ABSTRACT
Objective
To determine the economically justifiable price (EJP) of cenobamate to become a cost-effective alternative compared with third-generation anti-seizure medications in the treatment of focal-onset seizures (FOS) in adult patients with drug-resistant epilepsy (DRE) in Spain.
Methods
Cost-effectiveness analysis compared cenobamate with brivaracetam, perampanel, eslicarbazepine acetate, and lacosamide. Markov model simulation of treatment pathway over a 60-year time horizon is presented. We determined the effectiveness and quality-adjusted life-years (QALYs) of health status and disutilities associated with treatment-related adverse events. Acquisition costs and use of medical resources were obtained from published literature and expert opinion. Base-case of cenobamate’s EJP calculated applying a willingness-to-pay (WTP) threshold of €21,000/QALY. Analyses were performed at different thresholds, including dominant price scenario. Result robustness was assessed through sensitivity analyses.
Results
Base-case shows that cenobamate’s daily EJP of €7.30 is cost-effective for a threshold of €21,000/QALY. At a daily price of €5.45, cenobamate becomes dominant over all treatment alternatives producing cost-savings for the national health system (NHS). Sensitivity analyses supported the robustness of base-case findings.
Conclusions
Treatment with cenobamate produces incremental clinical benefit over third-generation ASMs, and at the base-case, EJP could represent a cost-effective option for the adjunctive treatment of FOS in adult patients with DRE in Spain.
1. Background
Epilepsy is one of the most common chronic neurological disorders affecting around 50 million people worldwide [Citation1,Citation2] with an estimated prevalence in Spain of 5,79 per 1,000 habitants [Citation3] corresponding to approximately 200,000 adult patients in Spain. It is characterized by recurrent spontaneous seizures, which can be classified as focal or generalized. Epilepsy with focal-onset seizures (FOS) is the most common type in the adult population, accounting for more than 60% of the patients with epilepsy in Spain [Citation4].
Conventional treatment for epilepsy is based on the long-term administration of oral anti-seizure medications (ASMs) [Citation5]. For most patients, treatment with ASMs results in long-term seizure freedom. However, approximately 40% of patients with epilepsy, particularly those with FOS, present drug-resistant epilepsy (DRE) despite the availability of more than 20 ASMs [Citation6]. The International League Against Epilepsy (ILAE) defines DRE as treatment failure to two tolerated, appropriately chosen and used ASM schedules (in monotherapy or in combination) to achieve sustained seizure freedom. Failure is considered to occur if the patient does not remain seizure-free for a minimum of three times the longest pre-treatment inter-seizure interval or 12 months, whichever is longer [Citation7].
Spanish experts in epilepsy consider DRE as a severe condition associated with a significant decrease in quality of life (QoL) for patients and their caregivers, the presence of associated comorbidities and an increased probability of early death compared with patients with seizure freedom [Citation8], which leads to important health-care resource consumption and economic burden for the Spanish National Health System (NHS) and society in general [Citation8–10].
Furthermore, DRE is associated with relevant unmet needs, mainly the availability of ASMs with improved efficacy and tolerability profiles that allow to reach treatment objectives [Citation8]. Despite the most recent ASMs approved in the last decade, the so-called ‘third-generation ASMs’, the response to pharmacological treatment has not been substantially modified compared to previous years, and the probability of achieving seizure freedom in DRE patients remains at approximately 4% [Citation6,Citation11]. Additionally, some of these treatments present numerous treatment-emergent adverse events (TEAEs) such as somnolence, dizziness, or even aggression or psychosis, as well as interactions that can complicate patient treatment and management, especially for those with DRE, which are usually treated with a combination of several ASMs [Citation8].
The lack of clinical practice guidelines and/or specific protocols for the management of DRE makes the choice of medication difficult, highlighting the need to individualize treatment according to patient’s profile [Citation12]. Third-generation ASMs, although not specifically indicated for DRE, have included patients refractory to previous therapies in their clinical development programs and currently represent the most commonly used option in these patients.
Cenobamate is a new and the first ASM approved in Spain (June 2021 [Citation13]) for the adjunctive treatment of FOS with or without secondary generalization in adult patients with epilepsy who have not been adequately controlled despite a history of treatment with at least two ASMs [Citation14]. To date, it is undergoing evaluation for pricing and reimbursement in Spain, so no public price is available. Cenobamate has demonstrated a high efficacy, with as much as a 20% rate of seizure-free patients, never reached before in comparable trials with third-generation ASMs [Citation15,Citation16], representing a new and efficacious treatment option for these patients [Citation17]. Furthermore, the latest update of the epilepsy guidelines by the Spanish Society of Neurology (2022) recognizes cenobamate as the only effective treatment for drug-resistant epilepsy [Citation18].
