ABSTRACT
Background
STN1013001 is an innovative latanoprost cationic emulsion for open-angle glaucoma/ocular hypertension (OAG/OHT) and ocular surface disease (OSD).
Methods and findings
A 5-year, 7 health states, 1-year cycle early Markov model-supported cost-utility analysis (CUA) of STN1013001 vs. other latanoprost formulations (Latanoprost) followed the Italian National Health Service (INHS) perspective.
One-way, probabilistic and scenario sensitivity analyses tested the uncertainty of the baseline results. Value of information analysis (VOIA) investigated the potential cost-effectiveness of collecting further evidence.
Results
Over 5 years, the Markov model-supported CUA predicts STN1013001 to be potentially highly cost-effective vs. Latanoprost (+€57.60 cost at €2020 values; +0.089 Quality-Adjusted Life Years).
The Incremental Cost-Utility Ratio (€647.65) falls well below the lower limit of the acceptability range proposed for Italy (€25,000-€40,000).
Sensitivity analyses confirmed the robustness of the baseline findings. VOIA highlighted that further information might only be cost-effective for OAG/OHT utilities and OSD-related disutility.
Conclusion
STN1013001 is potentially highly cost-effective and strongly dominant vs. Latanoprost for OAG/OHT+OSD patients from the INHS perspective. These findings should be re-assessed using the data from the ongoing Phase III trial (NCT04133311) comparing the efficacy and safety of STN1013001 vs. Latanoprost and with future real-world CUAs upon the availability of STN1013001 on the Italian market.
1. Introduction
Glaucoma is one of the most frequent causes of irreversible blindness and represents a critical issue to healthcare systems [Citation1]. By 2040, 112 million glaucoma prevalent patients expected worldwide (+ 74% vs. 2013) will bring about severe disease-related economic and social consequences [Citation1].
According to the results of a population-based survey, the prevalence of open-angle glaucoma (OAG) in Italy reaches 1.4% [Citation2] and accounts for over 80% of the glaucoma cases, causing severe vision loss in approximately 50,000 patients [Citation3].
Glaucoma generally progresses from stage 0 (ocular hypertension – OHT) to more advanced stages (stage 1 – early glaucoma; stage 2 – moderate glaucoma; stage 3 – advanced glaucoma; stage 4 – severe glaucoma; stage 5 – end-stage/blindness) [Citation3,Citation4]. Before patient becomes symptomatic in progressing to more advanced disease stages, irreversible vision damage is often already present. Therefore early diagnosis and appropriate therapies to maintain vision in OAG/OHT patients are of primary importance [Citation1].
Based on the most recent data currently available, the average annual cost per OAG/OHT patient in Italy reaches Euros (€) 788.70 and increases with disease severity (€572.00 for OHT, €734.30 for stages 1–2 and €1054.90 for advanced OAG/OHT), with glaucoma medications and specialist consultations being the cost-drivers [Citation5].
The European Glaucoma Society guidelines suggest initiating the treatment with monotherapy and assessing OAG/OHT patient characteristics and drug properties before prescribing the active agent [Citation6]. In consideration of the amount of medication, the intra-ocular pressure (IOP)-lowering efficacy, the systemic safety profile and the treatment regimen, prostaglandin analogues are often used as monotherapy [Citation6].
About 60% of OAG/OHT patients receiving topical IOP-lowering drugs experience concomitant ocular surface disease (OSD) symptoms (e.g. dry eye disease – DED) [Citation7]. OSD symptoms negatively impact patients’ health related quality of life (HRQoL), which is already worsened by OAG/OHT [Citation6] and affect their daily activities [Citation8,Citation9]. In addition, OSD may decrease therapy adherence and reduce IOP control, while potentially increasing the risk of OAG/OHT progression [Citation8,Citation10–12].
Therefore, improvement of treatment adherence, as well as the effective management of concomitant OSD remain unmet needs in this patient population, which should be hopefully fulfilled by new OAG/OHT therapies.
STN1013001 (Santen, Osaka, Japan), formerly DE-130A, is an innovative latanoprost cationic emulsion formulation (based on the Novasorb® technology) for the treatment of OAG/OHT with concomitant OSD [Citation13].
The cationic emulsion possesses tear film stabilization and anti-inflammatory properties and has the ability to reside on the ocular surface for a prolonged period of time due to its optimized interaction with the tear film [Citation14].
