ABSTRACT
Background
Hyperkalemia is associated with increased healthcare resource utilization (HRU). This study evaluated the impact of sodium zirconium cyclosilicate (SZC) use on HRU in outpatients with hyperkalemia.
Research design and methods
A retrospective noncomparative study using claims data from the HealthVerity warehouse, which included outpatients in the United States who initiated SZC between January and December 2019 (index date) with ≥6 months’ continuous coverage before (baseline) and after (follow-up) the index date (total coverage of 12 months). The study aimed to describe HRU with long-term and short-term SZC (defined as >90 and ≤90 days’ supply, respectively, during 180 days’ follow-up) and identify characteristics associated with long-term versus short-term therapy.
Results
Of 1153 patients, 748 (64.9%) received short-term and 405 (35.1%) received long-term therapy. During follow-up, lower proportions of patients on long-term versus short-term therapy had hyperkalemia-related hospitalizations (10.1% vs 15.1%; P < 0.05) and all-cause hospitalizations (22.5% vs 29.3%; P < 0.05). Hyperkalemia-related and all-cause hospitalization proportions were 33.0% and 23.3% lower, respectively. Predictors of long-term therapy included stage 3 chronic kidney disease.
Conclusions
Approximately one-third of patients with hyperkalemia received long-term SZC therapy. Hyperkalemia-related and all-cause hospitalization proportions were lower with long-term therapy, although further confirmatory studies are needed.
1. Introduction
Hyperkalemia, variably defined as abnormally elevated serum potassium levels of >5.0 mmol/L [Citation1], is associated with potentially life-threatening consequences [Citation2] as well as a significant economic and healthcare burden [Citation3–7]. Patients with hyperkalemia often experience increased healthcare resource utilization (HRU), including inpatient hospitalizations and emergency department (ED) visits [Citation3,Citation4,Citation6–9]. In addition, hyperkalemia-related hospitalizations are associated with high rates of readmission within 90 days post discharge [Citation5].
Management of chronic hyperkalemia usually includes dietary modifications to reduce potassium intake, as well as diuretic therapy, oral sodium bicarbonate, and discontinuation or dose reduction of renin–angiotensin–aldosterone system inhibitors (RAASis) [Citation4,Citation10]. These strategies for managing hyperkalemia are associated, however, with several limitations. Dietary potassium restriction alone is not particularly effective with regard to reducing hyperkalemia risk and may also deprive patients of nutrient-rich, heart-healthy foods from which they would otherwise benefit [Citation11]. Similarly, diuretic therapy is less effective in patients with advanced chronic kidney disease (CKD) or end-stage kidney disease (ESKD) [Citation12], and the use of oral sodium bicarbonate to manage hyperkalemia is restricted to patients with concurrent metabolic acidosis [Citation10]. Furthermore, discontinuation or dose reduction of RAASi therapy to reduce hyperkalemia risk may lead to worsening of cardiovascular and renal outcomes [Citation13]. Although oral anti-hyperkalemia therapy (also known as oral potassium binder or gastrointestinal cation exchanger therapy) may be used to treat chronic hyperkalemia, sodium polystyrene sulfonate (SPS) is associated with several toxicities, including hypomagnesemia, hypocalcemia, and gastrointestinal adverse events, and long-term use of SPS is rare [Citation14,Citation15]. Newer oral anti-hyperkalemia therapies, including sodium zirconium cyclosilicate (SZC) and patiromer, are recommended to manage hyperkalemia and optimize RAASi therapy in patients with CKD [Citation16] and/or heart failure (HF) with reduced ejection fraction [Citation17].
Despite clear evidence that hyperkalemia is associated with increased HRU, there is a lack of studies investigating whether long-term maintenance therapy with a newer oral anti-hyperkalemia treatment reduces hyperkalemia-related hospitalization rates in patients with hyperkalemia. The aim of the current real-world study was to evaluate the use of SZC for hyperkalemia treatment in the outpatient setting and describe the impact of SZC treatment duration on hyperkalemia-related and all-cause HRU in these patients. This study also examined which patient characteristics were associated with long-term versus short-term SZC therapy for hyperkalemia in the outpatient setting.
