33, Citation34]. Starting at Year 2, 6.4% of patients were assumed to receive subsequent reSET-O therapy and 71.25% were assumed to continue TAU in the reSET-O group. For the TAU only group, 57% were assumed to continue treatment ().
Table 2. Treatment costs for reSET-O + TAU and TAU only groups
2.1. Medical service utilization rates
For medical service utilization rates for reSET-O + TAU and TAU alone, the lower bound of the 95% confidence interval (CI) represents the incidence of abstinent patients and the higher bound of the 95% CI represents nonabstinent patients. All utilization rates were obtained from published data as shown in [Citation38, Citation39, Citation41, Citation42] For each year, an annual growth of 11% in medical service utilization rates for nonabstinent patients in each comparator group was assumed; different medical resource utilization rates were assumed for nonabstinent patients who continued treatments and those who stopped treatments. All treated patients incur the associated medical costs regardless of whether they had received treatment in the given year or had stopped subsequent treatment. Distributions of patients by abstinence and treatment status for each comparator group were informed by internal data from Pear Therapeutics, Inc. For reSET-O + TAU, 61.5% of patients were abstinent, 24.2% continued treatment but were nonabstinent, and 14.3% were nonabstinent and dropped out of treatment. For TAU alone, the percentages were 54.4%, 13.9%, and 31.7%, respectively. Patients in the no treatment group were assumed to be untreated and nonabstinent.
Table 3. Medical service utilization rates
The base-case and high-cost scenario inputs for hospitalizations, intensive care unit (ICU) stays, partial hospitalizations (PH), and emergency department (ED) visits are outlined in . The costs of fatal and nonfatal overdoses are captured in these cost inputs for both abstinent and nonabstinent patients. All costs were adjusted and reported in 2020 US dollars, with no discounting performed, per ISPOR guidelines for budget impact models.
Table 4. Medical costs per episode
2.2. Sensitivity analyses
To evaluate parameter uncertainties, a one-way sensitivity analysis and a scenario analysis were performed to test the robustness of the results. Parameters tested in the one-way sensitivity analysis included population characteristics, market shares, treatment costs, treatment cycles, and medical services utilization rates and costs, and each was varied by 10%. In the scenario analysis, an alternative set of costs per episode for medical service was used in the model.
3. Results
3.1. Base-case analysis
The projected total cost of treating OUD increased over 5 years. With the introduction of reSET-O into the market, total yearly costs were projected to be consistently lower than those of the current market (), with the magnitude of savings increasing steadily over the 5 years. The base-case total budget impact of reSET-O over 5 years was estimated as −$763,026, with a PTMPM budget impact of −$106.68 and a PMPM budget impact of −$0.0116 (). The projected total costs for each treatment comparator and the detailed breakdown by cost component are presented in .
Table 5. Budget impact results summary
3.2. Sensitivity analyses
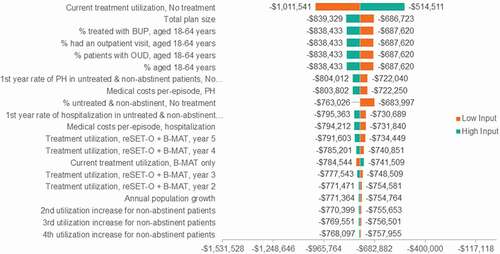
The one-way sensitivity analysis revealed that results were most sensitive to the proportion of patients in the untreated state. Other influential variables in the model were total plan size and proportion of patients aged 18 to 64 diagnosed or treated for OUD (). In the scenario analysis, where a higher set of per-episode costs for medical resource utilization (hospitalizations, ICU stays, partial hospitalizations, and ED visits) was assumed, the magnitude of savings with reSET-O over 5 years increased by an additional −$1,718,537 over the base case, with a PTMPM budget impact of −$346.94 and a PMPM budget impact of −$0.0378 (, ). The budget impact results were consistent with the base case, with greater savings with the introduction of reSET-O.