During a 3-month Phase 2 trial, STN1013001 proved to be as effective as Latanoprost at lowering IOP (−6.0% vs. −5.4%; p > 0.05) and significantly reduced OSD-related signs and symptoms (−36.0% vs. −7.0%; p < 0.05) vs. baseline in the per protocol study population [Citation15].
A Phase III trial investigating the comparative efficacy and safety of STN1013001 vs. Latanoprost (NCT04133311) is currently ongoing [Citation16].
As such, STN1013001 is not available on the market yet.
In order to provide decision makers with provisional evidence about the country-specific economic value of STN1013001 vs. other latanoprost formulations (henceforth Latanoprost) in OAG/OHT+OSD patients, three early Markov-model supported cost-utility analyses (CUA) [Citation17–23] for three relevant European markets (Germany [Citation24], France [Citation25], and Italy) were developed.
The aim of this early Markov-model supported CUA, that follows the Italian National Health Service (INHS) perspective, is to investigate the economic value of STN1013001 before it enters the Italian market.
2. Methods
2.1. Markov model
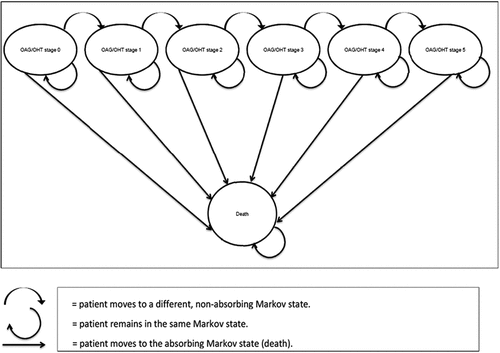
The Markov model, consisting of 1019 parameters, includes 7 mutually exclusive health states (OAG/OHT stages 0–5; age and gender-specific all-cause mortality) [Citation21–23, Citation26,Citation27] ().
The Markov model was developed in Excel Windows® 2010 (Microsoft, Redmond, WA, USA) and was mainly populated with pooled data collected from a convenience sample [Citation28] of 5 Italian ophthalmologists from public and private high-volume care practice centers (public teaching hospital: 3, private eye clinic: 1, and INHS-endorsed private hospital: 1).
Due to their extensive experience in managing OAG/OHT (1338 patients followed-up yearly), the glaucoma specialists were assumed to be authoritative sources of real world data concerning the disease.
The remaining data were retrieved from the literature.
Two cohorts of 1000 notional patients each were simulated for STN1013001 and Latanoprost. For both the monotherapies the Markov cohort simulation starts at OAG/OHT stage 0 [Citation21,Citation23].
The Markov model adopted a 1-year cycle length (365.25 days, correcting for leap years) [Citation29] and stretched over 5 years. Despite OAG/OHT being a chronic disease, a 5-year time horizon was consistent with the duration of a pivotal international, retrospective cost description on OAG/OHT that included, among other countries, Germany, France and Italy [Citation3]. In addition, in previous health economic evaluations supported by early decision models, a timespan varying between 1 and 5 years was considered sufficient to capture provisional costs and effectiveness results totaled by the healthcare programs under comparison [Citation17,Citation30].
For each Markov cycle, a transition probability matrix governed the distribution of each hypothetical cohort among the different OAG/OHT stages and all-cause mortality by age and gender (Supplementary [S] S1 Table) [Citation21,Citation23]. The probability of entering the absorbing state of the Markov model was calculated based on the Italian life tables [Citation26].
Consistent with the nature of the disease, the Markov model did not allow backward transitions from more to less severe OAG/OHT stages.
The half-cycle correction and a 3% real social discount rate were applied to costs, life-year saved (LYS), and Quality-Adjusted Life Years (QALYs) [Citation19–23,Citation31,Citation32]. The real social discount rate was set at 0% and 5% in one-way sensitivity analysis (OWSA) [Citation19–23, Citation31, Citation32].
The annual number of current and future OAG/OHT patients expected to be eligible to STN1013001 (20,000 per year during an assumed 5-year useful life of STN1013001 or 100,000 undiscounted patients in total) for Value of Information Analysis (VOIA) [Citation23,Citation33] was also discounted at 3% per year [Citation31,Citation32].