2. Patients and methods
2.1. Study design and objectives
RECOGNIZE I was a retrospective, observational, noncomparative, descriptive study that focused on outpatient management of hyperkalemia. The study used de-identified medical and pharmacy claims data from patients in the United States (US) from the HealthVerity data warehouse (Private Source 20 and Private Source 17), a for-profit data aggregation service that provides access to linked data from >70 different health data sources.
The study objectives were: (1) to describe the proportions of patients with hyperkalemia-related and all-cause HRU (inpatient hospitalizations, ED visits, and outpatient visits) in patients receiving long-term SZC therapy (defined as >90 days’ supply of SZC dispensed during 180 days’ follow-up) and short-term SZC therapy (defined as ≤90 days’ supply of SZC dispensed during 180 days’ follow-up); and (2) to identify patient characteristics associated with long-term versus short-term SZC therapy in the outpatient setting. Hyperkalemia-related hospitalization was defined as hospitalization with an International Classification of Diseases 10th Revision Clinical Modification (ICD-10-CM) diagnosis of hyperkalemia (diagnosis code of E87.5) in any diagnosis position. Similarly, hyperkalemia-related ED visits or outpatient visits were defined as visits with ICD-10-CM diagnosis of hyperkalemia in any diagnosis position.
As this study utilized de-identified data, no institutional review board waiver of informed consent approval or exemption was required, as per article 45 §CFR 164.514(e).
2.2. Study population
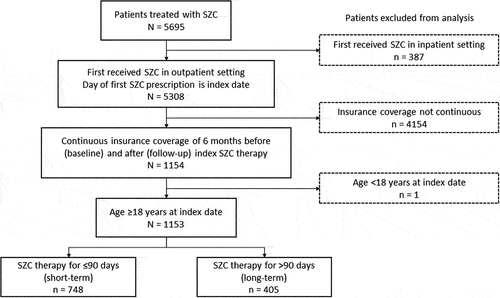
Eligible patients were aged ≥18 years and first received SZC in the outpatient setting between January 2019 and December 2019 (index date). Patients were required to have ≥6 months (≥180 days) of continuous medical and pharmacy insurance coverage before (baseline period) and after (follow-up period) the index date (total coverage of 12 months). Follow-up period claims data were analyzed through June 2020. Patients who first received SZC during inpatient hospitalization or did not have 12 months of continuous insurance coverage were excluded.
A pre-specified subgroup analysis of patients without ESKD was also conducted. Because the HealthVerity data warehouse does not contain data from fee-for-service Medicare (the major payer covering most medical services for patients with ESKD), claims data for these patients are likely to be incomplete in the analysis of the total population of patients on SZC therapy that included patients with ESKD. In addition, the US Food and Drug Administration (FDA) label for SZC did not provide any dosage and administration information for patients with ESKD on hemodialysis prior to expansion of its therapeutic indication to include these patients in April 2020 [Citation18]. As a result, clinicians would have lacked on-label guidance to prescribe SZC in patients with ESKD (e.g. administration on dialysis vs. non-dialysis days) during the study period. Therefore, a subgroup analysis of patients without ESKD was conducted to provide a more accurate description of SZC treatment patterns and their impact on HRU, including hospitalizations.
2.3. Statistical analysis
Patient characteristics were assessed using descriptive statistics; no formal statistical hypotheses were tested. Mean ± standard deviation (SD) were used to describe continuous variables and counts and percentages were used for categorical variables. For between-group comparisons, the Wilcoxon Rank Sum test was used for continuous variables, and the Chi-square test was used for categorical variables.
A logistic regression analysis was used to analyze patient characteristics associated with long-term versus short-term SZC therapy. The results of the regression analysis were reported as odds ratios (ORs) and their respective 95% confidence intervals (CIs). The potential predictors examined in the multivariable model included: age, sex, type of claims payer, geographic region, CKD stage, presence of comorbidities (i.e. congestive HF, diabetes, hypertension, or liver disease), any RAASi use, SPS or patiromer use, the number of inpatient hospital stays, ED visits, or outpatient visits, and the number of acute kidney injury (AKI)-related inpatient hospital stays.
Based on the findings of the logistic regression analysis, additional post-hoc subgroup analyses were conducted to account for differences at baseline between patients with characteristics associated with significant ORs for the likelihood of long-term versus short-term SZC therapy.