The increasing number of treatment options and the level of expenditure associated with the treatment and management of these patients underline the need for economic evaluations in this field [Citation19]. Cost‐effectiveness evaluations play an important role in supporting decision‐makers seeking to understand the value of competing health technologies and management strategies. Thus, the aim of this study is to determine the economically justifiable price (EJP) for cenobamate to represent a cost-effective alternative compared with standard of care third-generation ASMs (brivaracetam [Citation20], perampanel [Citation21], eslicarbazepine acetate [Citation22] and lacosamide [Citation23]) in the adjunctive treatment of FOS in patients with DRE in Spain.
2. Methods
2.1. Design and analysis perspective
A simulation Markov model was developed using Microsoft Excel® for the cost-effectiveness (CE) analysis of cenobamate versus the above mentioned third-generation ASMs. The analysis was performed from the Spanish healthcare system perspective. A 60-year time horizon was employed as it comprehensively captures the expected costs and health outcomes of patients over their remaining lifetime. The length of each treatment cycle was 28 days. The population entering the analysis included adult patients with FOS who have not been adequately controlled despite a history of treatment with at least two ASMs, in line with cenobamate’s approved indication [Citation14].
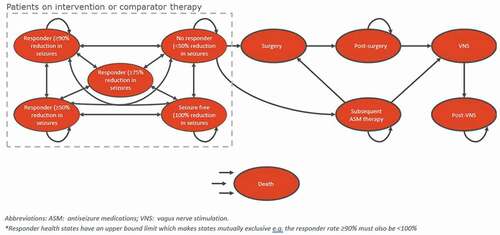
illustrates the model structure. Patients enter the model in the ‘No response’ health state where they initiate adjunctive ASM treatment. Whilst on treatment with cenobamate or with one of the comparators, patients move between the response health states. Following discontinuation, patients transition to a subsequent ASM therapy health state where they receive further combinations of ASM therapies to manage their condition. Patients may leave this state if they are suitable for surgery or vagus nerve stimulation (VNS) and proceed to these health states. Patients ineligible for invasive procedures remain on subsequent ASM therapy for the remainder of the model time horizon or until death.
The EJP of cenobamate was calculated based on a willingness-to-pay (WTP) threshold of €21,000/QALY, according to Spanish guidelines [Citation24] and it is expressed as daily cost of treatment. The key measure of effectiveness in the model was quality-adjusted life year (QALY), and the main outcome measure was the incremental CE ratio (ICER). ICERs below the WTP threshold are considered cost-effective. A 3.5% annual discount was applied both for costs and for health outcomes in line with Spanish guidelines [Citation24].
2.2. Comparators
There is currently no adequate price comparator for cenobamate since there is no other ASM authorized in Spain with the same indication. However, third-generation ASMs are the most commonly prescribed treatments in the third-line adjunctive setting (accounting for, approximately, 70% of prescriptions) [Citation25], so in order to be able to perform the CE analysis, the following four third-generation ASMs have been chosen: brivaracetam, perampanel, eslicarbazepine acetate, and lacosamide. This choice was validated by clinical experts in treating DRE patients in Spain [Citation25].
2.3. Clinical inputs
An expert panel composed of three Spanish clinical experts on DRE validated the parameters included in the model and contributed with complementary information from routine clinical practice whenever data could not be found in published literature. The evidence base for cenobamate derives from the C017 study [Citation16], its open-label extension (OLE) [Citation26] and the C021 study [Citation27]. The C013 study was not included in the economic analysis as its 6-week maintenance period, according to EMA guidance, is not considered sufficient to demonstrate long-lasting efficacy [Citation28].
The clinical efficacy of cenobamate, defined according to the level of response to treatment, was obtained from the C017 study [Citation16]. Response to treatment with cenobamate in each patient was parameterized according to the relative reduction in seizures compared to the screening period values over the last 28 days (duration of one cycle). A description of each response category and the distribution of patients among them is shown in . Transition probabilities for the first five cycles of the model were parameterized based on data from the C017 study [Citation16]. Transition probabilities among the response health states from cycle 6 onwards were extrapolated using the average transition probabilities over cycles 3–5 (which include the maintenance period) (Supplementary Table S1). Given the absence of head-to-head clinical trials between cenobamate and its comparators, an indirect treatment comparison (ITC) was performed [Citation29,Citation30]. Odds ratios from the ITC informed the treatment response for comparators (). Outcomes considered in the ITC were the proportion of patients with ≥50% reduction in seizures (moderate response) and the proportion of patients with seizure freedom (complete response).
