ABSTRACT
Objectives
To summarize healthcare resource utilization (HCRU) in patients with newly diagnosed (incident) paroxysmal or persistent atrial fibrillation (AF).
Methods
This retrospective, observational cohort study assessed HCRU among patients with incident paroxysmal or persistent AF using data from 1 January 2015 to 30 September 2019 in the IBM MarketScan® Research Databases.
Results
A total of 50,796 patients were identified in the overall incident AF cohort. Rates of all-cause inpatient hospital stays, all-cause emergency room visits, and all-cause outpatient visits in the overall incident cohort were 46.8, 114.7, and 2,752.7 events per 100 patient-years (PY), respectively. Rates of cardiovascular-related inpatient stays for the overall population were 11.3 events per 100 PY. During follow-up, 50.4% of the overall cohort filled prescriptions for direct-acting oral anticoagulants and 5.0% had catheter ablation.
Conclusions
Advances in anticoagulation and ablation have been realized since previously published HCRU analyses of patients with atrial fibrillation. This update suggests that HCRU among patients with incident AF in the US remains high with some subgroups of patients receiving more specialized care.
1. Introduction
Atrial fibrillation (AF) is the most commonly treated heart arrhythmia, occurring in 1.5–2.0% of the global population [Citation1]. The prevalence of AF is projected to affect 12 million people in the US by 2030, due to the growing numbers of older people in the population, as well as an increase in the incidence rate of AF [Citation2–4]. The increased incidence of AF is attributable, in part, to improved detection techniques and a greater application of screening. Additionally, the use of wearable technology, such as smartwatches [Citation5] is likely to alert more people to potential AF and lead them to seek medical advice.
AF is associated with an increased risk of cardiovascular morbidity and mortality [Citation6–8], and is an independent risk factor for ischemic stroke [Citation9] and death [Citation10]. Current treatment for AF includes a combination of stroke prevention, rate control, and rhythm control strategies along with management of underlying cardiovascular comorbidities [Citation11–14]. Rhythm control is achieved with the use of antiarrhythmic drugs (AADs), ablation procedures, surgery, and/or electrical or pharmacologic cardioversion [Citation11]. AF represents both a clinical challenge and an economic burden for healthcare systems due to the substantial costs of cardiovascular hospitalizations, which are expected to grow as the population ages. Hospitalization is a primary cost driver in AF management [Citation15–17], with direct national incremental costs reported to range between approximately $6 and $26 billion in 2011 over a 12-month period [Citation15]. Reports on healthcare resource utilization (HCRU) associated with the use of AADs in AF are limited [Citation15] and may not be representative of current treatment practices. Moreover, studies limited to younger patients with AF, or studies of small patient samples, may not be fully representative of the national AF population. The most recent in-depth publication estimating total healthcare costs for AF in the United States was published in 2011 [Citation15]. Ongoing updates to treatment management guidelines [Citation11,Citation14,Citation18], as well as emerging data on the benefits of early rhythm control from the Early Treatment of Atrial Fibrillation for Stroke Prevention Trial (EAST-AFNET 4) [Citation14,Citation19], dictate a need to describe current patterns of HCRU to understand how the evolving data can inform treatment decisions for AF management. Here, we provide a descriptive summary of HCRU events in a cohort of patients with newly diagnosed paroxysmal or persistent AF from a large, national commercial database.
2. Patients and Methods
2.1. Data source
This was a retrospective, observational cohort study of patients with newly diagnosed (incident) paroxysmal or persistent AF that included data collected from 1 January 2015 through 30 September 2019. Patients were identified using the IBM MarketScan® Research Databases, which capture person-specific clinical utilization, expenditures, and enrollment across inpatient, outpatient, prescription drug, and carve-out services; analyses from this database have been reported previously [Citation20]. The databases comprise a family of research datasets that provide de-identified patient-level health data, productivity data, laboratory results, health risk assessments, hospital discharges, and electronic medical records [Citation21,Citation22]. These data come from a selection of large employers, health plans, and government and public organizations; paid claims and encounter data are linked to detailed patient information across sites and types of providers [Citation21]. These annual medical databases include private-sector health data from approximately 350 payers. Scientific review committee approval was obtained; as the databases complied with all aspects of the Health Insurance Portability and Accountability Act of 1996 and all study data were de-identified, institutional review board approval was not required.