3. Results
3.1. Patients
In total, 1153 patients were included in this analysis (). In the total population, 748 patients (64.9%) had received short-term SZC therapy (≤90 days) and 405 patients (35.1%) had received long-term maintenance SZC therapy (>90 days). Of the 1153 patients on SZC, 266 patients (23.1%) and 799 patients (69.3%) were on mean daily SZC doses of 5 g and 10 g, respectively. The majority of patients (n = 1065; 92.4%) were on mean daily SZC doses of either 5 g or 10 g, while 88 patients (7.6%) were on a mean daily dose of 15 g. The non-ESKD subgroup included 822 patients, of whom 519 (63.1%) had received short-term SZC therapy and 303 (36.9%) had received long-term SZC.
Baseline demographic and clinical data for the total population and the non-ESKD subgroup are summarized by SZC treatment duration in . The overall data for each of these populations are summarized in Supplementary Table S1. In the total population, the mean ± SD age was 60.2 ± 13.9 years, 61.2% were male, 58.5% had stage 3–5 or unspecified stage CKD, and 57.1% had received RAASi therapy during the baseline period. The most common hyperkalemia-related comorbidities in the total population were hypertension (88.9%) and type 2 diabetes (62.6%). Patients in the non-ESKD subgroup had a mean ± SD age of 62.1 ± 13.7 years, 59.1% were male, 82.1% had stage 3–5 or unspecified CKD, and 61.3% had received RAASi therapy during the baseline period. Similar to the total population, the most common hyperkalemia-related comorbidities in the non-ESKD subgroup were hypertension (86.7%) and type 2 diabetes (62.3%).
Table 1. Baseline demographics and clinical characteristics according to duration of SZC therapy in the total patient population and in the subgroup of patients without end-stage kidney disease.
Statistical comparison of patient baseline characteristics between the short-term and long-term SZC therapy cohorts in the total population revealed that a significantly higher proportion of patients on long-term therapy had any CKD (stage 3–5 or unspecified), stage 3 CKD, and previous hyperkalemia treatment with patiromer (). A significantly lower proportion of patients who received long-term SZC therapy had congestive HF and inpatient hospitalizations during the baseline period (all-cause, hyperkalemia-related, and AKI-related; ) and follow-up period (all-cause and hyperkalemia-related; ) compared with patients on short-term therapy.
Table 2. Healthcare resource utilization during follow-up according to duration of SZC therapy in the total patient population and in the subgroup of patients without end-stage kidney disease.
In the statistical comparison of patient characteristics in the non-ESKD subgroup, a significantly higher proportion of patients who received long-term SZC therapy had any CKD (stage 3–5 or unspecified), stage 3 CKD, and previous hyperkalemia treatment with dialysis or patiromer compared with those on short-term therapy (). In this subgroup, a significantly lower proportion of patients had comorbid liver disease in the long-term versus short-term therapy cohort.
3.2. Healthcare resource utilization
In the unadjusted descriptive analysis of HRU, the proportion of patients with hyperkalemia-related inpatient hospitalizations during the 6-month follow-up period was 33.0% lower with long-term versus short-term SZC therapy in the total population (10.1% vs. 15.1%; P < 0.05), and 36.9% lower with long-term versus short-term therapy in the non-ESKD subgroup (6.9% vs. 11.0%; P = 0.07) (). Likewise, the proportion of patients with all-cause inpatient hospitalization was 23.3% lower with long-term versus short-term SZC therapy in the total population (22.5% vs. 29.3%; P < 0.05), and 25.4% lower with long-term therapy versus short-term therapy in the non-ESKD subgroup (17.8% vs. 23.9%; P = 0.05). Long-term SZC therapy was also associated with a significantly lower proportion of patients with all-cause ED visits compared with short-term therapy in the total population (20.0% vs. 26.3%; P < 0.05) and in the non-ESKD subgroup (16.8% vs. 23.1%; P < 0.05) (, ). However, there was no statistically significant difference in the proportion of patients with hyperkalemia-related ED visits between long-term and short-term SZC therapy in both the total population and in the non-ESKD subgroup.
Figure 2. Proportion of patients with (a) inpatient hospitalization and (b) emergency department visits during follow-up according to the duration of SZC therapy, in the total population and in the subgroup of patients without end-stage kidney disease.