Table 1. Distribution and description of response categories among cenobamate patients.
Table 2. Odd ratios of response to treatment and adverse events of comparators relative to cenobamate.
Patients enter the model with concomitant ASM treatment as background therapy. The model assumes that, after the introduction of cenobamate, concomitant ASMs are reduced by 5%, 10%, and 15% in the first, second, and subsequent years, respectively [Citation31].
The frequency of seizures per cycle quantified resource use associated with event management according to response category. Baseline seizure frequency rates per seizure type were obtained from the panel expert. The relative reduction of seizures by seizure type and response category were derived from C017 study data [Citation16] ().
Table 3. Seizure frequency per 28 days at baseline and median seizure reduction, by seizure type and response to treatment.
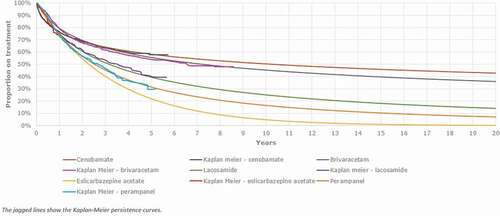
TEAEs reported in more than 5% of subjects treated with cenobamate were used with distinction between titration and maintenance phases [Citation16,Citation27]. Results from the ITC [Citation29,Citation30] were used to generate the rate of TEAEs for comparator treatments (). Data from the C017 study [Citation16], its OLE [Citation26] and the C021 study [Citation27] were used to inform the parametric distributions for Time-to-discontinuation (TTD) and the Kaplan-Meier curve for cenobamate. The generalized gamma was selected as the appropriate curve for estimating treatment discontinuation since it proved to be the most statistically efficient and consistent with the discontinuation rates observed in the studies (approximately 60% of patient retention rate after 4 years of treatment with cenobamate [Citation26]). The TTD for comparator treatments were obtained by digitizing published Kaplan–Meiers of each comparator [Citation32–35]. The data were then fitted to parametric distributions and curves to each Kaplan Meier. The resulting TTD is presented in .
The ‘Death’ health state accumulates patients who die due to all-cause mortality. HRs included were 1.6 and 2.4 for subgroups of patients who achieve seizure freedom and for those who do not achieve seizure freedom, respectively [Citation36].
2.4. Cost and resource use
In accordance with the analysis’ perspective, direct health-care costs were considered (), including pharmacological treatment, administration, routine monitoring, epilepsy event management, and TEAE management costs. Resource use in the treatment and management of epilepsy was obtained through panel expert opinion. Unit costs were obtained from the Spanish national healthcare cost databases [Citation37,Citation38] and from published literature [Citation9,Citation39]. Pharmacological costs were differentiated between titration and maintenance periods. The daily cost was calculated according to the average modal dose for each comparator obtained from the ITC [Citation29,Citation30]. The cost of administration includes neurologist outpatient visits during the titration phase whose frequency was determined by the expert panel [Citation25] and monitoring procedures in the case of lacosamide as stipulated in its Summary of Product Characteristics (SmPC) [Citation23]. The model considered that DRE patients routinely visit neurologist, general practitioner (GP) and other specialties such as psychiatrists. The frequency of these visits was determined by the panel expert [Citation25] according to patient response category and their real clinical practice experience. Resource use associated with the management of seizures was also estimated by clinical experts [Citation25] and calculated by seizure type and include attendance to accident & emergency (A&E), ambulance calls, GP and neurologist visit and hospitalization in the neurology department. It is considered that patients who attend the A&E and/or are hospitalized receive intravenous pharmacological treatment and other procedures such as routine tests. It is considered that TEAEs management would require treatment by a neurologist, so TEAEs management costs were assumed to be the cost of a visit to a neurologist.
Table 4. Costs and use of resources per treatment option included in the model.
2.5. Utility data
SF-6D values were sourced from a mapping study [Citation29] of patients with epilepsy retrospectively applied to data from the C017 study [Citation16] to reflect QoL according to health state: no response (0.50), moderate response (0.57), high response (0.61), very high response (0.61), and seizure freedom (0.65). Utility values for subsequent ASM treatment (0.55), post-surgery (0.61), and post-VNS (0.56) were calculated as weighted averages of the response rate utility values and patients’ distribution among different levels of response to treatment. The utility values for these health states were 0.5 for VNS, 0.56 for post-VNS, 0.5 for surgery, 0.61 for post-surgery, and 0.55 for subsequent ASM treatment. It was assumed that patients in the surgery and VNS health states would have the same utility as patients with no response to treatment (0.5). The TEAE disutility value (−0.06) and the disutility duration (28 days) were collected from the published literature to calculate the total QALY decrement (−0.0047) [Citation40].
