?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background
Physicians’ preferences for attributes of medical treatments for endometriosis-associated pain have not previously been quantified.
Methods
US obstetrician-gynecologists completed an online discrete-choice experiment survey. In a series of questions, physicians chose a medical treatment for a hypothetical patient with endometriosis experiencing severe, persistent dysmenorrhea, nonmenstrual pelvic pain, and/or dyspareunia. Each question presented two hypothetical medical treatments for endometriosis-associated pain, defined by seven attributes with varying levels. Preferences weights and conditional relative importance (CRI) were calculated using a random-parameters logit model.
Results
Respondents (N = 250) had an average age of 53 years; 36% were female. The most important attribute, conditional on the attributes and levels evaluated, was risk of moderate-to-severe hot flashes (CRI, 3.34). In descending order of importance, the CRIs of the other attributes were 2.13 for improvement in nonmenstrual pelvic pain, 2.04 for improvement in dyspareunia, 1.88 for improvement in dysmenorrhea, 1.16 for risk of pregnancy-related complications if pregnancy occurs during treatment, 0.62 for increased risk of bone fracture later in life, and 0.48 for mode of administration.
Conclusions
In addition to valuing pain reduction, respondents prioritized avoiding moderate-to-severe hot flashes, followed by less common and less immediate risks of pregnancy-related complications and bone fracture.
1. Introduction
Endometriosis, a heterogeneous disease with symptoms including dysmenorrhea, nonmenstrual pelvic pain, dyspareunia, painful bowel and/or bladder symptoms, and infertility, affects an estimated 6% to 10% of women of reproductive age in the United States (US) [Citation1–3]. A variety of surgical and medical interventions are used to treat pain associated with endometriosis [Citation4] Medical interventions include nonsteroidal anti-inflammatory drugs (NSAIDs), estrogen-progestin hormonal contraceptives, progestins (as an oral or injectable treatment or as an implantable progestin-releasing intrauterine device), gonadotropin-releasing hormone (GnRH) agonists, aromatase inhibitors, and the newer GnRH antagonists. In addition to GnRH antagonists, the landscape for medical treatments for endometriosis-associated pain includes a variety of multidisciplinary provisions, such as pelvic floor physical therapy and pain management/neuropathic pain treatments, as well as complementary therapies such as acupuncture. Despite known risks, narcotic medications also are a mainstay of endometriosis-associated pain management. Current medical treatments have varying levels of effectiveness, modes of administration, and treatment-related adverse events (AEs) [Citation5]. Because none of these treatments is clearly superior in terms of benefits and risks, the best treatment depends on the preferences of patients and physicians as identified in a shared decision-making framework.
A previous study examined patient preferences for the potential benefits and harms of treatments for endometriosis, the value patients place on pain relief relative to other treatment features, and likely choices among alternative treatments [Citation6,Citation7]. The patient sample’s treatment choices in the survey were most influenced by the risk of hot flash and improvements in endometriosis-related pain; however, side effect risks and mode and frequency of administration were relatively less influential. However, little is currently known about physicians’ preferences for attributes of treatments for endometriosis-associated pain in the US or how influential treatment attributes are in physicians’ treatment recommendations. Therefore, to complement the previous patient preference study, the current study examined physicians’ perspectives with the primary objective of quantifying the preferences of physicians practicing in the US for features and outcomes associated with medical treatments for patients presenting with endometriosis-associated pain. Additional objectives were to examine whether preferences varied with physician characteristics, to calculate the maximum level of risks of treatment-related AEs that physicians would be willing to accept for different levels of treatment benefit, and to calculate the probability that the average physician respondent would select one specific treatment over another.
2. Patients and methods
2.1. Study design
This study used a discrete-choice experiment (DCE) methodology to elicit preferences for features (attributes) of treatments used to treat patients with endometriosis-related pain. DCEs are an increasingly popular method of eliciting stated preferences for health [Citation8]. The study design was based on the patient preference study [Citation6] but was adapted so that the instrument was appropriate for the physicians and the attribute levels reflected the treatments available at the time of the study. Development of the survey, experimental design, and data analysis followed recommended research practices [Citation9–11], and the final survey was administered online via Survey Engine, a survey firm with a specialty in health research.
