ABSTRACT
Background
There is limited evidence on within-country discrepancies in biosimilar uptake. This study analyzes differences in timing and diffusion of biosimilar uptake across Portuguese NHS hospitals and explores possible determinants.
Research design and methods
We analyzed publicly accessible consumption data of originator biologic and biosimilar drugs for adalimumab, etanercept, infliximab, rituximab, and trastuzumab, by hospital and month for the years 2015–2021 (N = 9,467). We modeled the time to biosimilar adoption using survival regression models and the share of biosimilar consumption using generalized estimated equations with random hospital effects.
Results
Academic hospitals were characterized by a quicker uptake of adalimumab and infliximab biosimilars but lower shares for other drugs. A higher total consumption of biologics was related to a lower share of biosimilar uptake. A stronger participation in randomized controlled trials was linked to higher biosimilar shares and quicker uptake, except for rituximab. If all NHS hospitals had biosimilar shares equal to the highest ones, potential annual savings could reach 13.9 million euros.
Conclusion
The findings suggest a need for capacity-building on biosimilar prescribing, including for doctors of academic hospitals and those working in settings where high biosimilar use would be expected.
1. Introduction
In the last decade, health systems have been challenged by the market entry of new drugs with high prices [Citation1–4]. This is the case mainly for biologics and specialty drugs with extremely high prices in some therapeutical areas, such as oncology and rare diseases [Citation5–7]. In a context of tight public budgets, especially constrained by a sluggish economic environment, improved efficiency is crucial. Promoting competition in off-patent markets may contribute to this efficiency, with increasing penetration of generics and biosimilars [Citation8–11].
In 2021 biologic drugs accounted for 78.6 billion euros in spending, and 34% of drug expenditures in Europe [Citation12]. Among these, 80% of the main nine therapy classes were exposed to biosimilar competition. Biosimilars are highly similar to biological drugs and are approved with the ‘same high standards of quality, safety, and efficacy’ [Citation13]; their similar efficacy and safety outcomes have been shown in many observational and experimental studies [Citation14–16]. According to a joint statement published by the European Medicines Agency and the Head of Medicines Agencies in European Union Member States in September 2022, biosimilars approved in the EU are considered interchangeable [Citation17]. However, the savings potential appears not to have been fully exploited [Citation18]. There are some limitations on the supply side, such as patent litigation [Citation19], but a major hindering factor for best exploiting the savings potential is limited uptake of biosimilars in practice, for several reasons. These include physicians’ resistance to modify prescription choices based on economic arguments [Citation20], while questioning the similar efficacy and safety [Citation21,Citation22]; by originator pharmaceutical companies threatening to withdraw rebates in the event of therapeutic switch [Citation23]; and by patients’ reluctance to change well-functioning therapies, with the need, in some cases, to change the administration mode [Citation24–26]. Yet these behaviors are variable and strongly influenced by contextual factors, namely the policies favoring or not the uptake of biosimilars.
1.1. Cross-country variation and biosimilar policies
Substantial differences in the biosimilar market shares are observed across European countries, for instance in oncology, with biosimilar market shares ranging between 9% (Bulgaria) and 94% (Denmark) in 2021 [Citation12]. A major explanation for such discrepancies is the cross-country differences in policies related to biosimilar drugs, in terms of pricing policies (e.g. with or without linkage of biosimilar prices to originator ones; the specific design of external or internal reference pricing; and the use of tendering) and demand-side measures. Some countries have created incentives for biosimilar prescribing, based on quotas or prescription guidelines; direct replacement of the biologic originator with a biosimilar by pharmacists (allowed in a few countries); and the fact that the availability of educational programs directed to physicians, patients, and/or patients’ associations varies [Citation27,Citation28].
Recent contributions have highlighted the importance of coupling demand-side policies (e.g. guidelines, quotas, INN prescribing) with pricing policies (e.g. tendering and price linkage), to enhance competition and savings [Citation28,Citation29]. The recent example of Denmark was enlightening, with a large adoption of biosimilar adalimumab within a few weeks, based on a central multi-winner tendering on the supply side and specific guidelines before the biosimilar entry on the demand side [Citation30]. A survey for EU countries showed, also for the case of adalimumab, that tendering enhances competition and reduces prices, when coupled with early guidelines and physicians’ and patients’ education [Citation31]. Other policy dimensions are noticeable at an upper regulatory level, such as differences in attributing the interchangeability designation or differences in regulating patent litigation [Citation32].
1.2. Within-country variation and biosimilar policies
Adding to the differences across countries, biosimilar uptake may also vary within-country, across regions, or between health-care facilities, as shown by a body of evidence [Citation21,Citation33–35] that is, however, fairly limited.