2.6. Sensitivity analyses
A sensitivity analysis was performed by varying the WTP threshold. A lower threshold of €11,000 and a less strict threshold of €30,000 were included as recommended by Spanish guidelines [Citation24]. In addition, the maximum daily cost at which cenobamate remains to be the dominant (cost-saving) alternative was calculated.
One-way sensitivity analysis (OWSA) was conducted to reveal the impact of parameter variability on the ICER. The price was a fixed parameter. The EJP of cenobamate obtained from the base-case was included in this analysis. The OWSA was performed using a standard error approach. Whenever the standard error was not available for a given parameter, it was assumed to be 20% of the mean value. Based on its mean and the standard error, the parameter was then varied using a 95% confidence interval based on its distribution. The result of the OWSA was displayed as a tornado diagram ().
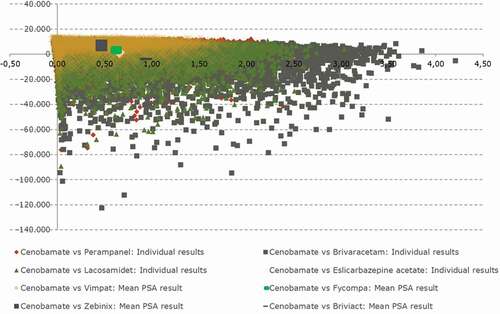
A probabilistic sensitivity analysis (PSA) was performed to assess the uncertainty of the input parameters in 10,000 simulations according to the Log-normal distribution for the OR sourced from the ITC analysis, beta distributions for clinical probabilities and resource use, health state utilities and gamma distribution for costs and seizure frequency. Where the standard errors for the parameters were unknown, they were assumed to be 20% of the parameter value for the purposes of defining the distributions for each parameter. Results of the PSA were presented in a CE plane (ICEP) ().
3. Results
3.1. Base-case analysis
Based on the WTP threshold of €21,000/QALY, an EJP of €7.30 was calculated. Over the lifetime period, at this EJP, treatment with cenobamate was associated with a total cost of €83,891 and 11.585 QALYs. In comparison with third-generation ASMs, cenobamate resulted in differential cost versus alternatives ranging from €6,715 for eslicarbazepine acetate to €-4,251 for brivaracetam and incremental QALYs ranging from 0.706 for brivaracetam to 0.320 for eslicarbazepine acetate. At an EJP of €7.30, cenobamate is not only cost-effective compared to all third-generation therapeutic alternatives but also dominant (cost-saving) compared to brivaracetam. The analysis resulted in ICER values of 21,000 vs. eslicarbazepine acetate, 8,389 vs. perampanel, 2,202 vs. lacosamide and −6,022 vs brivaracetam. Aggregated base-case results of the CE analysis are presented in .
Table 5. Cost-effectiveness analysis results (base-case and sensitivity analysis).
3.2. Sensitivity analyses
The threshold sensitivity analysis for the daily cost of cenobamate demonstrates that, for a WTP threshold of €11,000 per QALY, the daily cost per patient of cenobamate should be at €6.42 while for a WTP threshold of €30,000 per QALY, the daily cost of cenobamate should be at €8.09. For cenobamate to represent a cost-saving alternative and to dominate over all therapeutic alternatives, the daily cost should be at €5.45. shows the results of the CE analysis for the different WTP thresholds.
presents the tornado diagram assessing the variables most influential on the ICER from the CE analysis of cenobamate vs. eslicarbazepine acetate, the next cheapest comparator associated with the second highest QALYs gained after cenobamate. The key parameters identified as having the largest impact on the ICER were the odds ratio of seizure freedom and the odds ratio of response associated with eslicarbazepine acetate and the proportion of surgery leading to seizure freedom, highlighting these parameters as key drivers in the model.
shows the CEIP from the PSA, where 69.47% of the 10,000 simulations fell within the north-east quadrant, indicating that cenobamate is cost-effective compared to the alternatives. Moreover, 27.17% of simulations fell within the south-east quadrant, indicating that cenobamate is dominant over alternatives based on producing increased health gains and being less costly.
4. Discussion
This study represents a firsthand economic evaluation of cenobamate in Spain based on a CE study compared to standard treatment in drug-resistant epilepsy. CE analysis provides a formal mechanism to establish whether the incremental cost of a new technology is justified by its health gains and associated cost offsets, being critical for informed decision-making by health-care payers. This point of view is also reflected in recent projects spearheaded by the Spanish Ministry of Health such as REvalMED, which already includes economic evaluation as part of the Therapeutic Positioning Reports (TPR) [Citation41].