2.2. Patient selection
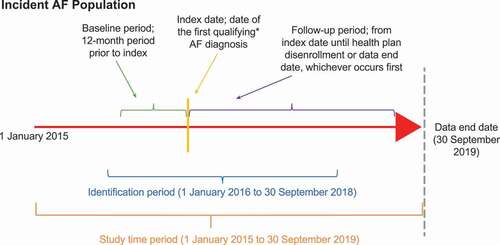
Patients initially diagnosed with paroxysmal or persistent AF during 1 January 2016 through 30 September 2018 were identified. This analysis period was selected to incorporate International Classification of Diseases, 10th Revision (ICD-10) coding, which allows for exclusion of patients with permanent AF and began in late 2015. Index date was defined as the date of the first qualifying incident AF diagnosis. A 1-year baseline period (defined as the 12-month period prior to index date) was used to exclude those with a prior diagnosis of AF and select only patients who were newly diagnosed with AF (). Patients eligible for inclusion had ≥1 inpatient or ≥2 outpatient claims (occurring on different days during the identification period) with a primary or secondary diagnosis of paroxysmal (ICD-10 I48.0) or persistent (ICD-10 I48.1) AF. Patients were required to be ≥18 years of age at the beginning of the baseline period and have continuous health plan enrollment during the baseline period. Patients were excluded if they had claims containing a primary or secondary diagnosis of permanent AF, had unspecified AF or atrial flutter (as indicated by ICD-9 or -10 codes), or were hospitalized for heart failure during the baseline period (as a proxy for severe heart failure). The follow-up period was measured from the index date until health plan disenrollment, last day of available data, or end of the study period (30 September 2019), whichever occurred first.
Figure 1. Study design. *Qualifying AF diagnosis; ≥1 inpatient or ≥2 outpatient claims with a primary or secondary diagnosis or paroxysmal (ICD-10 I48.0) or persistent (ICD-10 I48.1) AF occurring on different days during the identification period. Not to scale. Abbreviations: AF, atrial fibrillation.
An exploratory subgroup of patients with incident paroxysmal or persistent AF who filled prescriptions for dronedarone as first-line therapy during the study period, 1 January 2016 through 30 September 2019, was identified to represent a population receiving first-line rhythm control treatment. A 1-year baseline period was used to exclude those in this group with prior use of any AAD (amiodarone, sotalol, flecainide, propafenone, dofetilide, or dronedarone) to select only patients treated with dronedarone as first-line therapy for rhythm control.
2.3. Outcome measures
The primary objective of the current study was to characterize HCRU events in patients with incident paroxysmal or persistent AF.
Data on rates of all-cause HCRU events (all-cause inpatient stays, all-cause outpatient stays, and all-cause emergency room [ER] visits) and cardiovascular-related inpatient stays (any, AF, heart failure, acute coronary syndrome, and ischemic stroke) were reviewed. Event rates were stratified by AF type (paroxysmal versus persistent). Recent baseline medication use was determined by a patient’s claim of the medication on the day before or within 30 days prior to index. Medication use during follow-up was determined if the prescription fill date occurred on or after study index. Binary indicator variables were used to identify each medication for which patients had ≥1 pharmacy or medical claim during the follow-up period (e.g. medications were categorized as direct-acting oral anticoagulants [DOACs], warfarin, rate control medications, or AADs).
Screening for AF-related procedures and prescription drug use claims for anticoagulation medications were captured as observed during encounters containing a diagnosis code for AF in addition to a Current Procedural Terminology (CPT) or ICD-10 procedure code for the following: electrocardiogram, Holter/electrocardiogram monitoring, exercise stress test, spirometry, echocardiogram, or cardiac imaging (e.g. angiogram, computed tomography, chest x-ray, or magnetic resonance imaging). Cardioversion, AF catheter ablation, pacemaker insertion, and implantable cardioverter defibrillator insertion procedures were each captured using claims containing a CPT or ICD-10 procedure code (Supplemental Table 1).
2.4. Statistical analyses
The analyses in this study were descriptive. Categorical variables were summarized using patient counts with percentages, and continuous variables were summarized using means with standard deviations (SD), medians with interquartile ranges (IQR), and minimum and maximum values. All participants who met the inclusion criteria were included in the analyses. Patients with data missing were not excluded. Missing data for all study variables were quantified but not corrected (e.g. through imputation). HCRU was assessed during the follow-up period for each of the study cohorts, and event rates were calculated by dividing the mean number of events for each cohort by the mean number of years of follow-up for each cohort. Event rates were expressed as the number of events per 100 patient-years (PY), with initial and any subsequent events counted when calculating rates. Analyses were conducted using Python PySpark in the Sanofi Darwin Ecosystem.