2.2. Survey instrument
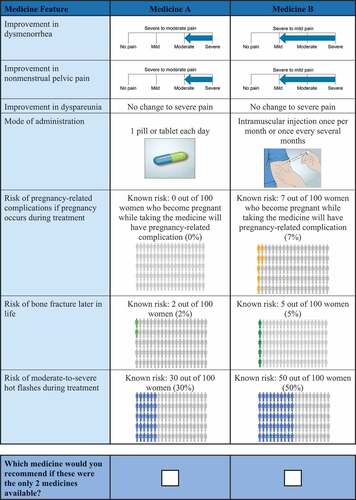
The survey presented each respondent with a series of choice questions in which they were asked to select the hypothetical treatment they would recommend for a hypothetical patient with specific characteristics from a pair of hypothetical treatments (Medicine A or Medicine B) for endometriosis-associated pain. presents an example choice question. Each hypothetical treatment was defined by seven clinically relevant attributes with varying levels; the DCE posits that each of the respondents’ treatment choices depends on the weight the respondent places on the attribute levels. The attributes and levels were the same as those used in the prior patient preference study [Citation6], with the addition of a mode of administration attribute level (i.e. two pills or tablets each day) not included in the patient study (). The attributes were chosen to represent the attributes of endometriosis treatments that are relevant to physicians making treatment recommendations and patients and that characterize and differentiate among medical treatments for endometriosis-related pain (including leuprolide and elagolix). Selection of the attributes and levels was informed by expert clinical opinion and by the prescribing information and clinical trial results for endometriosis treatments. For the physician DCE survey, the attribute descriptions were refined, as appropriate, from the patient-friendly descriptions used in the prior study to be relevant to physician respondents. In addition to the treatment-choice questions, the survey included questions about physicians’ demographic characteristics, practice setting, and endometriosis treatment patterns.
Table 1. Attributes and levels for the discrete-choice experiment.
To provide context for the treatment choice, physicians were asked to consider a hypothetical patient profile when completing the choice questions. This treatment profile was developed in collaboration with a clinical expert to describe a woman with severe endometriosis-related pain for whom all pharmacological treatments would be indicated. The hypothetical patient was described as a woman aged 18–49 years who is still menstruating; has surgically confirmed endometriosis; has severe endometriosis-related dysmenorrhea, nonmenstrual pelvic pain, and/or dyspareunia that has been inadequately treated with NSAIDs and oral contraceptive pills; and did not want to become pregnant within 24 months.
A draft version of the survey instrument was pretested in semistructured interviews with a convenience sample of 15 eligible physicians who treat patients with endometriosis in the US. The objectives of the pretest were to assess the understandability of the survey instrument, the appropriateness of descriptive information, and the difficulty of the trade-off choice questions. On the basis of the pretest findings, minor refinements were made to the survey instrument before the final version was administered online.
A statistically rigorous DCE requires an experimental design with known statistical properties. Following recommended research practices [Citation11], the experimental design consisted of combinations of the attribute levels that describe the full set of medicine alternatives included in the survey. The choice questions were determined by an experimental design with statistical properties that would allow estimation of the main-effects preference weights of interest through the use of a random-parameters logit (RPL) model [Citation9]. A fractional factorial experimental design was generated in SAS 9.4 software (SAS Institute Inc, Cary, North Carolina) using the attribute levels in and AD-optimal algorithm [Citation12,Citation13]. The full design included 48 unique choice questions, which were divided into 6 blocks of 8 questions such that each question appeared in only one block. Assessment of the experimental design revealed that the design had level balance and orthogonality that was comparable to other studies. Each respondent was randomly assigned to one block of choice questions.
2.3. Study population
Board-certified or equivalent obstetricians and/or gynecologists, recruited by Survey Engine, participated in this study. Eligible respondents treated at least ten patients with endometriosis per month in the US; at least two of whom had moderate-to-severe endometriosis-related pain. All respondents were able to read and understand English and provided informed consent electronically before completing the survey. The survey was granted an exemption from review by the institutional review board of RTI International.
