ABSTRACT
Background
Inflammatory bowel disease poses significant social and economic burdens. We assessed the budget impact of including the recently approved subcutaneous (SC) formulation of vedolizumab as maintenance therapy (MT) in patients with ulcerative colitis (UC) in France.
Methods
A decision-analytic model was developed from a French payer’s perspective over 5 years to assess budget impact of including vedolizumab SC as MT for UC following induction therapy with vedolizumab intravenous (IV), by subtracting outcomes of a ‘world without vedolizumab SC’ from a ‘world with vedolizumab SC.’ Comparators included approved therapies: infliximab (branded/biosimilar), adalimumab (branded/biosimilar), golimumab, ustekinumab, and vedolizumab IV. The model predicts drug, medical, and total costs, including indirect costs in a scenario analysis. A one-way sensitivity analysis explored the impact of varying individual parameters.
Results
Including vedolizumab SC as MT following vedolizumab IV induction yielded total cost savings of €59,176,842 (biologic-naïve) and €22,004,135 (biologic-experienced) versus a world without vedolizumab SC. Including indirect costs yielded cost savings in biologic-naïve (€62,600,716) and biologic-experienced (€24,314,915) populations in a world with vedolizumab SC.
Conclusions
Introducing vedolizumab SC as MT after IV induction is expected to have substantial cost savings to a health plan from a French payer’s perspective versus a world without vedolizumab SC.
1. Introduction
Ulcerative colitis (UC) is a form of inflammatory bowel disease (IBD) characterized by chronic inflammation and ulceration of the inner lining of the colon and rectum [Citation1]. Patients with UC experience symptoms such as bloody diarrhea, weight loss, and pain, which limit patients’ lifestyle and consequently affect patients’ quality of life [Citation1,Citation2].
It has been estimated that there are 6.8 million people globally with IBD (as of 2017), which represents a significant burden [Citation3]. The nationwide incidence of UC in France, calculated based on insurance data (January 2000 to December 2002), was 7.2 cases per 100,000 inhabitants, with even geographical distribution of UC throughout the country [Citation4]. The incidence rate of UC in Northern France from 1988 to 2007 has been estimated as 4.1 cases per 100,000 person-years; median age at diagnosis is 35 years, and significantly more men are affected than women [Citation5]. An increase in adolescent UC (age 10–16 years) of 156% has been observed from 1988–1990 to 2009–2011 [Citation6].
UC is associated with a high health-economic burden [Citation7]. Despite a potential reduction in costs associated with surgery and hospital stay, the increasing costs of therapies may contribute to an overall high-cost burden [Citation7]. The mean annual cost per patient with UC in France was estimated as €15,775 (standard deviation, €7,221) for patients treated with tumor necrosis factor inhibitors (anti-TNFs; infliximab or adalimumab) [Citation8]. Medication accounts for the majority of this cost (84%), with surgery and direct costs representing 11% and 2%, respectively [Citation8]. Reducing the cost of medications is important to allow more patients with UC access to treatment, which is currently limited in several countries [Citation9,Citation10].
Treatment options for UC focus on treating acute active disease, maintaining remission, and helping to avoid surgery, which itself is a treatment option and a likely outcome in patients with disease that is difficult to control with other treatments [Citation11]. The first-line treatment options depend on disease severity and include oral and/or rectal administration of 5-aminosalicylic acid or corticosteroids [Citation11]. Biologic agents, such as anti-TNFs, can be used in patients for whom conventional therapy is no longer efficacious [Citation11,Citation12]. Infliximab, adalimumab, golimumab, and ustekinumab have been approved for the treatment of UC in Europe [Citation13,Citation14]. The decision on the most appropriate therapy is usually made on an individual case basis. Despite the current knowledge and therapeutic advances in UC management, some patients do not achieve satisfactory outcomes [Citation7]. It was estimated that in France, during the induction phase, up to 26% of patients failed and discontinued first-line biologic, while switching to another anti-TNF was an independent predictor of greater indirect costs [Citation8,Citation15].