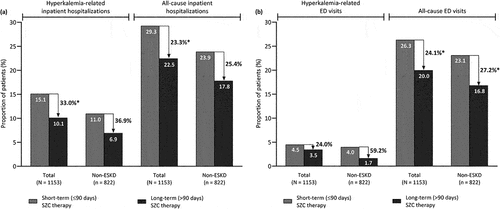
Among patients with hospitalizations for any cause during follow-up in the total population, 45.1% (41/91) of patients who were on long-term therapy and 51.6% (113/219) of those on short-term therapy had at least one hyperkalemia-related inpatient hospitalization (). In the non-ESKD subgroup, 38.9% (21/54) of patients on long-term therapy and 46.0% (57/124) of patients on short-term therapy who had inpatient hospitalizations, respectively, had one or more hyperkalemia-related inpatient hospital stay ().
During the baseline period, the proportion of patients with hyperkalemia-related inpatient hospitalizations was significantly lower with long-term versus short-term SZC therapy in the total population (11.9% vs. 19.9%; P < 0.001), whereas there was no significant difference in the hyperkalemia-related inpatient hospitalization rate between long-term and short-term therapy in the non-ESKD subgroup (10.6% vs. 13.5%; P > 0.05) (). Likewise, the proportion of patients with all-cause inpatient hospitalizations during the baseline period was significantly lower with long-term versus short-term SZC therapy in the total population (24.0% vs. 34.4%; P < 0.001) but was not significantly different between long-term and short-term therapy in the non-ESKD subgroup (22.4% vs. 26.4%; P > 0.05). There was also no significant difference in the proportion of patients with hyperkalemia-related or all-cause ED visits or outpatient visits between the long-term and short-term therapy cohorts in the total population, as well as in the non-ESKD subgroup, during the baseline period.
3.3. Predictors of long-term SZC therapy
In the total population, patient characteristics that were associated with significantly higher odds of long-term versus short-term SZC therapy were West (vs. Midwest) region (OR, 1.91; 95% CI, 1.25–2.94; P < 0.01) and stage 3 or unspecified stage CKD (vs. no CKD) (OR, 1.93; 95% CI, 1.14–3.37; P < 0.05) (). Patient characteristics associated with significantly lower odds of long-term versus short-term SZC therapy were Medicaid (vs. commercial or unknown) claims payer (OR, 0.44; 95% CI, 0.31–0.63; P < 0.001) and the number of all-cause inpatient hospitalizations at baseline (OR, 0.81; 95% CI, 0.65–0.97; P < 0.05).
Figure 3. Regression model of predictors of long-term (>90 days) versus short-term (≤90 days) SZC therapy in (a) the total patient population and (b) patients without end-stage kidney disease.
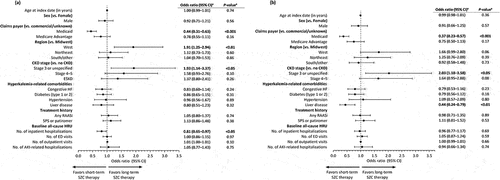
In the non-ESKD subgroup, the odds of long-term SZC therapy were significantly higher than for short-term therapy in patients with stage 3 or unspecified stage CKD (vs. no CKD) (OR, 2.03; 95% CI, 1.18–3.58; P < 0.05), and significantly lower in patients with Medicaid (vs. commercial or unknown) insurance (OR, 0.37; 95% CI 0.23–0.57; P < 0.001) or liver disease (vs. no liver disease) (OR, 0.44; 95% CI, 0.24–0.79; P < 0.01) ().
3.4. Additional post-hoc subgroup analyses
Comparison of all-cause and hyperkalemia-related HRU between the long-term and short-term SZC therapy groups showed that, as seen in the total population and the non-ESKD subgroup, the proportions of patients with inpatient hospitalizations and ED visits were always lower for patients receiving long-term SZC therapy than those for patients receiving short-term SZC (). HRU during the follow-up period for each subgroup is presented in Supplementary Tables S2-S6.
Table 3. Proportion of patients with hyperkalemia-related and all-cause healthcare resource utilization during follow-up according to duration of SZC therapy.