Reasons for the intra-country differences have been examined by only a few studies. Three recent studies may be highlighted, all based on mixed-method approaches. A study for Germany observed substantial regional differences in biosimilar market shares; the stakeholders who were interviewed attributed differences to the active use of quotas (i.e. with or without clear monitoring and sanctioning processes), and to active communication strategies by regional physician associations [Citation20]. A similar approach was used to examine the large cross-county differences in biosimilar market shares in Sweden. Interviewees attributed variations to price differences between biosimilars and originator drugs, to local guidelines and their follow-up, and to the possibility of keeping savings to be reallocated to other therapeutic areas, or to treat more patients (‘gain sharing’) [Citation34]. In the same study, a quantitative analysis also showed that price differences between the biosimilar and originator drug accounted for 59% of cross-county variation in biosimilar market shares. Finally, large regional discrepancies were observed in the UK. In interviews, stakeholders highlighted the role of price differences, gain sharing to providers prescribing biosimilars, and leadership by regional NHS offices [Citation33].
1.3. The Portuguese case
The case of Portugal is of special interest, because in the European Union it is among the bottom third group of countries in GDP per capita, while the country has been facing tight public budgets and recurring financial deficits. At the same time, the National Health Service (NHS, called Serviço Nacional de Saúde/SNS) guarantees universal coverage and charges low co-payments for a considerable number of pharmaceuticals, while medicines delivered or administered in hospitals, such as biologic drugs, are free of charge for the patients. This makes Portugal comparable to many countries worldwide in which the public health service is struggling to reduce costs while guaranteeing quality and universal access.
In the Portuguese NHS, new biologic drugs are approved for reimbursement based on a health technology assessment mechanism including an economic evaluation, and prices are set based on external price referencing (this topic and the following are regulated by the Decree-Law 97/2015, approved the 1st of June 2016). Biosimilar drugs can be financed provided that their price is at least 20% lower than that of the originator, similar to the linkage existing in other countries (e.g. Belgium, France, and Spain). Biosimilars are mainly provided in hospitals and are fully reimbursed. A centralized tendering is in place for inpatient biosimilars only, so most biosimilars are procured individually by hospitals, with the possibility of confidential discounts and ex-post rebates for the hospitals.
NHS hospitals are financed through global budgets, based on negotiated price-volume contracts. A small portion (5%) of the budget is attributed based on performance whereby the share of biosimilars is one indicator among several used to measure performance (high performance is attributed when the share is above 20%). Hospitals are residual claimants on potential profits, but this occurs very rarely in practice due to recurring deficits.
Close to what exists in most EU countries [Citation28], guidelines recommend the prescription of the lowest-priced drug for naïve patients, but substitution by the pharmacist is not allowed. In this sense, the Portuguese NHS case may be considered as representative of several other EU countries in terms of biosimilar policies. Note, however, that Portuguese NHS hospitals have been facing severe financing constraints in the recent past, which may trigger a quick uptake of efficiency measures. Also, clinical guidelines suffer from severe limitations: they are rarely produced in a timely fashion, and never before the biosimilar has been approved for financing; there is no systematic monitoring of the guidelines’ application at hospitals; and there is no management support to help hospitals implement the guidelines in practice.
1.4. Research questions
This study examines biosimilars’ adoption across the Portuguese NHS hospitals, determinants for possible differences, and the potential savings associated with adoption. In exploring the impact of public hospital characteristics on biosimilar uptake, we seek to answer four main questions, each of which is based on a hypothesis that may be contested. First, academic hospitals and those with higher consumption of biologic drugs are expected to be more willing to adopt biosimilars because of greater expected savings and more competences to acknowledge interchangeability. Second, hospitals performing more randomized controlled trials (RCT) related to originator drugs may be more reluctant to adopt biosimilars because of marketing activities and rebates from pharmaceutical firms. Third, we assume that hospitals with higher debts will be more inclined to adopt biosimilars in order to increase efficiency and reduce spending. Fourth, it is expected that biosimilar adoption may increase over time.
2. Methods
2.1. Data
We used data from the ‘Transparency Platform of the NHS’ (https://www.sns.gov.pt/transparencia/, accessed on 14 February 2022), which provides publicly accessible monthly information, by hospital, on the consumption of originator biologic and biosimilar drugs. The data refer only to the total consumption for the originator and biosimilar drugs, without distinguishing which biosimilar of usually several available biosimilar drugs was used. Therefore, we will refer hereinafter to ‘biosimilar drugs’ in a generic manner, without further details about brands or number of available presentations.
Data have been available since January 2015, meaning that we analyzed the biosimilars with a positive NHS reimbursement decision only after that date (i.e. a decision that guarantees NHS financing for the drug), and for which complete monthly data were available. We also excluded molecules for which the observation period was too small or with too many missing observations (sodic enoxaparine, epoietin), and one molecule for which the rate of biosimilar was almost 100% over the complete period (filgrastim). This limited our sample to the following five molecules: adalimumab (first consumption record in November 2018), etanercept (October 2016), infliximab (January 2015), rituximab (January 2016), and trastuzumab (May 2018). The last available month was July 2021, and our sample thus included information for 5 molecules over 79 months, for 39 NHS hospitals. Indeed, we removed from the sample hospitals with considerable missing data (data available for fewer than 100 of 345 per-hospital observations), all corresponding to small, specialized hospitals, and to public–private partnerships for which data were available only from 2021 on. Our final sample included 9,467 observations.