The minimum sample size for a DCE study depends on several criteria, including the format of the survey questions, the complexity of the choice task, the desired precision of the results, and whether subgroup analyses will be conducted [Citation14,Citation15]. A priori power calculations cannot be conducted without prespecifying the relative magnitude of the preference weights, which typically is not possible [Citation16]. Most published choice experiments have a sample size of 100 to 300 respondents, and an empirical analysis of DCE sample size indicates that 250 to 350 respondents who each answer 8 to 10 choice questions are likely to be sufficient for studies with 6 to 8 attributes [Citation17,Citation18]. A recruitment target of 250 physicians, which was considered sufficient to estimate preference weights for all treatment attributes and levels included in the DCE, was established for this study. Respondents were excluded from the analysis if they did not show variability in their answers to the DCE choice questions (i.e. they always selected ‘Medicine A’ or ‘Medicine B’) or if they completed the survey too quickly (i.e. in less than 6 minutes).
2.4. Statistical analysis
An RPL regression model was used to analyze the choice data collected from the DCE and to estimate the primary study endpoints (i.e. attribute-level preference weights). The RPL model has the advantage of accounting for unobserved heterogeneity in preferences, estimating both the mean preference weights for each attribute levels as well as the standard deviation in the mean preference weight. The RPL model parameter estimates are log-odds relative preference weights, indicating the relative strength of preference, for each attribute level included in the survey [Citation9]. If the 95% confidence intervals (CIs) do not overlap for pairs of levels for a particular attribute, the mean estimates are statistically significantly different from each other at the 5% level of significance. Despite producing specific preference-weight values for each attribute level, the scale of these weights is arbitrary and is not directly interpretable. To analyze the primary endpoints, we considered a main-effects utility function specification in which all independent variables were represented by effects-coded variables for each attribute level so that the mean effect for each attribute was normalized at zero.
The conditional relative importance (CRI) of an attribute can be interpreted to describe the influence changes in an attribute had on survey treatment choices, conditional on the attributes and levels evaluated in the study. The CRI of an attribute is calculated as the difference between the level of that attribute with the highest preference weight and the level with the lowest preference weight. This difference represents the maximum change in utility that can be achieved with any attribute, conditional on the levels chosen for the attributes in the study.
Subgroup analyses were used to determine whether average preferences varied systematically among respondents in mutually exclusive subgroups [Citation9]. Seven mutually exclusive subgroups were identified in post hoc analysis based on gender (male vs. female); physicians’ prescribing patterns for elagolix (those who prescribed >5 times the median in the last 3 months vs. those who prescribed less); physicians’ geographic area (urban/suburban vs. rural); physicians’ practice setting (private vs. hospital or academic or other); physicians’ region (southern US statesFootnote1 vs. the rest of the US); whether physicians would prescribe add-back therapy as additional treatment (those who stated they would vs. those who would not prescribe add-back therapy); and whether physicians would prescribe contraception as additional treatment (those who stated they would vs. those who would not prescribe contraception as additional treatment).
The measures of risk tolerance (mean maximum acceptable risk [MAR] of treatment-related AEs and the 95% CIs around these means) were calculated using the RPL regression parameters (log-odds relative preference weights) from the RPL model. The mean MAR of each treatment-related AE in exchange for specific improvements in treatment benefit was calculated as the negative ratio of the relative importance of an improvement in a treatment attribute to the value of a unit change in the risk of a treatment-related AE (accounting for the fact that this might vary across different absolute levels of risk). Because the use of effect-coded variables allows for risk preferences to be nonlinear, to calculate maximum acceptable risk, linear interpolation was used to estimate preference weights for attribute levels that lie between attribute levels included in the study. That is, the value of a unit change in the risk of a treatment-related AE was determined by the linear interpolation of the preference weight at the point at which the relative importance of an increase in the treatment-related AE was equal to the relative importance of an improvement in treatment effectiveness. In some cases, these calculations resulted in MAR estimates that were outside the risk ranges used in the study design. We could estimate these MARs only by making the strong assumption that risk tolerance is linear outside the range, and therefore we report it as the MAR being greater than the maximum level of risk shown in the survey.
Finally, the physicians’ estimated relative preference weights were used to calculate the average probability that a physician in the sample would choose an elagolix-like treatment profile over a depot leuprolide acetate (leuprolide)–like treatment profile. These results were used as a benefit-risk assessment of the profiles and replicated the benefit-risk assessment conducted with patient preference study results [Citation5].