4. Discussion
From a US third-party payer perspective, reSET-O is a cost-saving PDT from 12 weeks up to a cumulative 5-year period when added to TAU in the management of OUD in adults treated in an outpatient setting. This BIA suggested that the additional treatment costs from reSET-O were fully offset by the reduction in costs associated with HCRU. The savings start with the first treatment cycle due to a reduction in hospitalizations and ED visits, the inclusion of CM, and a 50% reduction in counseling sessions, and the cost savings increase as the proportion of patients treated with reSET-O increases. Findings were consistent across various sensitivity analyses. The one-way sensitivity analysis showed that the model was most sensitive to the proportion of patients not receiving treatment, while the scenario analysis showed additional cost reductions over the base case through the avoidance of high-cost treatment episodes.
In the previously published BIA conducted by Wang and colleagues (2021) [Citation28], reSET-O was budget neutral over a 12-week time horizon, which was consistent with the findings in the current analysis [Citation35]. With a hypothetical 1-million-member health plan, the incremental total cost of adding reSET-O ranged from $3,563 at 10% market share uptake to $17,815 at 50% market share uptake, which translated to $0.0012 PMPM and $0.006 PMPM, respectively. Under similar settings, the current analysis showed that 10% market share uptake resulted in a −$1,377 difference in total cost and −$0.0004 PMPM, while 50% market share uptake resulted in a −$6,884 difference in total cost and −$0.0022 PMPM. The Wang model showed minimal budget impact to US payers, which increased with the expansion of reSET-O [Citation28]. On the other hand, findings from the current analysis suggest increased cost savings associated with reSET-O with larger market shares. The different trends of budget impact between the models are driven by differences in the estimation of medical costs. For instance, in the analysis conducted by Wang, 12-week medical and pharmacy costs of $3,886 and $11,218 were reported for adherent and nonadherent patients, respectively [Citation28]. Cost estimates were combined with the retention rates reported by Christensen and colleagues (2014) to calculate the weighted-average 12-week per-patient HCRU costs for reSET-O + TAU ($5,323) and TAU only ($6,519) groups [Citation27]. In the current analysis, the data source and approach utilized estimates of HCRU reported by Velez and colleagues (2020) by site of care [Citation42]; in addition, unit cost for each healthcare type, which provided extra information to better understand the driver of medical costs, was also incorporated [Citation44]. Patients were also distinguished by abstinence status, realizing that nonabstinent patients incurred more HCRU and costs (Reutsch) [Citation6]. As such, the current model accounted for both retention and abstinence for each comparator and provided a more comprehensive picture of OUD treatment.
The per-episode cost of HCRU across sites of care may be an important factor affecting budget impact. A wide variation exists in unit costs for HCRU and thus a scenario analysis was conducted with a set of higher cost estimates from published literature. The results suggest that payers can potentially save more in circumstances where medical costs are higher, since patients treated with reSET-O + TAU reported lower medical resource utilization rates (Velez) [Citation42]. One-way sensitivity analysis revealed limited effect of variation (10%) in medical costs. Regardless of this variation, reSET-O + TAU remained a cost-saving regimen in the current analysis.
The one-way sensitivity analysis found that the model is most sensitive to the proportion of untreated patients. Untreated patients with OUD incur significantly higher total healthcare costs compared to treated patients ($17,477 per patient per year in 2008 US dollars) Lynch [Citation14]. Based on the 2018 National Survey on Drug Use and Health, 81.9% of patients with OUD received no treatment SAMHSA [Citation10]; therefore, targeting the large population of untreated patients can be an opportunity to reduce healthcare costs. For example, a model constructed by the Institute for Clinical and Economic Review suggested that each dollar spent on expanding maintenance treatment would return $1.80 in savings ($2.6B in savings from an additional 505 patients in treatment) [Citation50]. Cost reductions have also been observed in a real-world expansion of treatment in Texas [Citation46].
Several barriers have been identified for the delivery of psychosocial support services, which can potentially be overcome with PDTs such as reSET-O. Workforce shortages of clinical and psychosocial providers – including behavioral counselors and buprenorphine prescribers – can decrease the availability of TAU and patient access [Citation45]. According to the Bureau of Labor Statistics Occupational Employment Statistics, there were 283,540 behavioral counselors in the workforce in 2019 [Citation47], which represented a shortage of 357,821 counselors; an estimated 641,361-person workforce is needed to support patients with serious emotional disturbance, serious mental illness, or substance use disorder [Citation30]. The shortages in rural areas were even worse, as 17% of counties that were not part of a metropolitan or micropolitan area had no behavioral health providers [Citation51]. Psychosocial support can help patients manage the challenges of transitioning from illicit opioid to a partial agonist such as buprenorphine [Citation52]. Delivered on mobile devices, PDTs can help decrease barriers to treatment and increase patient access to OUD treatments, improve patient engagement with the treatment process, and motivate patients for longer [Citation53, Citation54]. The addition of reSET-O may increase the variety of treatment options available to patients, which could possibly increase the overall number of treated patients, while reducing the incidence of high-cost hospitalizations and emergency department visits.