2.2. Explanatory factors
We used two groups of explanatory variables, namely, the general hospitals’ characteristics, and their ties to originator firms (variables are detailed in ).
Table 1. List of variables.
In the first dimension, we included as explanatory variable the academic vs. non-academic hospital status. We also factored in the total monthly consumption of biological drugs (volume), as proxy of the number of patients under treatment and to reflect the dimension of potential savings. We also included information on each hospital case mix index (CMI), which is the average complexity of inpatient stays and outpatient consultations, based on the relative weights of Diagnosis Related Groups. This information was made available for the year 2015 only. In the absence of more recent data, we applied this value to all years.
In the second dimension, we included the total number of ongoing RCTs over the complete period as proxy of the hospital relationship to the pharmaceutical industry supplying originator products. We used the total number of RCTs for any drug, either biologic or not. This was used in the absence of more specific factors regarding the relationship, such as the marketing expenses by pharmaceutical firms for the hospital or the number of visits by these firms to the hospital. The participation in RCTs primarily signals the hospital’s interest in innovation and scientific activities and is accompanied by a close link to the manufacturer that developed the drug being evaluated, designed the study, influenced the selection of hospital sites, convinced their professionals to participate, financed the trial, and closely collaborated with professionals in the implementation of the study and interpretation of its results.
We also included the respective share per originator pharmaceutical firm in the expenditure data per hospital as a proxy of the firm portfolio in the hospital in order to gain an awareness of possible closer relationships. We retrieved contracts with pharmaceutical firms and eliminated all joint contracts, that is, those that involved various pharmaceutical firms, given that we could not identify the share related to the firm of interest. Overall, 1,478 AbbVie contracts were included, 3,525 MSD contracts, 3,136 Pfizer contracts, and 3,637 Roche contracts. This variable was divided by the total pharmaceutical expenditure by hospital/year, to obtain the share of each firm in each hospital portfolio, per year. Unfortunately, the same exercise could not be performed for biosimilar drugs, since we did not know which biosimilar was consumed, and which firm commercialized it. We also considered in the analysis of the biosimilar share, the time since the biosimilar was launched, in months.
Finally, an additional analysis was performed to investigate whether hospitals that are more indebted were more inclined to adopt biosimilars earlier and to a greater extent. To do so, we accessed data on overdue debt by the end of each year, which are publicly available through the Transparency Platform, and calculated its burden as the percentage of overdue debt on total annual expenditures. Unfortunately, we were able to obtain data on expenditures for only the years 2016 to 2019, so that this analysis was performed only for this shorter period.
2.3. Statistical analysis
We first performed a survival analysis on the time to adoption of the biosimilar. The month of adoption was defined as the earliest month with consumption of the biosimilar in any hospital. We could have opted instead for the official date of approval for NHS financing, but we rejected this option because time may elapse between approval and purchasing contracts and their use can be implemented.
Survival regressions were performed on the time to adoption. We first tested the proportional hazard assumption by checking if the association with explanatory variables varied with time (if this were the case, then the proportional hazard assumption would be rejected). We used parametric models because the assumption was not fulfilled (, appendix). In cases for which parametric models were adopted, we selected the most appropriate distribution using the Akaike Information Criterion (, appendix).
We then modeled the share of biosimilar consumption on the total consumption of biologic drugs. Since we had repeated observations per hospital and month, we used panel data analysis techniques, namely generalized estimated equations with random hospital effects. The choice of random effects was determined by all explanatory variables that were hospital-fixed characteristics. We modeled the share as function of the same explanatory variables used in the survival analysis, adding however a variable for the time since the biosimilar approval. We used negative binomial models, which were the most appropriate based on the AIC (, appendix) and because it is appropriate when there are many zero values in the data. Both survival and panel-data regressions were first performed by medicine (INN). The Stata13 software was used for all analyses.
2.4. Estimate of savings potential
For modeling the savings potential in different scenarios, we developed a base case scenario based on 2020 total hospital expenditure (latest year with complete data). The expenditure was determined using the consumption data of each drug multiplied by its average price, for the originator and the biosimilar drug, respectively. The price data of originator and biosimilar drugs were provided by APOGEN, for the year 2020 through data collected by IQVIA (confidential data on file). The prices are those that were effectively paid by hospitals, accounting for rebates in the case of originator drugs and for the biosimilar actually selected by the hospital. We calculated potential savings by measuring the total expenditures for each hospital for the year 2020, if they behaved as the hospital with the highest share of biosimilars during that year.