4. Discussion
In this retrospective study of US outpatients receiving SZC therapy, at least one-third of patients received long-term maintenance treatment (>90 days) over 6 months of follow-up. In our descriptive unadjusted analysis, significantly lower proportions of patients on long-term SZC therapy had hyperkalemia-related hospitalization (10.1% vs. 15.1%) and all-cause hospitalization (22.5% vs. 29.3%) during the follow-up period compared with those who received short-term therapy.
These data suggest that long-term maintenance SZC therapy may be associated with a reduced risk of hyperkalemia-related hospitalization compared with short-term therapy. Whether this reduction leads to reduced hyperkalemia-related healthcare costs should be investigated in future studies. For patients without ESKD, there were no statistically significant differences in the proportions of patients with hyperkalemia-related and all-cause hospitalization between the long-term and short-term SZC cohorts during the baseline period. During the follow-up period, the proportions of patients with hyperkalemia-related and all-cause hospitalization in the non-ESKD subgroup were numerically lower with long-term versus short-term SZC therapy; this is potentially attributable to the initiation of long-term maintenance SZC therapy, although no statistical comparison of within-group pre- and post-index HRU was done.
It should be noted that the proportions of patients in the total population with hyperkalemia-related and all-cause inpatient hospitalizations were already significantly lower in the long-term versus short-term therapy cohorts during the baseline period. Therefore, the significantly lower rates of hyperkalemia-related and all-cause hospitalization during the follow-up period with long-term versus short-term therapy could be partly caused by a continuation of baseline trends.
In the total population as well as the subgroups, the proportion of patients with all-cause and hyperkalemia-related outpatient visits during follow-up trended higher among patients who received long-term SZC therapy than among those who received short-term SZC therapy. This may indicate that these patients were more actively engaged with their outpatient healthcare providers, which could potentially have led to greater likelihood of receiving long-term SZC therapy. Increased outpatient visits with a diagnosis of hyperkalemia could be considered a desirable outcome in that it likely reflects close monitoring of blood potassium levels and could facilitate a more proactive approach for hyperkalemia management.
Further investigation of the benefits of long-term anti-hyperkalemia therapy in patients with hyperkalemia will require an additional study that compares outcomes among patients with hyperkalemia receiving long-term SZC therapy versus those not receiving anti-hyperkalemia therapy. In one such previous study of propensity score-matched Medicare Advantage patients with hyperkalemia, treatment with patiromer was associated with significantly lower HRU over 6 months compared with that of untreated patients, with 25% versus 37% of patients, respectively, requiring all-cause inpatient hospitalization or an ED visit [Citation19]. However, to our knowledge, the current real-world study is the first to show that long-term maintenance therapy with a newer anti-hyperkalemia treatment may be associated with a reduction in hyperkalemia-related and all-cause inpatient hospitalizations compared with short-term treatment. Further studies are needed to confirm whether hyperkalemia-related and all-cause hospitalization costs are reduced with long-term anti-hyperkalemia therapy and ideally would include a comparator group of patients with hyperkalemia not treated with anti-hyperkalemia therapy or treated with non-SZC therapy.
Another potential advantage of long-term anti-hyperkalemia therapy is optimization of RAASi therapy, as suggested by Kidney Disease: Improving Global Outcomes and American College of Cardiology guidelines [Citation16,Citation17]. Although the current study did not examine the impact of long-term SZC therapy on RAASi utilization, a previous real-world study of patiromer showed high rates (~80%) of RAASi therapy continuation among patients who received continuous patiromer treatment over 6 months [Citation20]. Further real-world studies are needed to investigate RAASi utilization with long-term SZC therapy.