Longer treatment with MOUD is associated with positive outcomes on mortality, abstinence, and healthcare utilization [Citation55–60]. In previous studies, reSET-O demonstrated cost-effectiveness, dominant cost-utility, improved treatment retention, abstinence, and decreased real-world HCRU [Citation42, Citation61, Citation62]. In a long-term real-world data analysis, Velez et al. (2021) demonstrated continued benefits for reSET-O in reducing HCRU in OUD, with inpatient stays and ED visits decreased by 50% and 27% at 9 months, respectively [NaN]. To capture the long-term benefits associated with MOUD, the time horizons in this BIA were 12 weeks up to 5 years [Citation49]. Our study provided additional evidence showing that the addition of reSET-O provides a cost-saving opportunity to US payers for a longer period.
As other modeling studies, this BIA was limited by key assumptions made around market shares, treatment patterns, and HCRU due to uncertainties. Specifically, a conservative annual market share increase of 1.5% was used and was taken proportionally from TAU only and no treatment. This assumption was based on the potential increase in access to OUD treatment due to the convenience of PDTs delivered on mobile devices. As discussed, increased market share of reSET-O + TAU has the potential for greater cost savings by having more patients initiate treatment. Due to the lack of definitive abstinence data associated with real-world HCRU, the current model assumed that abstinent patients incurred HCRU at the lower bound of the 95% CI of the reported utilization rate, while nonabstinent patients utilized HCRU at the upper bound of the 95% CI. The rationale for this was based on the patient cohort reported by Velez and colleagues (2020) [Citation42], which consisted of a mix of abstinent and nonabstinent patients, where nonabstinent patients incurred higher HCRU based on the existing literature [Citation6]. Despite best efforts to select the most accurate model inputs from existing studies, parameters and assumptions were varied through sensitivity analyses to assess the model’s robustness to these assumptions and uncertainties, and the cost-savings of reSET-O were still realized.
5. Conclusion
The results of this model suggest initial healthcare cost savings after only 1 treatment cycle. Additionally, healthcare cost will decrease with reSET-O and this decrease is greater with higher adoption of the technology. The findings align with current evidence that the increased availability and expanded access to recovery services is associated with cost savings [Citation32]. reSET-O represents a potential opportunity to avoid the substantial costs and use of inpatient and ED services by retaining patients who might otherwise drop out of therapy and return to the use of illicit opioids.
Declaration of Interests
F Velez is an employee of Pear Therapeutics. Pear Therapeutics contracted Xcenda to assist in the research and completion of this study, and D Huang and L Mody are employees of Xcenda. DC Malone is an employee of Strategic Therapeutics and is a consultant for both Pear Therapeutics and Xcenda. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Taylor JL, Johnson S, Cruz R, et al. Integrating harm reduction into outpatient opioid use disorder treatment settings. J Gen Intern Med. 2021;36(12):3810–3819.
- Food and Drug Administration (FDA). Opioid use disorder; 2018. [cited 2021 Jul 13]. Available from: https://www.fda.gov/downloads/ForIndustry/UserFees/PrescriptionDrugUserFee/UCM605959.pdf
- National Institute on Drug Abuse (NIDA). Drugs, brains, and behavior: treatment and recovery; 2020. Available from: https://www.drugabuse.gov/publications/drugs-brains-behavior-science-addiction/treatment-recovery. Cited 2021 Jul 14.
- Strain E, Saxon AJ, Hermann R. Opioid use disorder: epidemiology, pharmacology, clinical manifestations, course screening, assessment, and diagnosis; 2018. Available from: http://www.uptodate.com/contents/opioid-use-disorder-epidemiologypharmacology-clinical-manifestations-course-screeningassessment-and-diagnosis. Cited 2021 Jul 13.