3. Results
3.1. Study population
A total of 50,796 patients met the inclusion criteria for the overall incident AF cohort of the study (). As shown in , 58.3% of patients in the overall cohort were male, and 46.1% were <65 years of age. A total of 38.5%, 27.0%, 22.2%, and 12.1% of patients were located in the South, Midwest, Northeast, and West regions, respectively. The majority of patients were enrolled in preferred provider organization health plans (56.5%). CHA2DS2-VASc scores were ≥2 in 80.8% of patients. During the baseline period, 11.7% of patients received stress tests, 10.2% received cardioversion, and 1.1% received pacemakers; the mean (SD) number of electrocardiograms performed was 4.8 (4.6). Medication prescriptions filled at baseline (i.e. prior to AF diagnosis) for patients in this cohort included any rate control medication (60.0%), any DOAC (10.1%), and warfarin (5.5%).
Figure 2. Patient attrition for the overall incident AF population Abbreviations: AAD, antiarrhythmic drugs; AF, atrial fibrillation.
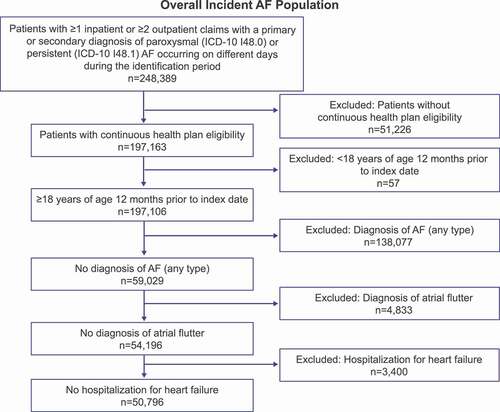
Table 1. Demographic and clinical characteristics
3.2. Healthcare resource utilization and event rates
All-cause inpatient hospital stays, all-cause ER visits, and all-cause outpatient visits, measured as events per 100 PY, were 46.8, 114.7, and 2,752.7, respectively, and were associated with a mean cost per patient of $55,918.55, $5,316.13, and $25,139.28 (). Median length of all-cause inpatient hospital stays was 5 days (IQR 2–10 days). Of the 28,310 all-cause inpatient hospital stays in the overall incident cohort, 4,521 (16.0%) events were readmissions that occurred within 30 days of discharge, and 6,853 (24.2%) were cardiovascular-related. These cardiovascular inpatient stays accounted for 11.3 events per 100 patient years (PY) with a median length of 3 days (IQR 2–6 days) and were associated with a mean cost per patient of $38,548.41.
Table 2. HCRU event rates during the follow-up period
Event rates for acute coronary syndrome and ischemic stroke were 1.5 per 100 PY and 2.0 per 100 PY, associated with a cost per patient of $55,156.77 and $43,872.07, respectively. Rates of hospitalization due to AF and heart failure were 8.1 and 0.3 events per 100 PY, with associated cost per patient of $32,565.83 and $54,465.21, respectively.
More patients were diagnosed with paroxysmal versus persistent AF (46,434 versus 4,362, respectively). The rates of inpatient all-cause hospital stays in the overall paroxysmal and persistent groups were similar (40.5 and 41.2 per 100 PY, respectively; ). Rates of ER visits were higher in the paroxysmal versus persistent AF groups (100.4 versus 88.6 events per 100 PY).
Table 3. HCRU event rates during the follow-up period for the overall population stratified by paroxysmal and persistent AF
Screening and initiation of AF-related procedures were performed at a rate of 166.1 events per 100 PY, with an associated mean cost of $700.54 per patient () and AF catheter ablation procedures were performed at a rate of 8.4 events per 100 PY, with a mean cost per patient of $19,980.22. There were 37,447 cardioversions in 12,536 patients in the overall cohort (62.0 events per 100 PY; mean cost per patient of $1,817.87). Pacemaker and implantable cardioverter defibrillators were implanted at low rates (2.6 and 0.1 events per 100 PY, respectively).