Although our study did not include an analysis of safety outcomes, other publications have reported evidence on the short-term and long-term safety of SZC. A phase 3, randomized, placebo-controlled study (NCT02088073; Hyperkalemia Randomized Intervention Multidose ZS-9 Maintenance [HARMONIZE]) was conducted in 258 patients with hyperkalemia [Citation21]. During the 28-day maintenance phase, the majority of adverse events were comparable in the SZC and placebo groups and there were no treatment-related serious adverse events. A dose-dependent occurrence of edema (including generalized and peripheral edema) was reported in 2 patients (2.4%) in the placebo group, 1 patient (2.2%) in the SZC 5 g group, 3 patients (5.9%) in the SZC 10 g group, and 8 patients (14.3%) in the SZC 15 g group. Constipation was the only gastrointestinal disorder occurring in ≥5% of patients in any group (n = 6 [7.1%] in the placebo group; n = 0 [0.0%], n = 1 [2.0%] and n = 1 [1.8%], respectively, in the SZC 5 g, 10 g and 15 g groups). Hypokalemia occurred in 5 patients (9.8%) in the SZC 10 g group and in 6 patients (10.7%) and was reported as an adverse event in 1 patient (1.8%) in the SZC 15 g group, but not in any patient in the SZC 5 g or placebo groups; all hypokalemia events were mild and resolved after dose reduction. Of the 14 patients who developed edema, 13 patients completed the study. None of the edema events were deemed as treatment-related by the investigators. However, given that edema was mostly seen in the SZC 15 g group in clinical trials, the relatively small proportion of patients (7.6%) in our study who were on a daily SZC dose of 15 g will likely be reassuring for clinicians.
In a phase 3 open-label, single-arm, long-term study (NCT02163499) of SZC therapy in 751 patients with hyperkalemia, adverse events were mostly mild or moderate in severity and did not result in treatment interruption [Citation22]. During the 12-month maintenance phase, the standardized Medical Dictionary for Regulatory Activities query (SMQ) for edema was reported in 113 patients (15.1%) and was considered related to SZC in 18 patients (2.4%). Only three SMQ edema events of fluid overload (n = 2; 0.3%) and peripheral edema (n = 1; 0.1%) led to treatment discontinuation. During the maintenance phase, hypokalemia as an adverse event was reported in 11 patients (1.5%) and led to treatment discontinuation in 1 patient (0.1%). The most common gastrointestinal adverse events during the maintenance phase were nausea (n = 56; 7.5% of patients), constipation (n = 48; 6.4%), vomiting (n = 36; 4.8%), and diarrhea (n = 33; 4.4%). The mean daily dose of SZC during the maintenance phase was 7.2 g, with the majority of patients receiving daily doses of 5 g or 10 g.
In our study, patients with CKD (vs. no CKD) or from the West (vs. Midwest) region had a higher likelihood of receiving long-term versus short-term SZC therapy. The association with CKD was statistically significant for stage 3 or unspecified CKD but not stage 4 and above; the study may be underpowered to show significance for the difference in these advanced CKD subgroups. Our study also found that patients with a higher number of baseline inpatient hospitalizations or with Medicaid insurance were less likely to receive long-term therapy. Medicaid health plans often have limited coverage of branded medications relative to patients’ ability to pay [Citation23], which may partly explain why long-term SZC therapy was less likely in patients with Medicaid insurance. Similarly, in the non-ESKD subgroup, long-term therapy was more likely in patients with CKD and less likely in patients with Medicaid insurance and those with liver disease. The higher odds of long-term versus short-term SZC therapy in patients with CKD (vs. no CKD) may be caused by an increased risk for hyperkalemia in patients with CKD, which is known to increase with advancing CKD stage [Citation24]. As continuous medical and pharmacy insurance coverage for 6 months after starting SZC was required for inclusion in the study, mortality was considered less likely to impact the duration of SZC therapy. Further studies are needed to confirm the predictors for long-term SZC therapy, especially the role of the severity of hyperkalemia.