2.2. Data collection
As the study population differed from the one considered in this research and no economic evaluation was planned alongside the 3-month Phase 2 trial comparing STN1013001 vs. Latanoprost [Citation15], the Markov model-supported CUA mainly relied on glaucoma specialists judgment.
Experts opinion can be elicited via different methods [Citation34,Citation35]. According to the literature [Citation34], a convenience sample [Citation28] of 5 experts was considered too small for a Delphi panel to be feasible. Therefore, a questionnaire aimed at data collection was developed and sent out by e-mail to the glaucoma experts separately between July-September 2020, along with the STN1013001 target product profile. The STN1013001 target product profile reported on the Novasorb® technology [Citation14], along with the therapeutic indication (OAG/OHT + concomitant OSD), daily posology (one drop per eye) and efficacy [Citation15] of this innovative latanoprost cationic emulsion formulation.
Upon their validation of both the research tools [Citation34], ophthalmologists were requested to fill in the questionnaire according to their experience [Citation35] (for Latanoprost) and target product profile (for STN1013001).
The questionnaire aimed at collecting the following OAG/OHT stage-specific data across a 5-year time horizon: patient anagraphics; annual probabilities of remaining in the same health state or transitioning to a more severe one; annual adherence probabilities to STN1013001 or Latanoprost monotherapy; potential add-on therapies and probabilities of their prescription in case of insufficient IOP control with the monotherapies under comparison; volume of other healthcare resource and probabilities of their consumption (e.g. medications, tests and specialist visits for diagnosis, management and follow-up of OAG/OHT and concomitant OSD); utilities.
All questionnaires were returned completed. When needed, follow-up teleconferences were scheduled with the experts for clarifications.
The parameters that populated the Markov model were calculated based on the weighted average of the data provided by glaucoma experts, the weight being the estimated annual number of patients eligible to STN1013001 and Latanoprost for each OAG/OHT stages ().
Table 1. Base case analysis – Methods – Unit cost for healthcare resources, utility and disutility values (Costs in €2020).
The annual probability of concomitant OSD was the only parameter derived from the Phase 2 trial comparing STN1013001 to Latanoprost [Citation15].
The 3-month reduction on eye dryness (STN1013001: 0.350 vs. Latanoprost: 0.420; p > 0.05) [Citation15] was considered by ophthalmologists as a reasonable proxy for the 3-month probability of concomitant OSD in the two hypothetical cohorts of patients. The annual probability of concomitant OSD (STN1013001: 0.762 vs. Latanoprost: 0.837; p > 0.05) was calculated converting the aforementioned 3-month probability over a 12-month timespan assuming a fixed rate with respect to time [Citation23].
As the Markov model-supported CUA did not require patients enrollment [Citation21,Citation23], no Ethics Committee approval of the questionnaire was required by the current Italian legislation [Citation36].
2.3. Utility, disutility and QALYs
No OAG/OHT utilities were available for the Italian setting at the time this research was performed. Therefore, following a previous CUA comparing two POAG surgical procedures that adopted the INHS viewpoint [Citation37], utility values were retrieved from a Dutch study (OAG/OHT stages 0 and 5) [Citation38] or elicited from the experts (OAG/OHT stages 1–4) according to their opinion about the expected difference in annual adherence to STN1013001 or Latanoprost monotherapy ().
The utility for death was set at 0 for both the hypothetical cohorts of patients [Citation19,Citation20].
OSD-related disutility, which was derived from literature [Citation39], was assumed to be the same for both STN1013001 and Latanoprost notional patients ().
QALYs were calculated based on LYS, OAG/OHT stage-specific utilities and OSD-related disutility.
2.4. Cost
Consistent with the CUA perspective, only INHS-funded healthcare resources were valued (). OSD-related medications, such as preservative-free lubricants/artificial tears, were therefore not included in the model, as these are not funded by the INHS but paid out of pocket by patients. Likewise, drug administration was not costed as all medications are self-administered by patients.
In addition, healthcare resources did not include non-pharmacological treatments (i.e. surgical or laser procedures) for two different reasons. First, the main goal of this research was to compare STN1013001 and Latanoprost in terms of costs and QALYs. In addition, according to experts opinion, during the 5-year timespan the probability of undergoing surgery or laser procedures was not expected to differ between the two monotherapies.
On average, one pack of STN1013001 and Latanoprost was assumed to last 30 and 28 days, respectively [Citation40,Citation45].