Similar results were seen when stratifying by paroxysmal or persistent AF (). Among patients with paroxysmal AF, the incidence of catheter ablation during the follow-up period was 4.1% (6.9 events per 100 PY); among patients with persistent AF, these rates were 5.8% (9.9 events per 100 PY). Rates of cardioversion were higher in the persistent AF group with 84.7 events per 100 PY compared with 50.7 events per 100 PY in the paroxysmal cohort.
A high proportion of patients filled prescriptions for rate control medications (70.8%). DOAC prescriptions were filled by 50.4% of patients (). Prescriptions for DOACs were higher in the persistent versus the paroxysmal AF group (66.7% versus 48.8%) ().
Characteristics of the first-line dronedarone cohort are reported in Supplementary Table 2, and results of exploratory analyses in this subgroup are included in Supplementary Tables 3–4.
4. Discussion
This analysis of HCRU from a large, national dataset offers insights into the complexities of the US healthcare system through capture of treatment and medication claims from multiple payers. As expected, the results of this analysis indicate that cardiovascular comorbidities were prevalent in patients with incident AF, with most patients at increased risk for stroke. Management of AF, which is driven by AF burden and symptoms in combination with comorbid conditions [Citation11,Citation14], is associated with substantial HCRU, as demonstrated by the data on inpatient hospital stays, ER visits, outpatient visits, and substantial costs reported herein.
This effort was undertaken to better understand the HCRU in contemporary patients with AF in the US. Prior research on this topic was conducted by Kim et al. more than 10 years ago in a population that was approximately 3 years older (mean 71 years, vs 67.9 years in the current study) [Citation15]. All-cause hospitalization rates observed in this study (35.6%) were similar to that observed in the 2011 study by Kim (37.5%); however, rates of cardiovascular-related hospitalization were lower in this study (12.0% vs 21.3%) [Citation15]. The reduction in cardiovascular-related inpatient visits in this study may be due to the younger age of the population included in the current study, updated treatment guidelines in the past decade [Citation18] or due to changes in ICD coding (for example, ablation for AF has been mainly coded as an outpatient procedure since Q4 2015). Comparing patients with paroxysmal and persistent AF in the current study, all-cause hospitalization rates were similar between groups. However, those with paroxysmal AF had higher rates of any cardiovascular- and AF-related inpatient stays, possibly due to higher rates of AF-related prescriptions observed in this group. They also had a higher rate of AF-related procedures overall and higher rates of AF-related prescription medications.
Most patients in the study cohort had a CHA2DS2-VASc score ≥2, and a greater number of prescriptions for DOACs were reported relative to AADs. This is generally aligned with reports of current medication usage [Citation23–25] as well as prevailing clinical practice guidelines during the study period (2016 to 2018), which recommend AADs for symptom control after evaluation of appropriate rate control, while DOACs are recommended regardless of symptoms in patients with CHA2DS2VASc score ≥2 (men) or ≥3 (women) upon diagnosis of AF [Citation14,Citation26,Citation27]. As the guidelines recommend use of AADs for rhythm control in patients with symptoms, the pattern of AAD use in the study population (<10% use of AADs in the baseline period and ~25% during follow-up) may reflect increasing symptoms of AF over the study period in that population or filtering of symptomatic patients to specialized providers more likely to prescribe an AAD.
In the recent EAST-AFNET 4 study, which compared early, comprehensive rhythm control (AADs or AF catheter ablation) to guideline-recommended standard of care limited to management of AF-related symptoms, early rhythm control was associated with a significant reduction in risk of adverse cardiovascular outcomes. Additionally, the mean number of nights spent in the hospital did not differ significantly between the groups [Citation19]. Almost identical rhythm control therapy was received by asymptomatic patients from EAST-AFNET 4 compared with symptomatic patients, including AF ablation in ~25% of patients in follow-up 2 years after randomization [Citation28]. The effect of rhythm control therapy on cardiovascular complications in asymptomatic patients with AF was not observed to be different than in symptomatic patients and was also not affected by severity of symptoms [Citation28]. Additionally, in another sub-analysis of EAST-AFNET 4, the clinical benefit of early rhythm control therapy was observed in patients with preserved, midrange, and reduced left ventricular ejection fraction [Citation28,Citation29]. While EAST-AFNET 4 included both AADs and ablation as early rhythm therapy, the majority of patients received an AAD. However, studies such as Cryoballoon Catheter Ablation in Antiarrhythmic Drug Naive Paroxysmal Atrial Fibrillation (STOP AF) and Early Aggressive Invasive Intervention for Atrial Fibrillation (EARLY AF) have also supported the early use of ablation in people with paroxysmal AF [Citation30,Citation31]. The Catheter Ablation vs Antiarrhythmic Drug Therapy for Atrial Fibrillation (CABANA) trial demonstrated that catheter ablation, compared with medical therapy, did not significantly reduce the primary composite end point of death, disabling stroke, serious bleeding, or cardiac arrest [Citation32]. Costs were recently reported from the CABANA trial and were significantly higher among those receiving catheter ablation than those who received AAD therapy in the first 3 months of therapy, however, no significant differences in cost beyond 12 months were observed between groups [Citation33]. Updates to guidelines based on the emerging evidence on the potential benefits of early rhythm control may affect HCRU in patients with AF. Thus, a follow-up to the current analysis is warranted.