Vedolizumab is the first gut-selective biologic therapy for the treatment of IBD. It was approved in 2014 for intravenous (IV) administration in patients with UC and Crohn’s disease [Citation16]. Subsequently, in 2020, vedolizumab SC (108 mg every 2 weeks) was approved in Europe, Canada, and Australia as a maintenance therapy in moderately to severely active UC [Citation16–19]. In France, vedolizumab SC is prescribed in general practice and then either administered at a hospital (day hospitalization) or at home (self-administered or by a nurse).
The aim of this study was to assess the potential budgetary impact of introducing vedolizumab SC as maintenance therapy following vedolizumab IV induction as a treatment for biologic-naïve and biologic-experienced patients with moderate to severe UC in France.
2. Methods
2.1. Model structure and patient population
A decision-analytic model was developed to assess the budget impact of including vedolizumab SC as maintenance therapy in UC following two doses of vedolizumab IV as induction therapy (hereafter referred to as ‘vedolizumab SC’), calculated by subtracting the outcomes of a ‘world without vedolizumab SC’ from the outcomes of a ‘world with vedolizumab SC’ (). The ISPOR guidelines for conducting budget impact analysis were followed [Citation20].
Figure 1. (A) Budget impact model structure and (B) model health states. 1L, first-line treatment; 2L, second-line treatment; SC, subcutaneous.
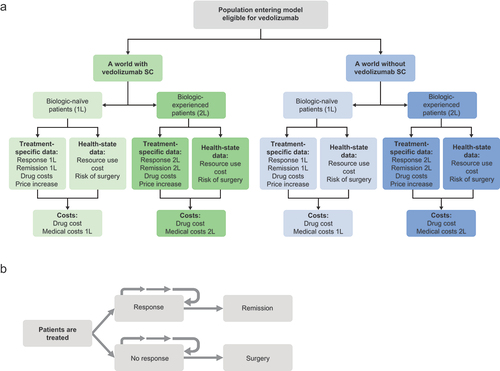
The target model population reflected the licensed population for vedolizumab IV. Vedolizumab IV is indicated for the second-line treatment of adult patients with moderately to severely active UC (Mayo score of 6–12). The model first funneled the population of the health plan through a series of steps to identify those with UC who were eligible to receive biologic treatment (i.e. those with moderate to severe UC, not all patients with UC). The model considered an initial total population of France of 67,063,703 enrollees, to which incidence and prevalence rates of UC were applied, and of those, patients diagnosed with moderate to severe UC who were eligible for biologic treatment were selected (). Treatment line in the model was defined by previous exposure to a biologic therapy: biologic-naïve patient population (first-line biologic treatment) and patients for whom prior treatment with biologic had failed (biologic-experienced patient population; second line of treatment). This classification was based on the patient status at the beginning of the model, when the first biologic was considered. Due to the non-differential switching of patients among treatments, we assumed a new incidence rate and a uniform market share for new patients and experienced patients.
Table 1. Population characteristics.
The model consisted of four health states based on which medical costs were calculated (). Patients entered the model as either responders or non-responders to treatment with biologic therapies. Responders could either remain as responders or enter remission, and non-responders could either remain as non-responders or undergo surgery.
2.2. Model perspective and time horizon
The model was developed from the French payer’s perspective (i.e. the Haute Autorité de Santé). The timespan of the model was 5 years. Annual drug and medical costs were estimated for the first year and cumulative annual costs for the following years.
2.3. Treatment comparators
The comparators included in the study were infliximab (branded and biosimilar), adalimumab (branded and biosimilar), golimumab, ustekinumab, and vedolizumab IV. This selection was based on a treatment pathway specific to France and guidelines on the current recommended treatments for the target population.
2.4. Model input parameters
2.4.1. Treatment efficacy
The model inputs considered probabilities of response and remission over 1 year and risk of surgery among patients with moderate to severe disease. Clinical response was defined as a reduction in complete Mayo score of ≥3 points and ≥30% from baseline, with an accompanying decrease in rectal bleeding score of ≥1 point or absolute rectal bleeding score of ≤1 point. Clinical remission was defined as a complete Mayo score of ≤2 points and no individual subscore of >1 point. Comparative efficacy of treatments () was estimated using a Bayesian network meta-analysis (NMA) [Citation25], which included 19 randomized controlled trials, three of which were treat-through trials and the remaining were re-randomized trials. All trials were placebo-controlled except for one (VARSITY), which reported results on vedolizumab versus adalimumab.