- Clark RE, Baxter JD, Aweh G, et al. Risk factors for relapse and higher costs among Medicaid members with opioid dependence or abuse: opioid agonists, comorbidities, and treatment history. J Subst Abuse Treat. 2015 Oct;57:75–80.
- Ruetsch C, Tkacz J, Nadipelli VR, et al. Heterogeneity of nonadherent buprenorphine patients: subgroup characteristics and outcomes. Am J Manag Care. 2017;23(6):e172–e179.
- Han B, Compton WM, Jones CM, et al. Nonmedical prescription opioid use and use disorders among adults aged 18 through 64 years in the United States, 2003-2013. JAMA. 2015 Oct 13;314(14):1468–1478.
- Florence CS, Zhou C, Luo F, et al. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care. 2016 Oct;54(10):901–906.
- Murphy SM. The cost of opioid use disorder and the value of aversion. Drug Alcohol Depend. 2020;217:108382.
- Substance Abuse and Mental Health Services Administration. Results from the 2019 national survey on drug use and health: detailed tables. Rockville: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2020. Available from: https://www.samhsa.gov/data/sites/default/files/reports/rpt29393/2019NSDUHFFRPDFWHTML/2019NSDUHFFR1PDFW090120.pdf Cited 2021 Jul 13.
- Centers for Disease Control and Prevention. 12 month-ending provisional number of drug overdose deaths by drug or drug class; 2021. Available from: https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm Cited 2021 Jul 13.
- Wakeman SE, Larochelle MR, Ameli O, et al., Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Network Open. 3(2): e1920622–e1920622. 2020.
- Boyett B. The individualized treatment of opioid use disorder. J Manag Care Spec Pharm. 2019 June;25(6):634–636.
- Lynch FL, McCarty D, Mertens J, et al. Costs of care for persons with opioid dependence in commercial integrated health systems. Addict Sci Clin Pract. 2014 Aug 14;9(1):16.
- Ronquest NA, Willson TM, Montejano LB, et al. Relationship between buprenorphine adherence and relapse, health care utilization and costs in privately and publicly insured patients with opioid use disorder. Subst Abuse Rehabil. 2018;9:59–78.
- Larochelle MR, Bernson D, Land T. Medical for opioid use disorder after nonfatal opioid overdose and association with mortality. Ann Intern Med. 2018;169(3):137–145.
- O’Connor AM, Cousins G, Durand L, et al. Retention of patients in opioid substitution treatment: a systematic review.PLoS One. 2020 [Published 2020 May 14];15(5):e0232086.
- Stein MD, Cioe P, Friedmann PD. Buprenorphine retention in primary care. J Gen Intern Med. 2005;20(11):1038–1041.
- Williams AR, Samples H, Crystal S, et al. Acute care, prescription opioid use, and overdose following discontinuation of long-term buprenorphine treatment for opioid use disorder. Am J Psychiatry. 2020;177(2):117–124.
- American Society of Addiction Medicine. National practice guideline for the use of medications in the treatment of addiction involving opioid use. Chevy Chase: American Society of Addiction Medicine; 2015. Available from: https://www.asam.org/docs/default-source/practice-support/guidelines-and-consensus-docs/asam-national-practice-guideline-supplement.pdf?sfvrsn=0 Cited 2021 Jul 13.
- Maricich YA, Bickel WK, Marsch LA, et al. Safety and efficacy of a prescription digital therapeutic as an adjunct to buprenorphine for treatment of opioid use disorder. Curr Med Res Opin. 2020;37(2):167–173.
- Budney AJ, Higgins ST. Therapy manuals for drug addiction, a community reinforcement plus vouchers approach: treating cocaine addiction. Rockville: National Institute on Drug Abuse; 1998 13 July 2021. Available from: https://archives.drugabuse.gov/sites/default/files/cra.pdf
- Bolivar HA, Klemperer EM, Colemann SRM, et al. Contingency management for patients receiving medication for opioid use disorder. JAMA Psychiatry. 2021;78(10):1–11.
- Becker SJ, Kelly LM, Kang A, et al. Factors associated with contingency management adoption among opioid treatment providers receiving a comprehensive implementation strategy. Subst Abus. 2019;40(1):56–60.
- Helseth SA, Janssen T, Scott K, et al. Training community-based treatment providers to implement contingency management for opioid addiction: time to and frequency of adoption. J Subst Abus Treat. 2018;95:26–34.