As dronedarone has been shown to reduce cardiovascular linked hospitalization [Citation34,Citation35], we conducted an exploratory analysis of HCRU in a small population of patients with AF receiving first-line dronedarone. In that population, the majority (57%) of patients were <65 years of age and were primarily covered by private insurance plans. Charlson comorbidity index values and CHA2DS2-VASc scores tended to be low in the dronedarone cohort. However, the dronedarone cohort had high rates of screening, indicating a possibly higher symptom burden in younger patients, likely reflecting a sub-population in need of specialized care [Citation36–38].
Limitations exist in the study due to the nature of claims data and the sample population. Biases may be unintentionally included as the population was not randomized [Citation21]. As data mostly come from large employers, patients not on an employer-sponsored health plan and patients with small- to medium-size employers may be underrepresented [Citation21]. The retrospective design of the study is also a potential limitation; although a 12-month baseline period is often used in retrospective analyses, this may have limited the capture of AF diagnoses or use of AADs, as well as preexisting conditions. Similarly, while a precedent exists for the 30-day pre-index period to assess prescription claims [Citation39], it may have limited capture of baseline medication use. Due to a lack of information available in the claims dataset, we were unable to identify severity of symptoms and disease burden and its impact on HCRU. While interventions during follow-up were evaluated, new diagnoses of underlying cardiopulmonary disease post-index were not evaluated, which is likely to have affected risks of hospitalizations and HCRU. Patients with postcardiac surgery-related AF were not excluded from the analysis and use of unique stroke-related measures such as left atrial appendage closure devices was not captured. Additionally, patient preferences were not directly accounted for in these data.
5. Conclusions
The results of this study present updated HCRU among patients with incident paroxysmal or persistent AF receiving care in the US and demonstrate that AF continues to be responsible for significant HCRU across a wide range of demographic and clinical groups. Hospitalization and inpatient stays continue to drive HCRU, however, rates of inpatient stays due to cardiovascular events may be lower than previously reported. Of note, rates of cardiovascular inpatient stays due to any cardiovascular event or AF were lower in those with persistent AF than those with paroxysmal. Exploratory analyses of a subpopulation of patients receiving first-line dronedarone suggest that different subpopulations in people with AF may receive different care patterns. Future work is needed to understand the long-term outcomes of variably intensive HCRU for AF in real-world practice, including the impact of increased use of early rhythm therapy on patient outcomes, whether specific subgroups of patients with AF are responsible for the high HCRU observed in this study, and whether these outcomes mirror those seen in contemporary clinical trials.
Since age is the most powerful risk factor for AF, the burden of AF is expected to continue to rise as the American population ages [Citation2,Citation3]. As the burden of AF on healthcare increases, HCRU is expected to increase as well, so effective and efficient management strategies are critically needed. This study takes a first step toward meeting this urgent need by establishing a baseline understanding of AF-related HCRU in a large, commercial, administrative database that is broadly representative of the US population and providing updated HCRU estimates in the overall incident AF cohort that reflect more contemporary AF management than prior publications.