Table 2. Percentage of patients achieving response or remission at first year from treatment for ulcerative colitis (network meta-analysis).a.
2.4.2. Other efficacy inputs
Classification of disease severity was based on the previous models [Citation26,Citation27]. Patients who responded to treatment (as defined above) were classified as being in remission if they had a Mayo score of 0–2. The surgery costs incurred by patients who required surgery (annual rate, 4.9%) were also captured in the model [Citation28].
2.4.3. Base case and scenario analysis
The base case analysis included direct drug costs and direct healthcare costs associated with each health state (including the cost of managing adverse events). Treatment outcomes (sourced from the NMA) as an efficacy input source were used. Health-state costs were taken from previously published economic analyses (adjusted to 2020 Euros [€] using the French consumer price index [France National Bureau of Statistics]) () [Citation29–32]. Costs for each biologic administration per year (hospital-specific and retail) were estimated in the base case for a standard dose (no up-dosing was assumed). For pharmacy costs of IV treatments, the model assumed that IV treatments were only administered on an inpatient basis (at a hospital; higher pharmacy cost due to higher administration costs), and only hospital prices were considered. For the SC treatments, only retail prices were considered.
Table 3. Mean annual cost by health state for patients with ulcerative colitis.
A scenario analysis was also performed, in which indirect costs of transportation and loss of productivity costs (societal costs) were included, in addition to the costs from the base case analysis (). The effect of dose escalation was also investigated in another scenario analysis. The percentage of patients receiving dose escalation was informed by the results of a systematic literature review conducted in 2019 (data on file), and up-dosing was included only for those treatments for which dose escalation was permitted in the product label (percentage of patients: vedolizumab IV, 28%; infliximab IV, 22%; adalimumab SC, 31%; golimumab SC, 13%).
Table 4. Cost of biologics during standard 1-year treatment.
2.5. Market share
The market share data for vedolizumab and the relevant comparators were provided by Takeda for the world with and without vedolizumab SC as a maintenance therapy (Supplementary Table S1). The market share reflects the actual number of biologic treatments in the first, second, and later lines of therapy. It also reflects the number of patients who are switching to different treatments.
2.6. Model assumptions
The model assumptions included assumption of full compliance to medication and monotherapy, and consideration of adverse event costs as part of the health-state costs. The modeled health states for UC were defined according to Mayo scores: remission, Mayo score of 0–2; response, >3-point reduction in Mayo score; no response, defined as 100% minus percentage responders minus percentage of those undergoing surgery; and surgery. Other model assumptions are summarized in Supplementary Table S2.
2.7. Sensitivity analysis
A one-way sensitivity analysis (OWSA) was performed by varying individual inputs to assess the impact of the model parameters on model results. The impact of the following parameters was examined: health-state costs, drug costs (along with the percentage annual increase in price), efficacy inputs, and the percentage of patients who require surgery. The results from scenario 2 (a world without vedolizumab SC) were deducted from scenario 1 (a world with vedolizumab SC) to generate the net budget impact. The individual parameters that varied by 20% in the OWSA are listed in the Supplementary data.
2.8. Data availability
The datasets, including the redacted study protocol, redacted statistical analysis plan, and individual participants’ data supporting the results reported in this article, will be made available within 3 months from initial request, to researchers who provide a methodologically sound proposal. The data will be provided after its de-identification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization. Data are available upon request via application at https://search.vivli.org.