The EJP reflects the maximum price that could be set for a health-care intervention, such that it will still be deemed to be an efficient use of limited health-care resources. This is often estimated as the price that would result in an ICER being equal to, or just below, the WTP threshold [Citation42].
The results from this study show that, at a daily price of €7.30, cenobamate results cost-effective considering a willingness-to-pay threshold of €21,000/QALY. The CE threshold of €21,000/QALY is considered as standard in the economic evaluation guidelines of drugs proposed by the Spanish Society of Hospital Pharmacy (SEFH) [Citation24] and has been previously established in the existing literature conducted within the Spanish context [Citation43,Citation44]. Hence, a daily price of €7.30 represents the EJP for cenobamate. At a price of €5.45, cenobamate would not only represent a cost-effective option but would also produce cost-savings to the Spanish NHS.
It is noteworthy that the introduction of cenobamate allows for a reduction in the number of concomitant ASMs [Citation31], which could benefit both the safety and tolerability profile for the patient and represent a saving in terms of pharmacological costs. The proven high efficacy of cenobamate translates into a lower use of health-care resources, mainly in terms of fewer visits to specialists and A&E services. The efficacy of cenobamate has been specifically studied and demonstrated in all types of focal seizures, including focal to bilateral tonic-clonic seizures, associated with an increased risk of morbidity and mortality [Citation45,Citation46], with a consequent reduced need for medical care, hospitalization, and pharmacological treatment and reduced social and psychological burden for patients and caregivers.
The incremental benefit of cenobamate reported by this study is aligned with and complements recently published evidence from a network meta-analysis (NMA) among third-generation ASMs for the treatment of FOS in adult patients. This NMA demonstrates that cenobamate is ranked best for efficacy compared to brivaracetam, perampanel, lacosamide, and eslicarbazepine acetate [Citation47].
Findings in this study are not in line with a previous study based on the CE analysis of brivaracetam compared to third-generation ASMs in the adjunctive treatment of patients with FOS in Spain [Citation39]. The analysis of brivaracetam includes perampanel, lacosamide, and eslicarbazepine acetate as comparators and its results show that brivaracetam is the dominant option. In contrast, our study has shown that brivaracetam is associated with the highest cost and least QALYs gained. The difference in results may be due to the different parameters included that impact on resource use and the associated costs, the utility values considered, and the time horizon used in both analyses, which makes comparison difficult between them.
In this study, the effectiveness and cost data were obtained from the literature [Citation9,Citation37–39] but also included first-hand data and personal experience as reported by three Spanish clinical experts [Citation25]. This not only provided a real clinical practice perspective on the use of health-care resources for the management of FOS in patients with DRE in Spain but made the model results more applicable to the Spanish NHS and patient population.
In addition, it must be highlighted that the model design, the selected comparators (third-generation ASMs), and the Markov model used in this analysis were validated by Spanish clinical experts, and they have also been used in other recent economic assessments of cenobamate in the United Kingdom by the National Institute for Health and Care Excellence (NICE) [Citation29] and in Sweden by the Dental and Pharmaceutical Benefits Agency (TLV) [Citation48]. Besides, review of the CE model by health economics and outcomes research experts validated the appropriateness and accuracy of the model and the use of the 60-year time horizon to capture the long-term CE of the intervention.
The results of this analysis are in line with the results of the economic analyses carried out by both NICE [Citation29] and TLV [Citation48], which conclude that cenobamate is a cost-effective alternative in the adjunctive treatment of FOS in adult patients with DRE.
4.1. Limitations
The study results should be interpreted in the context of some limitations. One of them is the lack of real-life data across treatment alternatives, with most of the data coming from the available literature supplemented by clinical experts’ input. Although these data are modeled, they can over- and under-estimate probabilistic values using the health-care payer perspective.
Given the absence of head-to-head comparative data between the alternatives, it was necessary to perform indirect comparisons using a Network Meta-Analysis (NMA) [Citation29,Citation30]. However, the studies for the different comparators included in the ITC differed in their duration, variables measured and patient population.
Indirect costs arising from out-of-pocket expenses associated with epilepsy and caregiver involvement due to their lost productivity have not been included in this model. CE studies can benefit from taking into account a broader societal perspective, in which the goal is to maximize social welfare, including broader impacts on society that fall outside the health-care sector (e.g. impact on productivity and minimization of need for social care services).
Finally, due to the limited published economic and resource use data in Spain, complementary data to fill evidence gaps were provided by clinical experts in epilepsy [Citation25]. Variability in resource use may exist depending on the choice of experts, their location, and practice context.