This study showed that hospitalizations continue to drive HCRU with all-cause hospitalization rates for people with AF similar to those previously reported [Citation15]. However, though these rates remained similar, a reduction in cardiovascular-related hospital stays was observed [Citation15]. The current study provides information for the current status of HCRU in AF management. Management of AF is evolving, and there is emerging evidence demonstrating benefits of early rhythm control in the treatment of newly diagnosed AF, so treatment guidelines for AF are expected to change to reflect these data and more strongly recommend early rhythm control for AF [Citation19,Citation32,Citation40]. Therefore, it is important to understand how changes in management affect HCRU and further studies of real-world data would be key in assessing this in early treatment regimens.
In addition to the main study population, we carried out a small exploratory analysis of patients receiving dronedarone as first-line therapy, as its use has been associated with improved cardiovascular event outcomes [Citation34]. We noted that patients receiving dronedarone were likely to have private medical coverage. In addition, the majority of those receiving dronedarone were under 65 years of age and had relatively high screening rates for AF. These results suggest that different groups of people with AF may receive specialized care management. Therefore, it is important to understand how subgroups of AF patients may drive HCRU observed in AF management to help identify opportunities for improvement with regard to clinical outcomes and HCRU efficiency. We believe that this study provides a benchmark of HCRU in AF in an evolving treatment landscape. Given the results of this study, we believe that further work is warranted evaluating HCRU for AF in real-world practice, with a focus on the impact of increased use of early rhythm therapy, and in specific patient subgroups.
Author contributions
All authors contributed to the concept and design of the analysis, interpretation of the data, drafting/reviewing this paper, and confirming accuracy, and approved the final manuscript for publication.
Declaration of interest
A Cockerham, D McKindley, and C Ronk are employees of Sanofi and hold share and/or stock options in the company. S Huse is an employee of Evidera. M Kim reports consulting support from Sanofi. E Zeitler reports travel and speaking support from Medtronic, travel support from Abbott, and consulting support from Sanofi. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (408.3 KB)Acknowledgments
Medical writing and editorial support was provided by Brooke Middlebrook, and Meenakshi Subramanian, Evidence Medical Affairs (Philadelphia, PA, USA) and was funded by Sanofi US Inc.
Data Availability Statement
Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient level data will be anonymized, and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://www.clinicalstudydatarequest.com/.
Supplementary material
Supplemental data for this article can be accessed here
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Ferreira C, Providencia R, and Ferreira MJ, et al. Atrial fibrillation and con-cardiovascular diseases: a Systematic review. Arq Bras Cardiol. 2015;105(5):519–526.
- Miyasaka Y, Barnes ME, and Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114(2):119–125.
- Colilla S, Crow A, Petkun W, et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112(8):1142–1147.
- Tsao CW, Aday AW, and Almarzooq ZI, et al. Heart disease and stroke statistics-2022 update: a Report from the American Heart Association. Circulation. 2022;145(8):e153–e639.
- Perez MV, Mahaffey KW, and Hedlin H, et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. 2019;381(20):1909–1917.
- Benjamin EJ, Wolf PA, D’Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98(10):946–952.
- Jabre P, Roger VL, and Murad MH, et al. Mortality associated with atrial fibrillation in patients with myocardial infarction: a systematic review and meta-analysis. Circulation. 2011;123(15):1587–1593.
- Wattigney WA, Mensah GA, Croft JB. Increased atrial fibrillation mortality: United States, 1980-1998. Am J Epidemiol. 2002;155(9):819–826.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–988.
- Vidaillet H, Granada JF, and Chyou P, et al. A population-based study of mortality among patients with atrial fibrillation or flutter. Am J Med. 2002;113(5):365–370.
- Hindricks G, Potpara T, and Dagres N, et al. ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2020;42(5):373–498.
- Lip GYH. The ABC pathway: an integrated approach to improve AF management. Nat Rev Cardiol. 2017;14(11):627–628.
- Proietti M, Romiti GF, Olshansky B, et al. Comprehensive management with the ABC (Atrial Fibrillation Better Care) pathway in clinically complex patients with atrial fibrillation: a post hoc ancillary analysis from the AFFIRM trial. J Am Heart Assoc. 2020;9(10):e014932.
- January CT, Wann LS, and Alpert JS, et al. AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1–e76.
- Kim MH, Johnston SS, and Chu BC, et al. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4(3):313–320.
- Kim MH, Lin J, Hussein M, et al. Cost of atrial fibrillation in United States managed care organizations. Adv Ther. 2009;26(9):847–857.
- Coyne KS, Paramore C, Grandy S, et al. Assessing the direct costs of treating nonvalvular atrial fibrillation in the United States. Value Health. 2006;9(5):348–356.