3. Results
3.1. Physician characteristics
In total, 250 physicians were recruited and completed the online survey. The average respondent age was 53 years, 36% were female, 72% were obstetrician-gynecologists, 76% were in private practice, and 74% had been practicing >15 years ().
Table 2. Demographic characteristics of physician respondents.
3.2. Relative preference weights and conditional relative attribute importance
The estimated preference weights indicate that on average, respondents preferred medical treatments that yield greater pain reduction and have lower risks of pregnancy-related complications, bone fracture, and moderate-to-severe hot flashes. In addition, daily oral treatments were preferred to treatments administered by injection once per month or less frequently.
shows the relative preference weights and conditional relative attribute importance of changing each attribute from the least-preferred level to the most-preferred level. Preference weights can be interpreted as weights indicating the relative strength of preference for each attribute level. More preferred outcomes have higher preference weights. All differences in preference weights corresponding to different levels of improvements in dysmenorrhea (all p ≤ 0.002), nonmenstrual pelvic pain (all p ≤ 0.001), dyspareunia (all p < 0.001), and changes in risk of moderate-to severe hot flashes (all p ≤ 0.05) were statistically significant, indicating that respondents differentiated between these levels when making treatment choices.
Figure 2. Relative preference weights and conditional relative attribute importance for respondents (N = 250).
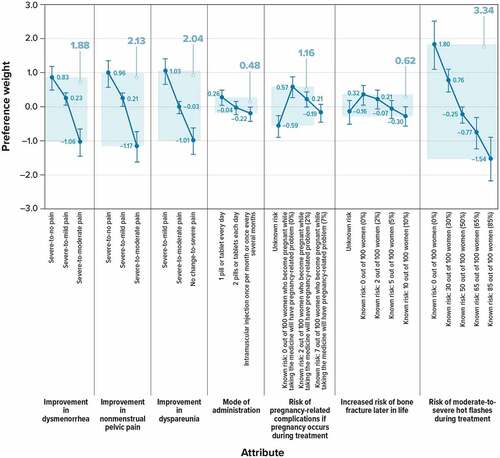
In general, oral treatments were preferred to injections; but some of the changes in mode of administration were not statistically significant, indicating that respondents did not, on average, differentiate between these levels when making treatment choices in the survey or that respondents were indifferent between those modes of administration. In particular, the change from injection once per month or less frequently to two pills or tablets each day yielded no statistically significant change in utility (p = 0.269), nor did the change from two pills or tablets each day to one pill or tablet each day (p = 0.117).
A change in the risk of pregnancy-related complications from unknown risk to 0%, from unknown risk to 2%, or from 0% to 7% yielded statistically significant changes in utility (p = < 0.001, p = 0.001, and p = 0.003, respectively), while all other risk changes yielded no statistically significant change in utility. A change in the increased risk of bone fracture later in life from 0% or from 2% to 10% yielded statistically significant changes in utility (p = 0.008 and p = 0.026, respectively), while all other risk changes yielded no statistically significant change in utility.
The attribute with the largest CRI was the risk of moderate-to-severe hot flashes (CRI, 3.34). The CRI of improvement in dyspareunia, nonmenstrual pelvic pain, and dysmenorrhea were all similar to one another. Results indicated that the CRI of improvements in all three types of pain were greater (had a greater influence on treatment choices in the survey) than the CRI of pregnancy-related complications, risk of bone fracture, or mode of administration (all p ≤ 0.001). Specifically, in descending order of importance, the CRIs for attributes other than the risk of hot flash were 2.13 for improvement in nonmenstrual pelvic pain, 2.04 for improvement in dyspareunia, 1.88 for improvement in dysmenorrhea, 1.16 for risk of pregnancy-related complications if pregnancy occurs during treatment, 0.62 for risk of bone fracture later in life, and 0.48 for mode of administration.
3.3. Subgroup analyses
Estimated preferences were not systematically different when compared across the following mutually exclusive subgroups (see ).
Table 3. Descriptions of the subgroups analyzed and results of tests for systematic differences in preferences (N = 250).