5. Conclusions
Treatment with cenobamate results in incremental clinical benefit over third-generation ASMs, demonstrating that it could be a cost-effective option for the adjunctive treatment of FOS in adult patients DRE in Spain.
Cenobamate represents an innovative treatment option in a disease with many unmet needs, and its clinical contribution to those has been demonstrated [Citation16,Citation26,Citation27]. The present study demonstrates its superior CE, producing more health per invested euro, which could be useful for evaluators and decision-makers in Spain who look at obtaining as much health as possible from new treatment alternatives. Results can also contribute to the performance of holistic value-based pricing and reimbursement evaluations and informed decision-making considering both the clinical and economic value contributions of cenobamate.
Declaration of interest
MA Calleja has received honoraria for participating in advisory boards/consultancy from Angelini, Amgen, Janssen, Pfizer, Roche, Novartis, Alexion, Lilly, Bayer, AstraZeneca, Galápagos, BMS, Almirall, UCB, MSD, Abbvie, Mylan, Sanofi, Teva. A Navarro has received honoraria for presentations and advisory boards from Angelini, GSK, Bristol, MSD, Pfizer and ASTRA and congresses registration fee from Janssen. JM Serratosa has received honoraria for participation in advisory boards or pharmaceutical industry-sponsored symposia from Angelini, Arvelle, BIAL, Eisai Inc, Esteve, GW Pharmaceuticals, Sanofi, UCB Pharma and UNEEG. M Toledo has received fundings and honoraria from Angelini, UCB Pharma, EISAI Inc, BIAL laboratorios, GSK, Esteve, Jazz Pharma, and Neuraxpharm. V Villanueva has received honoraria and/or research funds from Angelini, Arvelle, Bial, Eisai, Esteve, GW Pharma, NewBridge, Novartis, Takeda, UCB Pharma and Zogenix. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
S Subias and A Gil were responsible for the development of the economic analysis and the writing of the manuscript. MA Calleja, A Navarro, JM Serratosa, M Toledo and V Villanueva validated the study’s structure and premises and provided information on clinical practice in Spain. All the authors took part in the interpretation of the results and the review of the manuscript. All authors agree for the final version of the manuscript to be published.
Supplemental Material
Download MS Word (32.7 KB)Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14737167.2022.2107507
Additional information
Funding
References
- WHO. Epilepsy. [Internet]. 2019 [cited 2022 Mar 21]. Available from: https://www.who.int/en/news-room/fact-sheets/detail/epilepsy
- Beghi E. The epidemiology of epilepsy. Neuroepidemiology. [Internet]. 2020 [cited 2022 Mar 21];54(2):185–191. Available from: https://www.karger.com/Article/FullText/503831
- Serrano-Castro PJ, Mauri-Llerda JA, Hernández-Ramos FJ, et al. Adult prevalence of epilepsy in Spain: EPIBERIA, a population-based study. ScientificWorldJournal [Internet]. 2015 [cited 2022 Mar 21];2015:602710. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26783554.
- García-Ramos R, Pastor AG, Masjuan J, et al. FEEN: informe sociosantario FEEN sobre la epilepsia en España. [Internet]. Neurologia. 2011 [cited 2022 Mar 21]. 548–555. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21757265
- Goldenberg MM. Overview of drugs used for epilepsy and seizures: etiology, diagnosis, and treatment. P T. [Internet]. 2010 [cited 2022 Mar 21];35:392–415. Available from: http://professionals.epilepsy.com/page/
- Chen Z, Brodie MJ, Liew D, et al., Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs a 30-year longitudinal cohort study. JAMA Neurol. 2018;75(3):279–286.
- Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc task force of the ILAE commission on therapeutic strategies. Epilepsia. 2010;51(6):1069–1077.
- Villanueva V, Carreño M, Gil-Nagel A, et al. Identifying key unmet needs and value drivers in the treatment of focal-onset seizures (FOS) in patients with drug-resistant epilepsy (DRE) in Spain through multi-criteria decision analysis (MCDA). Epilepsy Behav. [Internet]. 2021 [cited 2022 Mar 21];122:108222. Available from: https://pubmed.ncbi.nlm.nih.gov/34371462/.