3. Results
3.1. Base case analysis
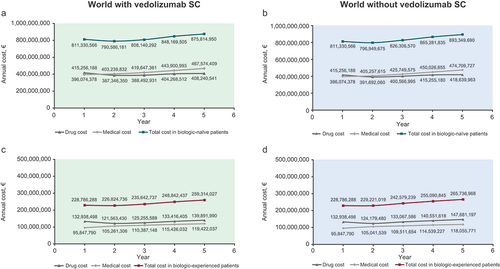
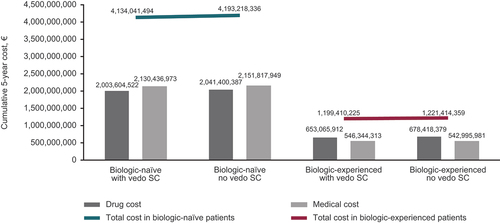
Including vedolizumab SC as a maintenance therapy following vedolizumab IV induction resulted in total cost savings of €59,176,842 and €22,004,135 for the biologic-naïve and biologic-experienced patient populations, respectively, over 5 years (). In biologic-naïve patients, the cost savings were mainly driven by lower costs of biologics in the world with vedolizumab SC compared with the world without (cost savings of €37,795,865). Medical costs in the biologic-naïve patients were also lower in the world with vedolizumab SC. In biologic-experienced patients, the medical costs were slightly higher (by €3,348,332) in the world with vedolizumab SC compared with the world without vedolizumab SC; however, the cost of biologics was low enough to offset the higher medical costs.
Figure 2. Cumulative 5-year healthcare spending for ulcerative colitis in biologic-naïve patients and in biologic-experienced patients. SC, subcutaneous; vedo, vedolizumab.
Summary of the annual costs is shown in . In biologic-naïve patients, drug costs increased on an annual basis (except for year 2) in both cases (with and without vedolizumab SC), and the medical costs also showed a slight tendency to increase on an annual basis (). The annual increase in medical cost was less in the world with versus without vedolizumab SC in biologic-naïve patients (mean annual increase of €17,875,008 and €19,658,837 in the world with and without vedolizumab SC, respectively), but the opposite was observed in the biologic-experienced patients (mean annual increase of €5,893,562 and €5,551,995 in the world with and without vedolizumab SC, respectively). In biologic-naïve and biologic-experienced patients, the total costs per annum were higher in the world without vedolizumab SC across all 5 years ().
3.2. Scenario analysis
In the scenario analysis (which included societal costs), treatment with vedolizumab SC as a maintenance therapy following vedolizumab IV also resulted in cost savings in both the biologic-naïve (€62,600,716) and biologic-experienced (€24,314,915) populations, similar to the base case analysis (Supplementary Table S3). Annual costs (drug, medical, and total) from the scenario analysis are reported in Supplementary Table S4.
In an up-dosing scenario, the total costs over 5 years were also lower in a world with vedolizumab SC compared with a world without vedolizumab SC in the biologic-naïve population (€88,090,369 total cost savings) and in the biologic-experienced population (€38,441,101 total cost savings) (Supplementary Figure S1).
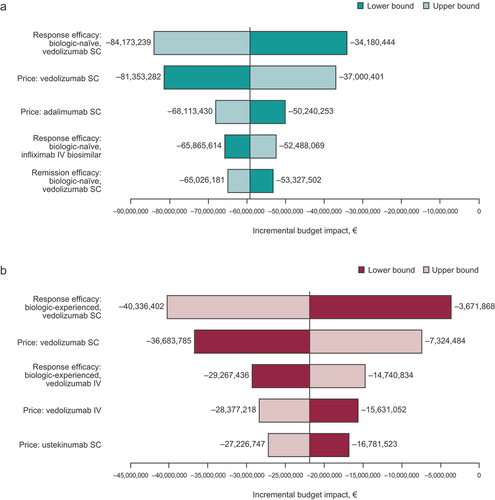
3.3. Sensitivity analysis
Efficacy of maintaining response and price of vedolizumab SC were the most sensitive parameters in both the biologic-naïve and biologic-experienced patient populations (). None of the parameters that were varied in the OWSA resulted in an increased incremental budget impact, except the lower bound efficacy of maintaining response with vedolizumab SC in the biologic-experienced population. This may have been due to wide confidence intervals in the biologic-experienced population.
4. Discussion
This model assessed the budgetary impact of including vedolizumab SC as maintenance therapy following vedolizumab IV induction for biologic-naïve and biologic-experienced patients with moderate to severe UC in France. The model demonstrated a decrease in overall payers’ budget in the world with vedolizumab SC (following vedolizumab IV induction) compared with the world without vedolizumab SC as maintenance therapy. These results are mainly driven by the lower cost of biologic treatment mix, as well as lower medical costs in the world with vedolizumab SC in biologic-naïve patients, due to a higher aggregate number of patients having a response or achieving remission when treated with vedolizumab SC.