3. Results
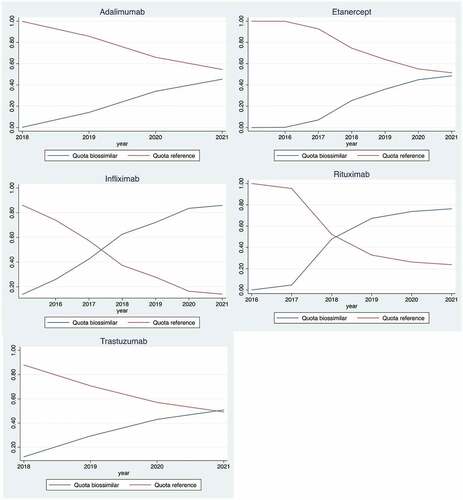
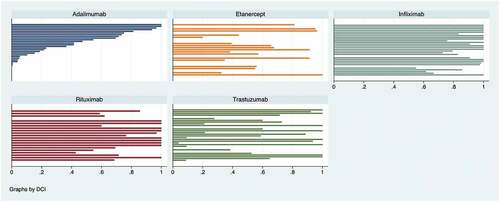
For the five medicines under analysis, we observed an increasing consumption quota for biosimilars over time, while the originator share decreased (). In July 2021 the biosimilar quota was more than 90% for infliximab and above 80% for rituximab. However, for adalimumab, etanercept, and trastuzumab, the shares of all their biosimilars used in hospitals was approximately the same as for the respective shares of the biological originator products. We observed a high inconsistency in the biosimilar consumption share by hospital, by June 2021 (the last month in our sample) (). In the case of adalimumab, etanercept, infliximab, and trastuzumab, values go from 0% to 100%, while they vary between 55% and 100% in the case of rituximab.
Figure 2. Biosimilar share, by hospital, June 2021* (own construction).*Each bar represents a hospital (its biosimilar share). Adalimumab biosimilar hospital decreasing shares, from 100% to zero %, are used to order shares for other drugs. The x-axis represents the biosimilar share, from 0 to 1 (100%).
The median time from first use (launch) to adoption was the lowest for adalimumab (22 months), followed by trastuzumab (27 months), etanercept (37 months), and rituximab and infliximab (46 months) (). The uptake time was slightly quicker at academic hospitals, and at hospitals with more RCTs. Results were variable regarding the case mix, the consumption of biologics, and the originator firms’ portfolio. The highest share over the period was observed for infliximab (54.2%) and rituximab (42.1%). Shares were generally larger at high-consuming hospitals, while no clear pattern emerges for the other variables.
Table 2. Median time to adoption and biosimilar quota, by hospital characteristic.
Survival analysis depicted a faster uptake at academic hospitals of adalimumab (HR = 1.75) and infliximab (HR = 1.27), but this status was not statistically associated with uptake for other medicines (). A greater consumption of biologic drugs was linked to a quicker uptake for trastuzumab (HR = 1.37) and etanercept (HR = 1.18), but to a slower uptake of infliximab (HR = 0.77). The relationship with case mix was never significant, except for infliximab, for which a lower CMI was linked to a quicker uptake. A greater participation in RCTs was linked to a more rapid adoption of etanercept (HR = 1.35) and infliximab (HR = 1.14) biosimilars, while the link was weak for the other medicines. The highest relationship with originator firm was significantly positive for rituximab (HR = 1.16) and not significant in the other cases.
Table 3. Hazard ratios of time to biosimilar adoption (standard errors between brackets).
For all drugs, we observed that the time after biosimilar first use was positively related to the share of biosimilars (). A lower share among academic hospitals was observed for all drugs except adalimumab, but none of the links were significant. A lower consumption of biologic drugs, a higher number of RCTs, and a lower case mix index were related to a higher share of biosimilars, except for rituximab. The share of originator firm in the hospital portfolio was inversely related to the adalimumab (beta = −1.14) and etanercept (beta = −1.07) biosimilar quotas, but positively related to other biosimilar medicines (although results were not significant).
Table 4. Panel data analysis of biosimilar quota (coefficients and standard errors between brackets).
The additional analysis on the overdue debt burden on the restricted sample (2016–2019 period) showed that this factor was significantly related to earlier uptake of adalimumab and rituximab, to a higher share of rituximab, but to a lower share of adalimumab and etanercept ().
Table 5. Association of uptake and shares with the burden of overdue debt.
We then calculated the potential savings if all hospitals performed as those with the highest share of biosimilars, and we obtained potential savings of 20.796 million euros for adalimumab, 20.439 million euros for etanercept, 20.692 million euros for infliximab, 33,200 euros for rituximab, and 12.915 million euros for trastuzumab (). Savings ranged from 9% (etanercept) to 32% (trastuzumab) on total expenditures for the drug. In total, potential annual savings would reach 13.9 million euros, only for the five drugs we evaluated (i.e. 1% of total hospital drug expenditures for the year 2020).