- Villanueva V, Girón JM, Martín J, et al. Impacto económico y en calidad de vida de la epilepsia resistente en España: estudio ESPERA. Neurologia. [Internet]. 2013 [cited 2022 Mar 21];28(4):195–204. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22743210
- Sancho J, Peña P, Rufo M, et al. Health and non-health care resources use in the management of adult outpatients with drug-resistant epilepsy in Spain: a cost-of-illness study (LINCE study). Epilepsy Res. [Internet]. 2008 [cited 2022 Mar 21];81(2–3):176–187. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18617369
- Perucca E, Brodie MJ, Kwan P, et al. 30 years of second-generation antiseizure medications: impact and future perspectives [Internet]. Lancet Neurol. Lancet Publishing Group; 2020 [cited 2022 Mar 21]. 544–556. Available from: https://pubmed.ncbi.nlm.nih.gov/32109411/
- Sociedad Española de Neurología. Manual de Práctica Clínica en Epilepsia. Recomendaciones diagnóstico-terapéuticas [Internet]. 2019 [cited 2022 Mar 21]. Available from: http://epilepsia.sen.es/wp-content/uploads/2020/06/Recomendaciones-Epilepsia-SEN-2019.pdf
- CIMA Centro de información de medicamentos [Internet]. [cited 2022 Mar 21]. Available from: https://cima.aemps.es/cima/publico/home.html
- EMA (The European Medicines Agency). Summary of product Characteristics Ontozry (Cenobamate). 2021
- Chung SS, French JA, Kowalski J, et al. Randomized phase 2 study of adjunctive cenobamate in patients with uncontrolled focal seizures. Neurology. [Internet]. 2020 [cited 2022 Mar 21];94(22):e2311–22. Available from: https://pubmed.ncbi.nlm.nih.gov/32409485/
- Krauss GL, Klein P, Brandt C, et al. Safety and efficacy of adjunctive cenobamate (YKP3089) in patients with uncontrolled focal seizures: a multicentre, double-blind, randomised, placebo-controlled, dose-response trial. Lancet Neurol. 2020;19(1):38–48.
- Steinhoff BJ. Cenobamate-a new perspective for epilepsy treatment. Nervenarzt. [Internet]. 2021 [cited 2022 Mar 21];92(2):150–160. Available from: https://pubmed.ncbi.nlm.nih.gov/32990790/
- Sociedad Española de Neurología. Guía de Epilepsia [Internet]. 2022 [cited 2022 Jul 05]. Available from: http://guiaepilepsia.sen.es/index.php/guia
- Simoens S. Pharmacoeconomics of anti-epileptic drugs as adjunctive therapy for refractory epilepsy. Expert Rev Pharmacoecon Outcomes Res. [Internet]. 2010 [cited 2022 Mar 21];10(3):309–315. Available from: https://pubmed.ncbi.nlm.nih.gov/20545595/
- EMA (The European Medicines Agency). Summary of product characteristics Briviact (brivaracetam). 2016
- EMA (The European Medicines Agency). Summary of product characteristics Fycompa (perampanel). 2012
- EMA (The European Medicines Agency). Summary of product Characteristics Zebinix (eslicarbazepine acetate). 2009
- EMA (The European Medicines Agency). Summary of product characteristics Vimpat (lacosamide). 2009
- GENESIS-SEFH. Guía de evaluación económica e impacto presupuestario en los informes de evaluación de medicamentos [Internet]. 2016 [cited 2022 Mar 21. Available from: https://gruposdetrabajo.sefh.es/genesis/genesis/Documents/GUIA_EE_IP_GENESIS-SEFH_19_01_2017.pdf
- Clinical expert opinion
- Klein P, Krauss G, and Aboumatar S, et al. Long-term efficacy and safety of adjunctive cenobamate in patients with uncontrolled focal seizures: open-label Extension of a Randomized Clinical Study. Neurology. 2020;94(1008).
- Sperling MR, Klein P, Aboumatar S, et al. Cenobamate (YKP3089) as adjunctive treatment for uncontrolled focal seizures in a large, phase 3, multicenter, open-label safety study. Epilepsia. [Internet]. 2020 [cited 2022 Mar 21];61(6):1099–1108. Available from: https://pubmed.ncbi.nlm.nih.gov/32396252/
- EMA (The European Medicines Agency). Guideline on clinical investigation of medicinal products in the treatment of epileptic disorders. 2018. Available from: https://www.ema.europa.eu/en/clinical-investigation-medicinal-products-treatment-epileptic-disorders.
- Single technology appraisal | cenobamate for focal onset seizures in epilepsy | committee papers | NICE [Internet]. 2021 [cited 2022 Mar 21]. Available from: https://www.nice.org.uk/guidance/ta753/evidence/appraisal-consultation-committee-papers-pdf-10894234285
- Privitera M, Richy FF, Schabert VF. Indirect treatment comparison of cenobamate to other ASMs for the treatment of uncontrolled focal seizures. Epilepsy Behav. 2022;126:108429.
- Gonzalez R. Efectividad a largo plazo de cenobamato en epilepsia focal refractaria. Presented at: LXXII Reunión Anual de la Soceidad Española de Neurología. 2020 26 Noviembre-3 Diciembre; Sevilla, España .