Add-on therapies, such as timolol, to be prescribed in case of insufficient IOP control on STN1013001 or Latanoprost monotherapy, were assumed to cover, on average, 29 days of treatment.
The unit cost per diem for STN1013001 was calculated based on its estimated ex-factory price provided by Santen.
The daily unit cost for Latanoprost was calculated based on the ex-factory price, obtained from the Agenzia Italiana del Farmaco (The Italian Medicines Agency) (November 2020) [Citation41], of all branded and generic latanoprost formulations available on the Italian market, weighted for their current market share, as per IQVIA MIDAS Sales data to moving annual total third quarter of 2020 [Citation42].
The same approach and sources were adopted to calculate the unit costs per diem of add-on therapies.
Tests and specialist visits for OAG/OHT diagnosis, follow-up and OSD management were valued at the current INHS or regional tariffs for outpatient healthcare procedures [Citation43,Citation44], which were assumed to represent fair proxies for the actual costs borne by the healthcare facilities to provide those healthcare services [Citation46] ().
Costs were expressed in €2020 values.
2.5. Statistical analysis
Mean and standard deviation (SD) were reported for notional patients in each one of the seven health states included in the Markov model.
A theoretical probability distribution was assigned to the majority of the parameters that populated the Markov model (700/1019 = 68.69%) [Citation22,Citation23].
The Beta distribution was fitted to most binomial data (e.g. probabilities of undergoing tests), as well as OAG/OHT stage-specific utility values.
Multinomial data, such as transition probabilities from less severe to more severe OAG/OHT stages, were assigned a Dirichlet distribution.
Volume of healthcare resource consumption (if different from drugs) and OSD-related disutility were modeled via a Gamma distribution.
The Normal distribution was fitted to the unit cost of healthcare resources different from drugs.
Being set by national medicines agencies [Citation31] or local guidelines [Citation32], drug costs and posology, as well as the real social discount rate, were not assigned a theoretical probability distribution, since they are not uncertain [Citation22].
A parametric 95% confidence interval (95% CI) was calculated for all the Markov model inputs that were assigned a statistical distribution. The 95% CIs for incremental cost (ΔC), LYS (ΔLYs), QALYs (ΔQALYs), monotherapies adherence probabilities and probabilities of undergoing tests and specialist visits for diagnosis, management and follow-up, as well as for OSD management, were calculated via the percentile method [Citation22].
As far as the construction of the 95% CIs is concerned, the standard error (SE) of the mean was calculated based on the number of patients in each OAG/OHT stage expected to be on STN1013001 or Latanoprost each year according to experts opinion ( and S1 Table).
Whenever the SE could not be calculated from the data collected, it was determined by imposing a coefficient of variation on the parameter sample estimate [Citation22,Citation47].
For parameters that were not assigned a theoretical probability distribution, a range was reported.
2.6. Sensitivity analyses
2.6.1. One-way sensitivity analysis
In OWSA, the 1019 parameters were changed one at a time by replacing their point estimate with the bounds of their 95% CI or range, while the remaining parameters were kept at their baseline values [Citation19,Citation20].
2.6.2. Probabilistic sensitivity analysis
Since varying each single parameter across its bounds may have a limited impact on the base case ICUR, a probabilistic sensitivity analyses (PSA) explored the joint uncertainty surrounding the baseline estimate of the parameters via a 10,000-iteration Monte Carlo simulation [Citation19,Citation20,Citation22,Citation23].
During each Monte Carlo trial, a random value for each parameter that was given a statistical distribution was drawn [Citation19,Citation20,Citation22,Citation23].
The cost-effectiveness plane [Citation48] presented the ΔC and ΔQALYs pairs obtained from the PSA. The non-parametric Cost-Effectiveness Acceptability Curve (CEAC) and the Cost-Effectiveness Acceptability Frontier (CEAF) were derived from the PSA results via the Net Monetary Benefit (NMB) [Citation19,Citation20,Citation22,Citation23,Citation49–51]. CEAC and CEAF showed the probability that the healthcare program under investigation is cost-effective (CEAC) or optimal (CEAF) vs. the comparator(s), against the unofficial acceptability range (€25,000-€40,000) for incremental LYS or QALY gained proposed for Italy by the Italian Association of Health Economists (AIES) [Citation31].