3.4. Maximum acceptable risks
Using the main RPL model results, the MAR of pregnancy-related complications, bone fracture, and moderate-to-severe hot flashes were calculated, with corresponding 95% CIs, for all possible changes in levels of efficacy changes in and mode of administration (). The results indicate that respondents were tolerant of side effect risks in exchange for improvements in pain. To improve from severe-to-moderate dysmenorrhea to severe-to-mild dysmenorrhea, the MAR estimates for the risk of pregnancy-related complications and bone fracture later in life were larger than the largest risk used in the DCE experimental design (i.e. a > 7% risk of pregnancy-related complications and a > 10% risk of bone fracture) and were 35% for the risk of moderate-to-severe hot flashes. Patients were less tolerant of risks in exchange for changes in mode and frequency of treatment. For a change in mode of administration from injection once per month or more frequently to a pill or tablet every day, respondents were willing to accept, on average, a 4% additional risk of pregnancy-related complications, a 7% risk of bone fracture, and a 14% increase in the risk of moderate-to-severe hot flashes.
Table 4. Maximum acceptable risk calculations (N = 250).
3.5. Preference shares
Results from the main RPL model were used to calculate the preference shares, i.e. the probability that an average respondent would select one of three treatment profiles using two choice sets: elagolix 150 mg daily versus leuprolide and elagolix 200 mg twice daily versus leuprolide (). When considering an elagolix 150-mg daily profile and a leuprolide profile, the likelihood that the average respondent would choose an elagolix 150-mg daily profile was approximately 49.3% (95% CI, 28.6%-69.9%). When considering an elagolix 200-mg twice-daily profile and the leuprolide profile, the average respondent’s likelihood of choosing the elagolix 200-mg twice-daily profile was 96.0% (95% CI, 91.2%-100.8%).
Table 5. Treatment profiles and preference share results: baseline analysis results (N = 250).
4. Discussion
This study is the first, to our knowledge, to have used a DCE to quantify US physicians’ preferences for attributes of treatments for endometriosis-associated pain. Based on the DCE results, the most important treatment attribute (considering those included in the survey) was risk of moderate-to-severe hot flashes (see ). The remaining attributes, in decreasing order of relative importance, were improvements in nonmenstrual pelvic pain, dyspareunia, dysmenorrhea, changes in risks of pregnancy-related complications and bone fracture, and mode of administration. Oral treatments were preferred to injections, but mode of administration attribute had the smallest effect on treatment choice. Respondents in this study did not differentiate between the changes in levels of mode of administration from injection once per month or less frequently to two pills or tablets each day or from two pills or tablets each day to one pill or tablet each day.
There are several possible explanations for these results. First, respondents may perceive the risk of pregnancy-related complications to be less common, because most patients with endometriosis are not trying to conceive or because they assume that the risk of pregnancy-related complications may be mitigated by prescribing contraception. Respondents also were asked to consider the profile of a hypothetical patient who did not want to become pregnant in the next 2 years when completing the survey. In addition, respondents may perceive the risk of bone fracture later in life to be less immediate and potentially mitigated with add-back therapy and/or weight-bearing exercise and therefore not a concern relative to the risk of moderate-to-severe hot flashes. Most of the respondents said they would prescribe add-back therapy (61%) or contraception (64%) to mitigate the risks of bone fracture or pregnancy-related complications. Furthermore, individuals place less value on outcomes that occur in the future than equivalent outcomes that occur sooner. In economic theory, this is known as discounting [Citation19]. In addition, some clinicians may place less value on outcomes not addressed within their area of specialization.
Mean MARs should be interpreted with caution. Although the MAR of pregnancy-related complications and bone fracture were calculated, most of the results were outside the range of risk levels observed in the study. These MARs may not precisely reflect respondent preferences, because there was no empirical information about preferences for risks above 7% and 10% for pregnancy-related complication and bone fracture, respectively. Furthermore, the 95% CIs around most of the mean estimates for MAR of pregnancy-related complications and bone fracture included zero (except for an improvement from severe-to-mild nonmenstrual pelvic pain to severe-to-no pain), meaning that the MAR estimates are not statistically significantly different from zero when applying a type I error threshold p value of 0.05. The results also suggested that physicians perceive value in the pain reduction provided by the hypothetical medical treatments for endometriosis; accordingly, mitigating the risks of the treatments’ short- and long-term side effects would be necessary.