- Krauss GL, Perucca E, Kwan P, et al. Final safety, tolerability, and seizure outcomes in patients with focal epilepsy treated with adjunctive perampanel for up to 4 years in an open-label extension of phase III randomized trials: study 307. Epilepsia. [Internet]. 2018 [cited 2022 Mar 21];59(4):866–876.
- Hufnagel A, Ben-Menachem E, Gabbai AA, et al. Long-term safety and efficacy of eslicarbazepine acetate as adjunctive therapy in the treatment of partial-onset seizures in adults with epilepsy: results of a 1-year open-label extension study. Epilepsy Res. 2013;103(2–3):262–269.
- Rosenfeld W, Fountain NB, Kaubrys G, et al. Safety and efficacy of adjunctive lacosamide among patients with partial-onset seizures in a long-term open-label extension trial of up to 8 years. Epilepsy Behav [Internet]. 2014 [cited 2022 Mar 21];41:164–170. Available from: https://pubmed.ncbi.nlm.nih.gov/25461210/
- Toledo M, Whitesides J, Schiemann J, et al. Safety, tolerability, and seizure control during long-term treatment with adjunctive brivaracetam for partial-onset seizures. Epilepsia. Internet]. 2016 [cited 2022 Mar 21];57(7):1139–1151. Available from: https://pubmed.ncbi.nlm.nih.gov/27265725/
- Trinka E, Bauer G, Oberaigner W, et al. Cause-specific mortality among patients with epilepsy: results from a 30-year cohort study. Epilepsia. [Internet]. 2013 [cited 2022 Mar 21];54(3):495–501. Available from: https://pubmed.ncbi.nlm.nih.gov/23167828/
- Oblikue. eSalud - Información económica del sector sanitario [Internet] Available from: http://esalud.oblikue.com/index.asp
- Botplusweb.portalfarma.com. BOT Plus 2. base de datos de medicamentos [Internet]. [cited 2022 Mar 21]. Available from: https://botplusweb.portalfarma.com/botplus.aspx
- Barrachina-Martinez I, Vivas-Consuelo D, Reyes-Santias F Cost-utility model of brivaracetam in the adjunctive treatment of patients with epilepsy in Spain. Expert Rev Pharmacoeconomics Outcomes Res [Internet]. 2020 [cited 2022 Mar 21]. Available from: https://pubmed.ncbi.nlm.nih.gov/33074031/
- de Kinderen RJA, Wijnen BFM, van Breukelen G, et al. From clinically relevant outcome measures to quality of life in epilepsy: a time trade-off study. Epilepsy Res. 2016;125:24–31.
- REvalMed S. Plan para la consolidación de los informes de posicionamiento terapéutico de los medicamentos en el Sistema Nacional de Salud. 2020.
- York Health Economics Consortium. Economically Justifiable Price [Internet]. 2016 [cited 2022 Mar 21]. Available from: https://yhec.co.uk/glossary/economically-justifiable-price/
- Vallejo-Torres L, García-Lorenzo B, Serrano-Aguilar P. Estimating a cost-effectiveness threshold for the Spanish NHS. Health Econ. [Internet]. 2018 [cited 2022 Mar 21];27(4):746–761. Available from: https://pubmed.ncbi.nlm.nih.gov/29282798
- Sacristán JA, Oliva J, Campillo-Artero C, et al. What is an efficient health intervention in Spain in 2020?. Gac Sanit. 2020;34(2):189–193.
- Whitney R, Donner EJ Risk factors for sudden unexpected death in epilepsy (SUDEP) and their mitigation [Internet]. Curr. Treat. Options Neurol. Current Science Inc.; 2019 [cited 2022 Mar 21]. Available from: https://pubmed.ncbi.nlm.nih.gov/30758730/
- Harden C, Tomson T, Gloss D, et al. Practice guideline summary: sudden unexpected death in epilepsy incidence rates and risk factors: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. [Internet]. 2017 [cited 2022 Mar 21];88(17):1674–1680. Available from: https://pubmed.ncbi.nlm.nih.gov/28438841/
- Lattanzi S, Trinka E, Zaccara G, et al. Third-generation antiseizure medications for adjunctive treatment of focal-onset seizures in adults: a systematic review and network meta-analysis. Drugs. [Internet]. 2022 [cited 2022 Mar 21];82(2):199–218. Available from: https://pubmed.ncbi.nlm.nih.gov/35061214/
- Dental and Pharmaceutical Benefits Agency (TLV) [Internet]. [cited 2022 Mar 21]. Available from: https://www.tlv.se/om-oss/om-tlv.html