This study has limitations that are attributable to the nonrandomized observational nature of its design, the lack of a non-SZC comparator group, and the use of a retrospective insurance claims data warehouse that was not created for research purposes. In addition, patient data, such as laboratory test results and electrocardiograph findings, were not available, limiting our ability to account for the confounding effect of hyperkalemia severity on hospitalizations and the likelihood of long-term SZC therapy. Given the descriptive aim of the study, multivariable adjustment, difference-in-difference, time-varying, or propensity score matching to account for patient differences were not done. However, additional post-hoc subgroup analyses to address patient differences were conducted based on the logistic regression findings related to which patient characteristics were associated with long-term versus short-term SZC therapy. We found that the results were mostly consistent with those of the total population in terms of directionality and numerical trend. Some of the subgroup analyses may have been underpowered to show statistical significance. However, confounding with regard to risk for baseline hospitalization may exist as there was no statistically significant difference in hospitalizations during follow-up with long-term versus short-term therapy in the subgroup of patients with baseline hospitalization. Furthermore, there was potential confounding by indication because of the need for outpatient visits to receive the medication; patients who received long-term SZC therapy had increased outpatient visits compared with those who received short-term therapy, which may suggest greater engagement with clinicians led to increased likelihood of long-term treatment. Within claims data, hyperkalemia is seldom reported as a primary diagnosis. Therefore, a hyperkalemia-related hospitalization (or ED/outpatient visit) does not necessarily mean that hyperkalemia was the primary reason for hospitalization (or ED/outpatient visit). Finally, as our analysis of HRU during the baseline and follow-up periods was unadjusted due to the absence of a comparison group of patients not treated with SZC, these results should be interpreted with caution. The strengths of this study include its large, geographically representative dataset that comprises all adult age groups and multiple claims payers and that it is the first real-world study of its kind for SZC.
5. Conclusions
In this real-world study of US outpatients, about one-third of patients received long-term SZC therapy; stage 3 CKD was a predictor of long-term SZC therapy. The proportions of patients with hyperkalemia-related and all-cause hospitalizations were significantly lower in patients receiving long-term versus short-term therapy during the follow-up period; however, additional confirmatory studies are needed. The potential decrease in hospitalizations with long-term SZC therapy may lead to reduced healthcare costs in patients with hyperkalemia, although the effect of SZC therapy on healthcare costs requires further research.
Declaration of interest
C Pollack, Jr has acted as a scientific consultant for AstraZeneca. A Agiro and Y Brahmbhatt are employees and stockholders of AstraZeneca. F Mu, E Cook, and K Betts are employees of Analysis Group, an economic consulting firm, which received funding from AstraZeneca for the conduct of this study; E Wirtz and J Young were employees of Analysis Group at the time this study was conducted. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
One peer reviewer declares: consultant and holds stock options in Vifor (patiromer). Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Author contributions
Study conception and design: C Pollack Jr, A Agiro, F Mu, E Cook, E Wirtz, J Young, K Betts, Y Brahmbhatt. Analysis: F Mu, E Cook, E Wirtz, J Young, K Betts. Interpretation of data: C Pollack Jr, A Agiro, F Mu, E Cook, E Wirtz, J Young, K Betts, Y Brahmbhatt. Drafting of the paper or revising it for critical intellectual content: C Pollack Jr, A Agiro, F Mu, E Cook, E Wirtz, J Young, K Betts, Y Brahmbhatt. Final approval of the version to be published: C Pollack Jr, A Agiro, F Mu, E Cook, E Wirtz, J Young, K Betts, Y Brahmbhatt. All authors (C Pollack Jr, A Agiro, F Mu, E Cook, E Wirtz, J Young, K Betts, Y Brahmbhatt) agree to be accountable for all aspects of the work.
Prior presentation
Abiy Agiro, Esteban Lemus Wirtz, Joshua A. Young Impact on hospitalizations of long-term versus short-term sodium zirconium cyclosilicate therapy during routine care for patients with hyperkalemia: RECOGNIZE I. Virtual poster presentation at the NKF 2022 Spring Clinical Meeting, April 6–10, 2022, Boston, MA + virtual meeting.
Ethical conduct of research
As this study utilized de-identified data, no institutional review board waiver of informed consent approval or exemption was required, as per article 45 §CFR 164.514(e).
Supplemental Material
Download MS Word (55.3 KB)Acknowledgments
Sarah Greig, PhD (Auckland, NZ) and Raewyn M. Poole (Philadelphia, PA, USA) of inScience Communications provided medical writing support funded by AstraZeneca.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14737167.2023.2161514
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Fried L, Kovesdy CP, Palmer BF. New options for the management of chronic hyperkalemia. Kidney Int Suppl. 2017;7:164–170.
- Montford JR, Linas S. How dangerous is hyperkalemia? J Am Soc Nephrol. 2017;28:3155–3165.
- Betts KA, Woolley JM, Mu F, et al. The cost of hyperkalemia in the United States. Kidney Int Rep. 2018;3:385–393.
- Desai NR, Reed P, Alvarez PJ, et al. The economic implications of hyperkalemia in a medicaid managed care population. Am Health Drug Benefits. 2019;12:352–361.