- January CT, Wann LS, and Calkins H, et al. AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140(2):e125–e151.
- Kirchhof P, Camm AJ, and Goette A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383(14):1305–1316.
- Kim MH, Lin J, Jhaveri M, et al. Impact of dronedarone treatment on healthcare resource utilization in patients with atrial fibrillation/flutter. Adv Ther. 2014;31(3):318–332.
- IBM Watson Health. White Paper: IBM MarketScan Research Databases for life sciences researchers. (2018).
- IBM MarketScan. IBM MarketScan Research Databases, (Ed.^(Eds), 2022 https://www.ibm.com/products/marketscan-research-databases/databases. Accessed January 2022.
- Kim H, Kim TH, and Cha MJ, et al. A prospective survey of atrial fibrillation management for real-world guideline adherence: cOmparison study of Drugs for symptom control and complication prEvention of Atrial Fibrillation (CODE-AF) registry. Korean Circ J. 2017;47(6):877–887.
- Chang AY, Askari M, and Fan J, et al. Association of healthcare plan with atrial fibrillation prescription patterns. Clin Cardiol. 2018;41(9):1136–1143.
- Proietti M, Laroche C, and Opolski G, et al. ‘Real-world’ atrial fibrillation management in Europe: observations from the 2-year follow-up of the EURObservational Research Programme-Atrial Fibrillation General Registry Pilot Phase. Europace. 2017;19(5):722–733.
- Kirchhof P, Benussi S, and Kotecha D, et al. ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–2962.
- Mansour M, Heist EK, and Agarwal R, et al. Stroke and cardiovascular events after ablation or antiarrhythmic drugs for treatment of patients with atrial fibrillation. Am J Cardiol. 2018;121(10):1192–1199.
- Willems S, Borof K, and Brandes A, et al. Systematic, early rhythm control strategy for atrial fibrillation in patients with or without symptoms: the EAST-AFNET 4 trial. Eur Heart J. 2021. DOI:https://doi.org/10.1093/eurheartj/ehab593.
- Rillig A, Magnussen C, and Ozga AK, et al. Early rhythm control therapy in patients with atrial fibrillation and heart failure. Circulation. 2021;144(11):845–858.
- Andrade JG, Wells GA, and Deyell MW, et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med. 2021;384(4):305–315.
- Wazni OM, Dandamudi G, and Sood N, et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med. 2021;384(4):316–324.
- Packer DL, Mark DB, and Robb RA, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321(13):1261–1274.
- Chew D, Cowper P, and Li Y, et al. Economic outcomes of ablation versus drug therapy in CABANA. 2021 Heart Rhythm Society annual meeting. Boston and Virtual; 2021.
- Hohnloser SH, Crijns HJ, and van Eickels M, et al. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med. 2009;360(7):668–678.
- Torp-Pedersen C, Crijns HJ, and Gaudin C, et al. Impact of dronedarone on hospitalization burden in patients with atrial fibrillation: results from the ATHENA study. Europace. 2011;13(8):1118–1126.
- Reynolds MR, Lavelle T, Essebag V, et al. Influence of age, sex, and atrial fibrillation recurrence on quality of life outcomes in a population of patients with new-onset atrial fibrillation: the Fibrillation Registry Assessing Costs, Therapies, Adverse events and Lifestyle (FRACTAL) study. Am Heart J. 2006;152(6):1097–1103.
- Bhatia S, Sugrue A, Asirvatham S. Atrial fibrillation: beyond rate control. Mayo Clin Proc. 2018;93(3):373–380.
- Rodríguez-Mañero M, Sarkozy A, and Chierchia GB, et al. Prophylactic antiarrhythmic drug therapy in atrial fibrillation. J Atr Fibrillation. 2013;5(5):108–123.
- Steen DL, Khan I, Ansell D, et al. Retrospective examination of lipid-lowering treatment patterns in a real-world high-risk cohort in the UK in 2014: comparison with the National Institute for Health and Care Excellence (NICE) 2014 lipid modification guidelines. BMJ Open. 2017;7(2):e013255.
- Blomström-Lundqvist C, Marrouche N, and Connolly S, et al. Efficacy and safety of dronedarone by atrial fibrillation history duration: insights from the ATHENA study. Clin Cardiol. 2020;43(12):1469–1477.