Annual increases in the total costs in biologic-naïve patients were observed in both worlds (with and without vedolizumab SC), but were smaller in the world with vedolizumab SC, showing cost savings with vedolizumab SC as a maintenance therapy. Although the medical costs were slightly higher in biologic-experienced patients in the world with vedolizumab SC, these were offset by the lower drug costs.
To the best of our knowledge, there are no published studies that have previously estimated the budget impact of including vedolizumab SC as maintenance therapy following vedolizumab IV induction for UC. In the United Kingdom, vedolizumab IV has been found to be cost-effective or dominant in biologic-naïve patients with moderate to severe UC when compared with infliximab, golimumab, adalimumab, and conventional therapy [Citation14,Citation35]. The opposite findings were made in an analytic decision model from a payer’s perspective in the United States, aiming to identify whether self-injectables, such as adalimumab SC, are more cost-effective (first year costs) than medications delivered via IV (infliximab and vedolizumab) [Citation36]. Despite the original hypothesis that adalimumab would be cost-effective due to its lower non-drug cost, infliximab IV was the most cost-effective first-line biologic in patients with moderate to severe UC (lower cost per mucosal healing) owing to its efficacy and lower drug costs [Citation36]. In that analysis, vedolizumab IV was better positioned as a second-line biologic therapy [Citation36]. The focus on mucosal healing as the efficacy endpoint precludes more direct comparison of this analysis with our model. In a 3-year model assessing the potential budget impact of including vedolizumab IV as a first- or second-line treatment on a health plan’s formulary in the United States, including vedolizumab IV as a first-line biologic to preferred first-line treatment options for UC (adalimumab, infliximab, and golimumab) resulted in cost savings of up to $1.63 million in year 1 and up to $4.68 million in year 3 compared with including vedolizumab IV as a second-line biologic [Citation37].
The limitations of this study include lack of head-to-head trials comparing multiple biologics. To overcome the lack of data, indirect comparisons of treatment efficacy were estimated based on a Bayesian NMA. The model used strong comparative effectiveness evidence from NMA that incorporated a direct head-to-head controlled trial among a menu of up-to-date available treatment options in France (both branded and biosimilar). A second limitation is scarce data on dose escalation. In this study, different dose escalation patterns and their impact on costs were accounted for using recent real-world data from patients with UC and from product labels. Thirdly, due to lack of available efficacy data for combination therapy, monotherapy was assumed. Fourthly, due to limited data, cost offsets of corticosteroid-free remission and mucosal healing were not considered in the model. Finally, the costs included in the model were accurate at the time the study was conducted, so changes in pricing are expected.
5. Conclusion
The introduction of vedolizumab SC as a maintenance therapy following vedolizumab IV induction for biologic-naïve and biologic-experienced patients with UC from a French payer’s perspective is expected to have a substantial cost-savings impact on a health plan compared with a world without vedolizumab SC.
Declaration of interest
M Oppe was an employee of Axentiva Solutions when this work was carried out, which was contracted by Takeda to perform this analysis. B Muresan and K Chan were employees of IQVIA when this work was carried out, and X Radu is a current employee of IQVIA, which was contracted by Takeda to perform this analysis. B Schultz, R Turpin, A Nucit, and E Fenu are employees of Takeda and hold stock/stock options in Takeda. Takeda were involved in the study design, data analysis, statistical input, review of drafts, writing of the article, and identification of papers for inclusion. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
M Oppe, B Muresan, K Chan, and X Radu were involved in study conception and design; acquisition of the data; analysis of data; interpretation of data; drafting or critically revising the article for intellectual content, and approval of the final version for submission. B Schultz, R Turpin, A Nucit, and E Fenu were involved in study conception and design; acquisition of the data; interpretation of data; drafting or critically revising the article for intellectual content, and approval of the final version for publication.
Abbreviations
1L, first-line treatment; 2L, second-line treatment; anti-TNF, tumor necrosis factor inhibitor; CD, Crohn’s disease; IBD, inflammatory bowel disease; IV, intravenous; N/A, not available; NMA, network meta-analysis; OWSA, one-way sensitivity analysis; SC, subcutaneous; UC, ulcerative colitis; VDZ, vedolizumab.