Table 6. Potential savings associated to biosimilars.
4. Discussion
4.1. Key findings
Overall, hospital biosimilar utilization shares reached 50% or more in the 3 years following their first use, for the five molecules studied. Yet, these shares were characterized by high heterogeneity, ranging from no use in some hospitals to exclusive use of biosimilars in others.
A longer delay in biosimilar adoption was observed for rituximab and infliximab, which nevertheless experienced a greater biosimilar share throughout the period; although their uptake was quicker, the lowest shares were observed for etanercept and adalimumab. Academic hospitals were characterized by a quicker uptake of adalimumab and infliximab biosimilars but lower shares of biosimilars for all except one drug. Except for rituximab, a higher consumption of biologics was related to a lower share of biosimilar uptake. More RCTs were linked to a greater biosimilar share except for rituximab, and a quicker uptake for all drugs; a greater share of originator portfolio was linked to quicker uptake for all drugs, while the link with shares was negative for adalimumab and infliximab but positive for the other drugs. A higher burden of hospital overdue debt was related to an earlier uptake of adalimumab and rituximab, but the link with biosimilar share was ambiguous. Finally, huge discrepancies across hospitals, ranging from 0% to 100% biosimilar shares, suggest that substantial savings could be achieved if all hospitals performed as the best.
4.2. Interpretation
4.2.1. The high heterogeneity in biosimilar uptake
First and foremost, there are substantial differences in the uptake of biosimilars across public hospitals in Portugal, for all the drugs investigated. These findings are in line with results from other countries, where heterogeneity was also observed across regions in Germany, Sweden, and, although to a lower extent, the UK [Citation12,Citation34]. Some of the causes of heterogeneity (e.g. the degree of quotas application) across German, Swedish, and the UK regions are not comparable to those observed across Portuguese hospitals, which have no competence on setting quotas or defining guidelines. Portuguese hospitals can define local guidelines, may have drug formularies, or may adapt stricter rules and support policies to favor the biosimilar uptake. Yet, there are neither strong incentives (weak budget constraints) nor recommendations to do so by the central administration, so that these initiatives depend on the local commitment, interest, and power of hospital managers. Finally, findings from other countries highlight the key role of price differentials, discounts, and gainsharing (in Sweden, 59% of the variation was explained by differences in prices). These economic dimensions may also play an important role in Portuguese hospitals, but we could not analyze this issue. Note, however, that the absence of a relationship with hospital debts suggests that gainsharing, and more generally financial constraints, may have a low priority in prescription choices.
The lower shares of etanercept and adalimumab biosimilars have several possible explanations. First, contrary to the other drugs, they are administered by the patient, using a subcutaneous injection, and they therefore require the patient’s involvement to define the treatment option, which may be more difficult to obtain. In particular, the treatment switch always results from a shared decision between the practitioner and the patient, and thus requires a strong motivation on both sides when the switch is demanding in terms of administration. In comparison, rituximab and trastuzumab are administered through intermittent treatment by intravenous injection at the hospital, so that the decision resides more among practitioners, requiring a lower involvement of the patient. Second, a 2010 Ordinance mandates public hospitals to deliver biologic drugs for rheumatic diseases free of charge to patients of their catchment area upon any out-of-hospital prescription, including at private settings (Despacho 14,919/2010, 2nd of December 2010). It becomes virtually impossible for the hospital to opt for a biosimilar for naïve patients or to switch therapy since the prescription was issued in the outpatient setting. Third, etanercept and adalimumab are prescribed for auto-immune disorders, which require more complex treatments and whose outcomes are more difficult to measure. Outcome measurement for rheumatic diseases is more difficult to assess and less consensual since it is more related to quality of life (compared, e.g. to cancer outcomes, which are related to survival). This may lead to a greater reluctance to modify a well-functioning therapy, especially by rheumatologists [Citation21,Citation36]. Fourth, the literature mentions that originator companies have adopted particularly aggressive competition strategies in the case of etanercept [Citation20]. In the Netherlands, also on etanercept, the competition authority launched a preliminary investigation on procurement practice of the originator firm to offer large discounts to hospitals, with the aim of discouraging switch (https://www.acm.nl/en/publications/drug-manufacturer-pfizer-discontinue-its-steering-pricing-structure-enbrel-following-discussions-acm, accessed on 13 October 2022). Finally, this result cannot be related solely to the shorter observation period for adalimumab (33 months) and etanercept (57 months), since the period was also shorter for trastuzumab (38 months), which yielded different results.
Noticeably, 3 years after its first use, the share of etanercept was slightly below 40%, well below the 56% observed in Germany after a similar period. By contrast, the share of infliximab was around 70%, while 61% in Germany.
In this paper, we sought to answer four main questions, each of which is based on a hypothesis that may be contested. We next detail how we answer those questions.