- Rash CJ, DePhillipis D. Considerations for implementing contingency management in substance abuse treatment clinics: the Veterans Affairs initiative as a model. Perspect Behav Sci. 2019;42(3):479–499.
- Christensen DR, Landes RD, Jackson L, et al., Adding an Internet-delivered treatment to an efficacious treatment package for opioid dependence. J Consult Clin Psychol. 82(6): 964–972. 2014.
- Wang W, Gellings Lowe N, Jalali A, et al. Economic modeling of reSET-O, a prescription digital therapeutic for patients with opioid use disorder. J Med Econ. 2021;24(1):61–68.
- Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 budget impact analysis good practice II task force. Value Health. 2014;17(1):5–14.
- Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2018 national survey on drug use and health; 2018. Available from: https://www.samhsa.gov/data/sites/default/files/cbhsqreports/NSDUHNationalFindingsReport2018/NSDUHNationalFindingsReport2018.pdf Cited 2021 Jul 13.
- Pharmacy Benefit Management Institute (PBMI). 2018 trends in drug benefit design 13 July 2021. Available from: https://www.pbmi.com/ItemDetail?iProductCode=BDR_2018&Category=BDR
- Wickizer TM, Mancuso D, Huber A. Evaluation of an innovative Medicaid health policy initiative to expand substance abuse treatment in Washington State. Med Care Res Rev. 2012;69(5):540–559.
- Velez FF, Ruetsch C, Maricich Y. Evidence of long-term real-world reduction in healthcare resource utilization following treatment of opioid use disorder with reSET-O, a novel prescription digital therapeutic. Expert Rev Pharmacoeconomics Outcomes Res. Jun 2021. https://doi.org/10.1080/14737167.2021.1939687.
- Truven Health Analytics. Use of medication-assisted treatment for opioid use disorders in employer-sponsored health insurance: out-of-pocket costs; 2019 13 July 2021. Available from: https://aspe.hhs.gov/basic-report/use-medication-assisted-treatment-opioid-use-disorders-employer-sponsored-health-insurance-out-pocket-costs
- DePhilippis D, Petry NM, Bonn-Miller MO, et al. The national implementation of Contingency Management (CM) in the department of veterans affairs: attendance at CM sessions and substance use outcomes. Drug Alcohol Depend. 2018;185:367–373.
- Premier Inc. Opioid overdoses costing U.S. hospitals an estimated $11 billion annually 13 July 2021. Available from: https://www.premierinc.com/newsroom/press-releases/opioid-overdoses-costing-u-s-hospitals-an-estimated-11-billion-annually
- Mallow PJ, Belk KW, Topmiller M, et al. Geographic variation in hospital costs, payments, and length of stay for opioid-related hospital visits in the USA. J Pain Res. 2018;11:3079–3088.
- Shah A, Duncan M, Atreja N, et al. Healthcare utilization and costs associated with treatment for opioid dependence. J Med Econ. 2018;21(4):406–415.
- RED BOOK®. IBM Micromedex®, IBM Watson health™. Cited October 13, 2020.
- Campbell ANC, Nunes EV, Matthews AG, et al. Internet-delivered treatment for substance abuse: a multisite randomized controlled trial. Am J Psychiatry. 2014;171(6):683–690.
- Devlin J, Duprey M, Roberts R, et al. 461: epidemiology, opioid exposure, and outcomes for ICU patients admitted with known opioid use disorder. Crit Care Med. 2018;46(1):214.
- Velez FF, Colman S, Kauffman L, et al. Real-world reduction in healthcare resource utilization following treatment of opioid use disorder with reSET-O, a novel prescription digital therapeutic. Expert Rev Pharmacoecon Outcomes Res. 2021 Feb. ;21(1):69–76. This analysis investigated the impact of reSET-O on HCRU in patients with OUD treated with buprenorphine. The addition of reSET-O resulted in a 60% reduction in hospitalizations and 20% reduction in emergency department visits.
- CMS. Hospital outpatient prospective payment-notice of final rulemaking with comment, CMS-1717-FC 2020 – 2020 NFRM OPPS Cost Statistics Files; 2019. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/Hospital-Outpatient-Regulations-and-Notices-Items/CMS-1717-FC
- McCollister K, Yang X, Sayed B, et al. Monetary conversion factors for economic evaluations of substance use disorders. J Subst Abuse Treat. 2017;81:25–34.