Supplemental Material
Download MS Word (95.8 KB)Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14737167.2023.2160322
Additional information
Funding
References
- Gajendran M, Loganathan P, Jimenez G, et al. A comprehensive review and update on ulcerative colitis. Dis Mon. 2019;65(12):100851.
- Ghosh S, Mitchell R. Impact of inflammatory bowel disease on quality of life: results of the European Federation of Crohn’s and Ulcerative Colitis Associations (EFCCA) patient survey. J Crohns Colitis. 2007;1(1):10–20.
- GBD 2017 group, Alatab S, Sepanlou SG, Ikuta K, et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5(1):17–30.
- Nerich V, Monnet E, Etienne A, et al. Geographical variations of inflammatory bowel disease in France: a study based on national health insurance data. Inflamm Bowel Dis. 2006;12(3):218–226.
- Chouraki V, Savoye G, Dauchet L, et al. The changing pattern of Crohn’s disease incidence in northern France: a continuing increase in the 10- to 19-year-old age bracket (1988-2007). Aliment Pharmacol Ther. 2011;33(10):1133–1142.
- Epimad Group, Ghione S, Sarter H, Fumery M, et al. Dramatic increase in incidence of ulcerative colitis and Crohn’s disease (1988-2011): a population-based study of French adolescents. Am J Gastroenterol. 2018;113(2):265–272.
- Danese S, Allez M, van Bodegraven AA, et al. Unmet medical needs in ulcerative colitis: an expert group consensus. Dig Dis. 2019;37(4):266–283.
- Lawton J, Achit H, Pouillon L, et al. Cost-of-illness of inflammatory bowel disease patients treated with anti-tumour necrosis factor: a French large single-center experience. United European Gastroenterol J. 2019;7(7):908–913.
- Baumgart DC, Misery L, Naeyaert S, et al. Biological therapies in immune-mediated inflammatory diseases: can biosimilars reduce access inequities? Front Pharmacol. 2019;10:279.
- Kostic M, Djakovic L, Sujic R, et al. Inflammatory bowel diseases (crohn s disease and ulcerative colitis): cost of treatment in Serbia and the implications. Appl Health Econ Health Policy. 2017;15(1):85–93.
- Harbord M, Eliakim R, Bettenworth D, et al. European Crohn’s and Colitis Organisation [ECCO]. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017;11(7):769–784.
- Armuzzi A, DiBonaventura MD, Tarallo M, et al. Treatment patterns among patients with moderate-to-severe ulcerative colitis in the United States and Europe. PLoS One. 2020;15(1):e0227914.
- Janssen Biologics BV. Ustekinumab (summary of product characteristics). Leiden The Netherlands: European Medicines Agency; 2021.
- Wilson MR, Bergman A, Chevrou-Severac H, et al. Cost-effectiveness of vedolizumab compared with infliximab, Adalimumab, and golimumab in patients with ulcerative colitis in the United Kingdom. Eur J Health Econ. 2018;19(2):229–240.
- Gordon JP, McEwan PC, Maguire A, et al. Characterizing unmet medical need and the potential role of new biologic treatment options in patients with ulcerative colitis and Crohn’s disease: a systematic review and clinician surveys. Eur J Gastroenterol Hepatol. 2015;27(7):804–812.
- Takeda Pharma A/S. Vedolizumab (summary of product characteristics). Taastrup Denmark: European Medicines Agency; 2020.
- Takeda Pharmaceuticals Australia Pty Ltd. Entyvio® (vedolizumab). Australian product information. Sydney Australia: GuildLink; 2020.