5. Academic and high-consuming hospitals
We initially postulated that academic hospitals and those with higher consumption of biologic drugs would be more willing to adopt biosimilars because of greater expected savings and more competences to acknowledge interchangeability.
The quicker uptake but lower shares of biosimilar at academic hospitals does not support this hypothesis; in this case, the adoption of biosimilar drugs was more likely to occur later, after months or years of treatment with the originator, which makes the switch more complex. Another consideration is that there may be a stronger long-term fidelity to originator firms. Another possible explanation is the 2010 Ordinance mentioned above, which obliges public hospitals to provide biologic drugs free of charge for rheumatic diseases prescribed at private facilities, without the possibility of modifying that prescription. Anecdotal evidence suggests that academic hospitals are the ones in greater demand, so they may be among those more subjected to external prescriptions that they cannot alter.
Surprisingly, a greater consumption of biologic drugs was not substantially linked to a quicker uptake or higher share of biosimilar drugs, despite the expected greater savings. We may hypothesize that greater consumption also permits larger rebates from originator firms, which we could not assess. A possible hypothesis would be that both academic and high-consuming hospitals may also be treating more complex patients, i.e. they may face a larger pool of patients for which biosimilar drugs are not recommended, either for clinical reasons or because switching may cause adherence problems. However, this hypothesis is challenged by the outcome on the case mix variable, which did not show significant relationships with uptake and shares.
That academic and high-consuming hospitals are not characterized by a greater and quicker uptake of biosimilars suggests a need for a stronger and earlier management, communication, and education effort at the national level and in academic hospitals before the originator is widely selected for most patients. In other words, such activities should consider the possible anticipation of the biosimilar arrival into the market, presenting this market entry and its potential economic gains at an early phase. The communication and education strategies, coupled with the creation of guidelines, were demonstrated as effective policies in the three countries where these were evaluated (Germany, Sweden, and the UK [Citation20,Citation33,Citation34]).
6. Ties with firms commercializing originator drugs
We then postulated that hospitals performing more randomized controlled trials (RCT) related to originator drugs may be more reluctant to adopt biosimilars because of marketing activities and rebates from pharmaceutical firms. Yet our findings show that stronger links with the pharmaceutical industry in research and development did not seem to be detrimental to the consumption of biosimilars, even though in some cases it appeared to favor the rapid uptake and consumption. We may argue that links with originator firms are related to a greater interest in adopting new – potentially costly – therapies, prompting the need for savings with older molecules. This result is aligned with those for the UK showing the role of gainsharing as driver of biosimilar adoption [Citation33]. It may also be that these are the hospitals more oriented toward scientific innovation and research, which promotes interest and knowledge about new drugs and biosimilars.
7. The role of financing constraints
We initially assumed that hospitals with higher debts would be more inclined to adopt biosimilars to increase efficiency and reduce spending. We found, though, that more-indebted hospitals were not clearly more likely to adopt biosimilars, contrary to expectations. However, the Portuguese hospital financing scheme explains this result; indeed, over the last decade there has been a dramatic under-budgeting of hospitals, which has provoked high debt compensated by regular bailouts. That is, due to these bailouts, debts have not been viewed by hospital managers as a major issue; in other words, the application of a soft budget constraint has not at all encouraged management practices seeking efficiency and cost containment.
This hesitancy of hospital managers to encourage biosimilar uptake suggests limited knowledge, or awareness of efficiency gains for the whole SNS, as highlighted by the savings potential analyzed in this study.
8. The role of time
As expected, the share of biosimilars increases with time since their first use. This result confirms expectations, as physicians and patients gain experience about the use of biosimilars and their therapeutic equivalence, as more biosimilars enter the market, and guidelines about biosimilar use get more widely diffused and known.
8.1. Strengths and limitations
The study stands out for its novelty. While differences across regions of a few European countries have been addressed (however, this has not been done for Portugal before), no quantitative analysis has measured how such differences were related to providers’ characteristics. The major strength of this study is its use of a large dataset of biologic consumption over 6 years for the complete universe of Portuguese public hospitals, which supports the validity of the results.
The major limitation is the use of aggregate data at the hospital level. No information was available about the detailed clinical characteristics of the patients nor about the exact indication for which the medicine was prescribed. It may well be that variations in uptake are related to patients’ diseases and their severity. Also, we had no information on the prescribing physicians’ characteristics, which would have allowed us to refine the analysis, relating the biosimilar consumption to the physician experience and practice, for instance. Although it is unlikely that patients’ profiles for each hospital change substantially on a yearly basis, the use of a single-case mix value from a single year might be a limitation, i.e. we might have underestimated the case complexity in later years, which might make the biosimilar uptake more difficult.