- Office of the Assistant Secretary for Planning and Evaluation. Psychosocial supports in medication-assisted treatment: site visit findings and conclusions; July 2019. Available from: https://aspe.hhs.gov/basic-report/psychosocial-supports-medication-assisted-treatment-site-visit-findings-and-conclusions Cited 2021 Jul 13.
- Texas Health and Human Services. Evaluation of Medicaid spending and outcomes for substance use disorder treatment; 2017. [cited 2021 Jul 13]. Available from: https://hhs.Texas.gov/sites/default/files/documents/laws-regulations/reports-presentations/2017/substance-abuse-disorder-treatment-nov-2017.pdf
- Bureau of Labor Statistics. CPI - All Urban Consumers - U.S. Medical Care Services 13 July 2021. Available from: https://data.bls.gov/cgi-bin/surveymost
- Stevens JP, Wall MJ, Novack L, et al. The critical care crisis of opioid overdoses in the United States. Ann Am Thorac Soc. 2017;14(12):1803–1809.
- Center for Substance Abuse Treatment. Substance abuse: clinical issues in intensive outpatient treatment. Rockville (MD): Substance Abuse and Mental Health Services Administration (US); 2006 13 July 2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK64093/
- Institute for Clinical and Economic Review. Management of patients with opioid dependence: a review of clinical, delivery system, and policy options. Available from: https://icer.org/wp-content/uploads/2020/10/CEPAC-Opioid-Dependence-Final-Report-For-Posting-July-21.pdf Cited July 13, 2021.
- Larson EH, Patterson DG, Garberson LA, et al. Supply and distribution of the behavioral health workforce in rural America. Data Brief #160; 2016. Available from: https://depts.washington.edu/fammed/rhrc/wp-content/uploads/sites/4/2016/09/RHRC_DB160_Larson.pdf Cited 2021 Jul 13.
- World Health Organization. Guidelines for the psychosocially assisted pharmacological treatment of opioid dependence. Geneva: World Health Organization; 2009 13 July 2021. Available from: https://www.who.int/substance_abuse/publications/9789241547543/en/
- Marsch LA. Digital health and addiction. Curr Opin Syst Biol. 2020;20:1–7.
- Marsch LA, Campbell A, Campbell C, et al. The application of digital health to the assessment and treatment of substance use disorders: the past, current, and future role of the national drug abuse treatment clinical trials network. J Subst Abuse Treat. 2020;112(suppl):4–11.
- Bhatraju EP, Grossman E, Tofighi B, et al. Public sector low threshold office-based buprenorphine treatment: outcomes at year 7. Addict Sci Clin Pract. 2017;12(1):7.
- Lo-Ciganic WH, Gellad WF, Gordon AJ, et al. Association between trajectories of buprenorphine treatment and emergency department and in-patient utilization. Addiction. 2016;111(5):892–902.
- Ma J, Bao YP, Wang RJ, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry. 2018. https://doi.org/10.1038/s41380-018-0094-5.
- Monico LB, Gryczynski J, Mitchell SG, et al. Buprenorphine treatment and 12-step meeting attendance: conflicts, compatibilities, and patient outcomes. J Subst Abuse Treat. 2015;57:89–95.
- Shcherbakova N, Tereso G, Spain J, et al. Treatment persistence among insured patients newly starting buprenorphine/naloxone for opioid use disorder. Ann Pharmacother. 2018;52(5):405–414.
- Stone AC, Carroll JJ, Rich JD, et al. Methadone maintenance treatment among patients exposed to illicit fentanyl in Rhode Island: safety, dose, retention, and relapse at 6 months. Drug Alcohol Depend. 2018;192:94–97.
- Velez FF, Luderer HF, Gerwien R, et al. Evaluation of the cost-utility of a prescription digital therapeutic for the treatment of opioid use disorder. Postgrad Med. 2021 May;133(4):421–427.
- Maricich YA, Gerwien R, Kuo A, Malone DC, Velez FF. Real-world use and clinical outcomes after 24 weeks of treatment with a prescription digital therapeutic for opioid use disorder, Hospital Practice. 2021;49(5):348-355. DOI: https://doi.org/10.1080/21548331.2021.1974243