- Betts KA, Woolley JM, Mu F, et al. Postdischarge health care costs and readmission in patients with hyperkalemia-related hospitalizations. Kidney Int Rep. 2020;5:1280–1290.
- Fitch K, Woolley JM, Engel T, et al. The clinical and economic burden of hyperkalemia on medicare and commercial payers. Am Health Drug Benefits. 2017;10:202–210.
- Mu F, Betts KA, Woolley JM, et al. Prevalence and economic burden of hyperkalemia in the United States medicare population. Curr Med Res Opin. 2020;36:1333–1341.
- Dashputre AA, Gatwood J, Sumida K, et al. Association of dyskalemias with short-term health care utilization in patients with advanced CKD. J Manag Care Spec Pharm. 2021;27:1403–1415.
- Sharma A, Alvarez PJ, Woods SD, et al. Healthcare resource utilization and costs associated with hyperkalemia in a large managed care population. J Pharm Health Serv Res. 2021;12:35–41.
- Palmer BF, Carrero JJ, Clegg DJ, et al. Clinical management of hyperkalemia. Mayo Clin Proc. 2021;96:744–762.
- Palmer BF. Potassium binders for hyperkalemia in chronic kidney disease-diet, renin-angiotensin-aldosterone system inhibitor therapy, and hemodialysis. Mayo Clin Proc. 2020;95:339–354.
- Kovesdy CP. Management of hyperkalaemia in chronic kidney disease. Nat Rev Nephrol. 2014;10:653–662.
- Rosano GMC, Tamargo J, Kjeldsen KP, et al. Expert consensus document on the management of hyperkalaemia in patients with cardiovascular disease treated with renin angiotensin aldosterone system inhibitors: coordinated by the working group on cardiovascular pharmacotherapy of the European society of cardiology. Eur Heart J Cardiovasc Pharmacother. 2018;4:180–188.
- Laureati P, Xu Y, Trevisan M, et al. Initiation of sodium polystyrene sulphonate and the risk of gastrointestinal adverse events in advanced chronic kidney disease: a nationwide study. Nephrol Dial Transplant. 2020;35:1518–1526.
- Rahman S, Marathi R. Sodium polystyrene sulfonate. StatPearls. Treasure Island (FL): StatPearls Publishing; 2021.
- Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2021;99:S1–S87.
- Heidenreich PA, Bozkurt B, Aguilar D, et al. AHA/ACC/HFSA guideline for the management of heart failure: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. 2022;145:e876–94.
- AstraZeneca. Lokelma US label updated to include dosing guidance for the treatment of hyperkalaemia in patients with end-stage renal disease on haemodialysis. 2020. [cited 2022 Feb 10]. Available from: https://www.astrazeneca.com/content/astraz/media-centre/medical-releases/lokelma-us-label-updated-to-include-dosing-guidance-for-the-treatment-of-hyperkalaemia-in-patients-with-end-stage-renal-disease-on-haemodialysis.html.
- Desai NR, Alvarez PJ, Golestaneh L, et al. Healthcare utilization and expenditures associated with hyperkalemia management: a retrospective study of Medicare Advantage patients. J Med Econ. 2021;24:1025–1036.
- Kovesdy CP, Gosmanova EO, Woods SD, et al. Real-world management of hyperkalemia with patiromer among United States veterans. Postgrad Med. 2020;132:176–183.
- Kosiborod M, Rasmussen HS, Lavin P, et al. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: the HARMONIZE randomized clinical trial. JAMA. 2014;312:2223–2233.
- Spinowitz BS, Fishbane S, Pergola PE, et al. Sodium zirconium cyclosilicate among individuals with hyperkalemia: a 12-month Phase 3 study. Clin J Am Soc Nephrol. 2019;14:798–809.
- Cohen MJ, Shaykevich S, Cawthon C, et al. Predictors of medication adherence postdischarge: the impact of patient age, insurance status, and prior adherence. J Hosp Med. 2012;7:470–475.
- Gasparini A, Evans M, Barany P, et al. Plasma potassium ranges associated with mortality across stages of chronic kidney disease: the Stockholm CREAtinine Measurements (SCREAM) project. Nephrol Dial Transplant. 2019;34:1534–1541.