2.6.3. Scenario sensitivity analysis
The uncertainty surrounding the baseline findings was further investigated via two scenario sensitivity analyses (SSA) [Citation19,Citation20].
Time horizon may be a source of uncertainty for early decision models [Citation17]. Therefore, a first SSA tested whether variations in the timespan of the Markov model (from 1 up to 19 years) changed the base case cost-effectiveness ranking of the two monotherapies under comparison due to discounting, increasing fraction of notional patients progressed to the absorbing state and non-linear relationships among parameters [Citation23].
A second SSA explored the structural uncertainty [Citation19,Citation20] concerning three parameters that potentially played a relevant role in driving the baseline ICUR, that is the annual probability of concomitant OSD for notional patients on STN1013001 or Latanoprost and the OSD-related disutility. A set of assumptions was applied to the annual probability of concomitant OSD: no concomitant OSD for both the hypothetical cohorts of patients; notional patients on STN1013001 or Latanoprost sharing the same annual probability of concomitant OSD; reverse annual probability of concomitant OSD between the two monotherapies. Eventually, OSD-related disutility was set at 0.
Consistent with the literature on structural uncertainty [Citation19], a PSA (10,000 Monte Carlo iterations) was performed for each assumption included in the second SSA.
2.7. Value of information analysis
Regulatory agencies and health technology assessment bodies decide whether a given healthcare technology should be reimbursed given the current information or whether further evidence is required to fund its adoption [Citation23,Citation33].
In order to investigate the necessity and the potential cost-effectiveness of future research on CUA results, a VOIA was performed [Citation23,Citation33].
VOIA included the expected value of perfect information (EVPI) and the EVPI calculated on the 10 most uncertain parameters (EVPPI) for both medications among those which were given a theoretical probability distribution (OAG/OHT stage-specific utilities and OSD-related disutility; unit cost for healthcare resources different from drugs; transition probabilities to OAG/OHT stages or death; probabilities of being classified as OAG/OHT stage 0–5 at diagnosis; annual adherence probabilities to monotherapies; annual probability of concomitant OSD, probabilities of undergoing diagnostic, follow-up and OSD-related tests and visits).
While the EVPI was calculated via 10,000 Monte Carlo iterations, due to computational expense the number of Monte Carlo iterations for EVPPI calculation was reduced (outer loop: 200; inner loop: 100) [Citation23].
EVPI and EVPPI functions were scaled up to a population of 94,342 patients (i.e. 100,000 in total over 5 years discounted at 3%) [Citation23,Citation31–33] and contrasted against €0-€40,000 threshold values, that includes the aforementioned informal acceptability range for the ICUR (€25,000-€40,000) suggested by AIES [Citation31].
3. Results
3.1. Markov model
Both the hypothetical cohorts of patients were assumed to enter the Markov model in OAG/OHT stage 0 at 57.60 years of age (range: 33.00;82.00) (S2 Table).
During the 5-year time horizon, the Markov model traces of both the hypothetical cohorts of patients are similar (), with a relevant share of the notional patients remaining in OAG/OHT stage 0 (STN1013001: mean: 440; SD: 324; Latanoprost: mean: 429; SD: 329) and a negligible number of notional patients who progress to OAG/OHT stage 5 (STN1013001: mean: 34; SD: 28; Latanoprost: mean: 37; SD: 31).
Table 2. Base case analysis – Results – Markov trace – Hypothetical cohorts of patients on STN1013001 and on Latanoprost (Costs in €2020).
All-cause mortality is identical (STN1013001: mean: 240; SD: 170; Latanoprost: mean: 241; SD: 171).
Across all disease stages treatment adherence is similar for STN1013001 and Latanoprost (S3 Table).
3.2. Base case analysis
3.2.1. Healthcare resources consumption – Diagnosis
Most notional patients receive at least three ophthalmologist visits for the diagnosis of OAG/OHT (STN1013001: 99.55%; mean: 4.15; 95% CI: 3.08;5.38; Latanoprost: 97.50%; mean: 3.95; 95% CI: 2.93;5.12) (S4 Table) and four tonometries (STN1013001: 100.00% notional patients; mean: 5.06; 95% CI: 3.91;6.36; Latanoprost: 99.95%; mean: 4.90; 95% CI: 3.90;6.01).