A comparison of the patient and physician study results – although limited by the differences in the experimental design and decision context – indicates that preferences are likely to be similar in these two sampled groups. Compared with the patient study [Citation6], the results showed that the overall CRIs of the treatment attributes led to very similar rankings of each attribute’s importance. The MARs and preference shares for physician respondents were similar to those for patient respondents. Specifically, a treatment with a profile similar to a twice-daily 200-mg dose of elagolix, relative to other treatment profiles that are similar to leuprolide, would likely be preferred both by a majority of physicians and by a majority of patients. Prior research comparing patient and physician treatment preferences from DCE studies has found more evidence of discordance than concordance in these stakeholders’ preferences [Citation20].
The strengths and limitations of this analysis should be considered. The survey was carefully designed in line with good study practices [Citation10,Citation11] and was pretested using in-depth interviews. In addition, the treatment-choice data were analyzed using advanced RPL methods that followed good research practices [Citation9] and were consistent with methods used in previous work conducted by the Center for Devices of Radiological Health [Citation21]. Our methods avoided estimation bias from unobserved variation in preferences across the sample and from within-sample correlation in the choice sequence for each respondent. Nonetheless, there are some limitations with this type of assessment. One limitation is that respondents evaluated hypothetical medical treatments for a hypothetical patient, and their choices do not have the same significance as choices involving actual treatment decisions. Further, recruitment method and study design are subject to potential selection and volunteer biases. The majority of respondents were male (>60%) and in practice for more than 15 years (>70%), with an average age over 50 years, and their preferences may not be generalizable to those of US obstetricians and/or gynecologists treating women with severe endometriosis-associated pain. Another possible limitation is that there are no empirical data from this study regarding preferences for risks outside the range obtained by extrapolation. Regarding the statistical methods, RPL provides average relative preference estimates at a population level and cannot be used to estimate preferences for an individual. Subgroup analyses did not reveal statistically significant differences in preferences between groups of respondents, potentially as a result of insufficient subgroup sizes to detect differences in preferences between subgroups rather than a true lack of difference in preferences between subgroups. Lastly, the final DCE survey instrument was administered online. Research has shown that results from online DCEs are, in general, not statistically significantly different from those elicited through face-to-face interviews [Citation22,Citation23]. However, potential information bias may still be present.
5. Conclusion
Among physicians treating women with severe endometriosis-associated pain, tolerance for treatment-related risks of moderate-to-severe hot flashes are relatively lower than tolerance of risks of pregnancy-related complications and bone fracture in exchange for pain relief. These results suggested that physicians perceive value in the pain reduction and they may be willing to trade off risks of side effects in exchange for efficacious treatment of pain.
Declaration of interest
C Poulos and C Leach are full-time employees of RTI Health Solutions, an independent nonprofit research organization, which was retained by AbbVie to conduct the research which is the subject of this manuscript. Their compensation is unconnected to the studies on which they work. W Botha is a past employee of RTI Health Solutions. Y Xu is an employee of AbbVie and may hold shares and/or stock options in the company. K Kahle-Wrobleski was a past employee of AbbVie and may hold shares and/or stock options in the company; she is now employed by GSK. K Gordon was a past employee of AbbVie and may hold shares and/or stock options in the company; he is now employed by Organon. S Estes is an employee of Penn State Health, has received research funding from AbbVie, Ferring, and Obseva, and is a consultant for AbbVie and Upsilon. S Missmer is an employee of Michigan State University and has been an advisory board member for AbbVie and Roche and receives research funding from the National Institutes of Health, the Department of Defense, the J. Willard and Alice S. Marriott Foundation, and AbbVie. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Acknowledgments
The authors would like to acknowledge Kimberly Moon of RTI Health Solutions for providing project management and to thank Melissa Mehalick and Kate Lothman of RTI Health Solutions for medical writing assistance.
Data availability statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (eg, protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent, scientific research, and will be provided following review and approval of a research proposal, Statistical Analysis Plan (SAP), and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time after approval in the US and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
Additional information
Funding
Notes
1. Delaware, District of Columbia, Florida, Georgia, Maryland, North Carolina, South Carolina, Virginia, West Virginia, Alabama, Kentucky, Mississippi, Tennessee, Arkansas, Louisiana, Oklahoma, and Texas.