We did not consider the official date of approval for financing because delays were observed between approval and launch to the market. Instead, we used the month in which the first adoptions were recorded, which may be later than when access was effectively guaranteed. As a result, we might have underestimated the median time of adoption. Nevertheless, the underestimation is equal for all providers, so that our findings on hospital determinants are not affected. Another limitation is that our dataset includes no data on the individual biosimilars consumed, but for the purpose of the study, we would not consider this as a limitation.
Finally, we did not have access to the individual hospital policies and initiatives regarding biosimilars, or the relevance of efficiency consideration in hospital decisions and its consequences, such as the inclusion of biosimilars in drug formularies, the internal incentives to prescribers, the existence of training or discussions about rational prescribing, or the guidelines in terms of patients’ involvement in treatment decisions. These issues could only be addressed through specific surveys at providers’ level. While this is beyond the scope of this paper, such policies might be examined in future research that builds on this study.
9. Conclusions
As expected, the longer the time following approval, the higher was the biosimilar uptake. Even so, half of the SNS hospitals in the cases we assessed took more than 2.5 years to adopt etanercept biosimilar, 3.5 years for rituximab biosimilar, and almost 2 years for trastuzumab biosimilar, despite the potential savings that reached 46% per dose in the case of trastuzumab.
In contrast to what might be expected, the biosimilar potential was not fully exploited at academic and large hospitals, which would likely benefit most from the biosimilar use. This finding suggests a need for a stronger and earlier management, communication, and education effort before the originator is widely selected for most patients.
Involvement in clinical trials does not hinder the biosimilar uptake, and even promotes it. Thus, it seems that involvement in scientific activities and interest in innovative drugs is not incompatible with the search for efficiency in prescription, so that these activities could be promoted.
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Concept and design: J Perelman, S Vogler, C Mateus. Analysis and interpretation of data: J Perelman, F Duarte-Ramos, A Gouveia, L Pinheiro, F Ramos, S Vogler, C Mateus. Drafting of manuscript: J Perelman. Critical revision of the paper for important intellectual content: F Duarte-Ramos, S Vogler, C Mateus. All authors read and approved the final manuscript for publication.
Additional information
Funding
References
- Kesselheim AS, Avorn J, Sarpatwari A. The high cost of prescription drugs in the United States: origins and prospects for reform. J Am Med Assoc. 2016;316(8):858–871.
- Prasad V, Jesús K D, Mailankody S. The high price of anticancer drugs: origins, implications, barriers, solutions. Nat Rev Clin Oncol. 2017;14(6):381.
- Rome BN, Egilman AC, Kesselheim AS. Trends in Prescription drug launch prices, 2008-2021. JAMA. 2022;327(21):2145–2147.
- Rintoul A, Colbert A, Garner S, et al. Medicines with one seller and many buyers: strategies to increase the power of the payer. BMJ. 2020;369. 10.1136/bmj.m1705.
- Belloni A, Morgan D, Paris V. Pharmaceutical expenditure and policies [Internet]. OECD iLibrary; 2016. (OECD Health Working Papers). Report No.: 87. Available from: 10.1787/5jm0q1f4cdq7-en
- Dranitsaris G, Zhu X, Adunlin G, et al. Cost effectiveness vs. affordability in the age of immuno-oncology cancer drugs. Expert Rev Pharmacoecon Outcomes Res. 2018;18(4):351–357.
- Vokinger KN, Hwang TJ, Grischott T, et al. Prices and clinical benefit of cancer drugs in the USA and Europe: a cost–benefit analysis. Lancet Oncol. 2020;21(5):664–670.
- Jang M, Simoens S, Kwon T. Budget impact analysis of the introduction of rituximab and trastuzumab intravenous biosimilars to EU-5 markets. BioDrugs. 2021;35(1):89–101.
- Kanters TA, Stevanovic J, Huys I, et al. Adoption of biosimilar infliximab for rheumatoid arthritis, ankylosing spondylitis, and inflammatory bowel diseases in the EU5: a budget impact analysis using a Delphi panel. Front Pharmacol. 2017;8:322.
- Vogler S. Can we achieve affordable cancer medicine prices? Developing a pathway for change. Expert Rev Pharmacoecon Outcomes Res. 2021;21(3):321–325.
- Stiff KM, Cline A, Feldman SR. Tracking the price of existing biologics when drugs enter the market. Expert Rev Pharmacoecon Outcomes Res. 2019;19(4):375–377.
- Troein P, Newton M, Scott K, et al. The impact of biosimilar competition in Europe - IQVIA report. Brussels; 2021.
- European Commission and European Medicine Agency. Biosimilars in the EU: information guide for health professionals. 2019. https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_en.pdf
- Cohen HP, Blauvelt A, Rifkin RM, et al. Switching reference medicines to biosimilars: a systematic literature review of clinical outcomes. Drugs. 2018;78(4):463–478.
- Luber RP, O’Neill R, Singh S, et al. An observational study of switching infliximab biosimilar: no adverse impact on inflammatory bowel disease control or drug levels with first or second switch. Aliment Pharmacol Ther. 2021;54(5):678–688.