- Takeda Canada Inc. Entyvio® vedolizumab (product monograph). Toronto Ontario Canada; 2020. Available from: https://www.takeda.com/4a11e2/siteassets/en-ca/home/what-we-do/our-medicines/product-monographs/entyvio/entyvio-pm-en.pdf
- Takeda Pharmaceutical Company Limited. Update on the U.S. development program for the investigational subcutaneous formulation of ENTYVIO® (vedolizumab) as a maintenance therapy in adults with moderate to severe ulcerative colitis 2020 [ updated 2020 Sep 1; cited 2021 Feb 24]. Available from: https://www.takeda.com/newsroom/statements/2020/update-on-the-u.s.-development-program-for-the-investigational-subcutaneous-formulation-of-entyvio-vedolizumab-as-a-maintenance-therapy-in-adults-with-moderate-to-severe-ulcerative-colitis/
- Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 budget impact analysis good practice II task force. Value Health. 2014;17(1):5–14.
- Haute Autorité de Sante. Transparency Committee Opinion, 3 October 2012. Adalimumab, ATC code L04AB04 (TNF inhibitor) cited 2021 Jun 8]. Available from 2021 Jun 8: https://www.has-sante.fr/upload/docs/application/pdf/2013-07/humira_ct_12238.pdf
- Ducimetière P. Apport du registre EPIMAD. De l’épidémiologie descriptive à l’analytique 2015 cited 2021 Apr 19]. Available from 2021 Apr 19: http://www.observatoire-crohn-rch.fr/wp-content/uploads/2015/09/EPIMAD-donne%CC%81es-re%CC%81centes.pdf
- GEMINI 1 Study Group, Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369(8):699–710.
- de Saint Pol T. Institut national de la statistique et des études économiques. Corps et appartenance sociale: la corpulence en Europe. Données sociales - La société française. 2006: 649–656
- Jairath V, Chan K, Lasch K, et al. Integrating efficacy and safety of vedolizumab compared with other advanced therapies to assess net clinical benefit of ulcerative colitis treatments: a network meta-analysis. Expert Rev Gastroenterol Hepatol. 2021;15(6):711–722.
- Bodger K, Kikuchi T, Hughes D. Cost-effectiveness of biological therapy for Crohn’s disease: Markov cohort analyses incorporating United Kingdom patient-level cost data. Aliment Pharmacol Ther. 2009;30(3):265–274.
- Tsai HH, Punekar YS, Morris J, et al. A model of the long-term cost effectiveness of scheduled maintenance treatment with infliximab for moderate-to-severe ulcerative colitis. Aliment Pharmacol Ther. 2008;28(10):1230–1239.
- Frolkis AD, Dykeman J, Negrón ME, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology. 2013;145(5):996–1006.
- Cohen R, Skup M, Ozbay AB, et al. Direct and indirect healthcare resource utilization and costs associated with ulcerative colitis in a privately-insured employed population in the US. J Med Econ. 2015;18(6):447–456.
- Cohen RD, Yu AP, Wu EQ, et al. Systematic review: the costs of ulcerative colitis in Western countries. Aliment Pharmacol Ther. 2010;31(7):693–707.
- Malone DC, Waters HC, Van Den Bos J, et al. A claims-based Markov model for Crohn’s disease. Aliment Pharmacol Ther. 2010;32(3):448–458.
- Rubin DT, Mody R, Davis KL, et al. Real-world assessment of therapy changes, suboptimal treatment and associated costs in patients with ulcerative colitis or Crohn’s disease. Aliment Pharmacol Ther. 2014;39(10):1143–1155.
- Sécurité Sociale l’Assurance Maladie. Codage website, France 2021 cited 2021 Feb 25]. Available from 2021 Feb 25: http://www.codage.ext.cnamts.fr/codif/bdm_it/index.php?p_site=AMELI
- Aide au Codage. Aide au codage website, France 2021 [ cited 2021 Feb 25]. Available from: https://www.aideaucodage.fr
- Essat M, Tappenden P, Ren S, et al. Vedolizumab for the treatment of adults with moderate-to-severe active ulcerative colitis: an evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics. 2016;34(3):245–257.
- Yokomizo L, Limketkai B, Park KT. Cost-effectiveness of Adalimumab, infliximab or vedolizumab as first-line biological therapy in moderate-to-severe ulcerative colitis. BMJ Open Gastroenterol. 2016;3(1):e000093.
- Wilson M, Lucas A, Cameron A, et al. Budget impact of adding vedolizumab to a health plan formulary as another first-line biologic option for ulcerative colitis and Crohn’s disease. Am Health Drug Benefits. 2018;11(5):253–262.