References
- Fuldeore MJ, Soliman AM. Prevalence and symptomatic burden of diagnosed endometriosis in the United States: national estimates from a cross-sectional survey of 59,411 women. Gynecol Obstet Invest. 2017;82(5):453–461.
- Goldstein DP, deCholnoky C, Emans SJ, et al. Laparoscopy in the diagnosis and management of pelvic pain in adolescents. J Reprod Med. 1980 Jun;24(6):251–256.
- Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997 Jun;24(2):235–258.
- Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med. 2020 Mar 26;382(13):1244–1256.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017 Jul 6;377(1):28–40.
- Poulos C, Soliman AM, Renz CL, et al. Patient preferences for endometriosis pain treatments in the United States. Value Health. 2019 Jun;22(6):728–738.
- Poulos C, Soliman AM, Tekin S, et al. Patient preferences for elagolix and leuprolide for treating endometriosis-related pain in the United States. Expert Rev Pharmacoecon Outcomes Res. 2021 Oct;21(5):1091–1099.
- Soekhai V, de Bekker-Grob EW, Ellis AR, et al. Discrete choice experiments in health economics: past, present and future. Pharmacoeconomics. 2019 Feb;37(2):201–226.
- Hauber AB, Gonzalez JM, Groothuis-Oudshoorn CG, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR conjoint analysis good research practices task force. Value Health. 2016 Jun;19(4):300–315.
- Bridges JF, Hauber AB, Marshall D, et al. Conjoint analysis applications in health–a checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value Health. 2011 Jun;14(4):403–413.
- Johnson FR, Lancsar E, Marshall D, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR conjoint analysis experimental design good research practices task force. Value Health. 2013 Jan-Feb;16(1):3–13.
- Kuhfeld W, Tobias F, Garratt M. Efficient experimental design with marketing research applications. J Market Res. 1994;31(4):545–557.
- Kuhfeld W. Marketing research methods in SAS: experimental design, choice, conjoint, and graphical techniques. Cary NC: SAS Institute Inc; 2010.
- Louviere JJ, Hensher DA, Swait JD. Stated choice methods: analysis and applications. New York: Cambridge University Press; 2000.
- Johnson FR, Yang J-C, Mohamed AF. In defense of imperfect experimental designs; statistical efficiency and measurement error in choice-format discrete-choice experiment. Presented at the Proceedings of the Sawtooth Software Conference. March, 2012. p. 195–205.
- de Bekker-Grob EW, Donkers B, Jonker MF, et al. Sample size requirements for discrete-choice experiments in healthcare: a practical guide. Patient. 2015 Oct;8(5):373–384.
- Marshall D, Bridges JF, Hauber B, et al. Conjoint analysis applications in health – How are studies being designed and reported?: an update on current practice in the published literature between 2005 and 2008Reported? The Patient: Patient-Centered Outcomes Research. 2010 Dec 1;3(4):249–256.
- Yang JC, Johnson FR, Kilambi V, et al. Sample size and utility difference precision in discrete-choice experiments: a meta-simulation approach. J Choice Model. 2015;16(C):50–57.
- Green L, Myerson J. A discounting framework for choice with delayed and probabilistic rewards. Psychol Bull. 2004 Sep;130(5):769–792.
- Harrison M, Milbers K, Hudson M, et al. Do patients and health care providers have discordant preferences about which aspects of treatments matter most? Evidence from a systematic review of discrete choice experiments. BMJ Open. 2017 May 17;7(5):e014719.
- Ho MP, Gonzalez JM, Lerner HP, et al. Incorporating patient-preference evidence into regulatory decision making. Surg Endosc. 2015 Oct;29(10):2984–2993.
- Marta-Pedroso C, Freitas H, Domingos T. Testing for the survey mode effect on contingent valuation data quality: a case study of web based versus in-person interviews.Ecol Econ. 2007 [2007 May 15];62(3):388–398.
- Nielsen JS. Use of the Internet for willingness-to-pay surveys: a comparison of face-to-face and web-based interviews. Resour Energy Econ. 2011;33(1):119–129.