- Schreiber S, Ben-Horin S, Leszczyszyn J, et al. Randomized controlled trial: subcutaneous vs intravenous infliximab CT-P13 maintenance in inflammatory bowel disease. Gastroenterology. 2021;160(7):2340–2353.
- European Medicines Agency. Statement on the scientific rationale supporting interchangeability of biosimilar medicines in the EU [Internet]. Amsterdam; 2022 [cited 2022 October 19]. Available from: https://www.ema.europa.eu/en/documents/public-statement/statement-scientific-rationale-supporting-interchangeability-biosimilar-medicines-eu_en.pdf
- Hübel K, Kron F, Lux MP. Biosimilars in oncology: effects on economy and therapeutic innovations. Eur J Cancer. 2020;139:10–19.
- Moorkens E, Vulto AG, Huys I. An overview of patents on therapeutic monoclonal antibodies in Europe: are they a hurdle to biosimilar market entry? In: MAbs. Taylor & Francis; 2020. p. 1743517.
- Moorkens E, Barcina Lacosta T, Vulto AG, et al. Learnings from regional market dynamics of originator and biosimilar infliximab and etanercept in Germany. Pharmaceuticals. 2020;13(10): 324.
- Beck M, Michel B, Rybarczyk-Vigouret MC, et al. Rheumatologists’ perceptions of biosimilar medicines prescription: findings from a French web-based survey. BioDrugs. 2016;30(6):585–592.
- Sarnola K, Merikoski M, Jyrkkä J, et al. Physicians’ perceptions of the uptake of biosimilars: a systematic review. BMJ Open. 2020;10(5):e034183.
- Hakim A, Ross JS. Obstacles to the adoption of biosimilars for chronic diseases. J Am Med Assoc. 2017;317(21):2163–2164.
- Frantzen L, Cohen JD, Tropé S, et al. Patients’ information and perspectives on biosimilars in rheumatology: a French nation-wide survey. Joint Bone Spine. 2019;86(4):491–496.
- Azevedo A, Bettencourt A, Selores M, et al. Biosimilar agents for psoriasis treatment: the perspective of Portuguese patients. Acta Med Port. 2018;31(9):496–500.
- Varma M, Almarsdóttir AB, Druedahl LC. “Biosimilar, so it looks alike, but what does it mean?” A qualitative study of Danish patients’ perceptions of biosimilars. Basic Clin Pharmacol Toxicol. 2022;130(5):581–591.
- Moorkens E, Vulto AG, Huys I, et al. Policies for biosimilar uptake in Europe: an overview. PLoS One. 2017;12(12): e0190147.
- Vogler S, Schneider P, Zuba M, et al. Policies to encourage the use of biosimilars in European countries and their potential impact on pharmaceutical expenditure. Front Pharmacol. 2021;12:1479.
- Ferrario A, Dedet G, Humbert T, et al. Strategies to achieve fairer prices for generic and biosimilar medicines. Br Med J. 2020;368:l5444.
- Jensen TB, Kim SC, Jimenez-Solem E, et al. Shift from Adalimumab originator to biosimilars in Denmark. JAMA Intern Med. 2020;180(6):902–903.
- Moorkens E, Godman B, Huys I, et al. Market exclusivity and the entry of adalimumab biosimilars in Europe: an overview of pricing and national policy measures. Frontiers in Pharmacology. 2021;11. 10.3389/fphar.2020.591134.
- Bennett CL, Schoen MW, Hoque S, et al. Improving oncology biosimilar launches in the EU, the USA, and Japan: an updated policy review from the southern network on adverse reactions. Lancet Oncol. 2020;21(12): e575–88.
- Moorkens E, Vulto AG, Kent J, et al. A look at the history of biosimilar adoption: characteristics of early and late adopters of infliximab and etanercept biosimilars in subregions of England, Scotland and wales-A mixed methods study. BioDrugs. 2021;35(1): 75–87.
- Moorkens E, Simoens S, Troein P, et al. Different policy measures and practices between Swedish counties influence market dynamics: part 1—biosimilar and originator infliximab in the hospital setting. BioDrugs. 2019;33(3):285–297.
- Moorkens E, Simoens S, Troein P, et al. Different policy measures and practices between Swedish counties influence market dynamics: part 2—Biosimilar and originator etanercept in the outpatient setting. BioDrugs. 2019;33(3):299–306.
- van Overbeeke E, De Beleyr B, de Hoon J, et al. Perception of originator biologics and biosimilars: a survey among Belgian rheumatoid arthritis patients and rheumatologists. BioDrugs. 2017;31(5):447–459.
Appendix
Table A1. Test proportional hazard assumption, complete sample
Appendix
Table A2. AIC results for various distributions for survival model, complete sample
Appendix
Table A3. AIC results for various distributions for GEE, complete sample