3.2.2. Healthcare resources consumption – Add-on therapies and follow-up
The annual probability of ≥1 add-on therapies to lower IOP in case this is not sufficiently controlled with STN1013001 or Latanoprost monotherapy, varies from 20% to 95% for OAG/OHT stages 0 and 5, respectively (S5 Table). Timolol (ranging from 85% to 60% in OAG/OHT stage 0 and 2, respectively) and timolol+dorzolamide (40% in OAG/OHT stages 3–5) are the most prescribed add-on therapies.
In all the OAG/OHT stages, tonometry is the most frequent follow-up test (STN1013001 notional patients: from 99.91% (stage 0) to 99.88% (stage 5); Latanoprost notional patients: from 99.50% (stage 0) to 99.87% (stage 5)) (S6 Table).
Both hypothetical cohorts of patients have a similar probability of OAG/OHT follow-up visits with the ophthalmologist (STN1013001: 59.68% (stage 3) to 90.81% (stage 0); Latanoprost: 58.55% (stage 3) to 89.00% (stage 0)).
3.2.3. Healthcare resources consumption – OSD management
Across OAG/OHT stages 1–5, patients in the STN1013001 hypothetical cohort have a lower chance of requiring a tear film breakup time test for the management of OSD vs. Latanoprost, varying from −2.98% (95% CI: −5.65%;-0.92%) in OAG/OHT stage 1 to −8.21% (95% CI: −12.03%;-4.80%) in OAG/OHT stage 5, respectively (S7 Table).
Additionally, in OAG/OHT stages 2–4 STN1013001 notional patients are less likely to undergo an optometrist assessment compared to those on Latanoprost. This difference ranges from −3.58% (95% CI: −6.07%;-1.60%) in OAG/OHT stage 2 to −1.93% (95% CI: −3.72%;-0.57%) in OAG/OHT stage 4.
Across all disease stages, slit lamp examination is the most frequently used test for OSD management (STN1013001: from 81.99% (stage 0) to 94.11% (stage 5); Latanoprost: from 85.50% (Stage 0) to 97.74% (stage 5)).
Notional patients on STN1013001 and Latanoprost have a similar probability to be referred to an ophthalmologist for the management of OSD (STN1013001: from 55.44% (stage 5) to 70.00% (stage 0); Latanoprost: from 55.44% (stage 4) to 69.50% (stage 0)).
3.2.4. Cost-utility analysis
The Markov model-supported CUA predicts STN1013001 to have the potential to be highly cost-effective vs. Latanoprost. The ICUR of €647.65 per QALY, resulting from an incremental cost of €57.60 divided by an incremental QALY gain of 0.089, falls well below the lower limit of the unofficial acceptability range (€25,000-€40,000) proposed for Italy by AIES [Citation30].
Over the 5-year time horizon, the mean cost per notional patient is similar for STN1013001 and Latanoprost (€1878.89 vs. €1821.29; ΔC STN1013001: €57.60; 95% CI: -€67.26;€188.60) (). For both the hypothetical cohorts of patients, the cost-drivers are add-on therapies and the healthcare resources consumed during follow-up (STN1013001: 50.52%; Latanoprost: 52.33%).
Table 3. Base case analysis – Results – Costs per patient and cost-utility analysis (€2020).
Higher cost for OAG/OHT monotherapy incurred by notional patients on STN1013001 vs. notional Latanoprost patients (difference: €89.27; 95% CI: €82.43;€99.00) is partially offset by lower costs for the management of OSD compared to their Latanoprost counterparts (difference: -€42.47; 95% CI: -€87.84;-€1.72).
After 5 years, both hypothetical cohorts of patients total similar LYS (3.603 vs. 3.596; ΔLYS STN1013001: 0.006; 95% CI: −0.066;0.087), and QALYs (2.080 vs. 1.991; ΔQALYs STN1013001: 0.089; 95% CI: −0.028;0.207) (). The statistically insignificant 5-year incremental LYS gain for STN1013001 is due to a small difference in the percentage of female notional patients in OAG/OHT stage 0 as reported by experts (43.56% vs. 43.25% for STN1013001 and Latanoprost, respectively) (). The female advantage in life expectancy [Citation26] carries over its negligible effect for STN1013001 from year 1 to year 5 ().
The progression of cumulated costs, LYS and QALYs during the 5-year timespan is also comparable between the two monotherapies ().
3.3. Sensitivity analyses
3.3.1. One-way sensitivity analysis
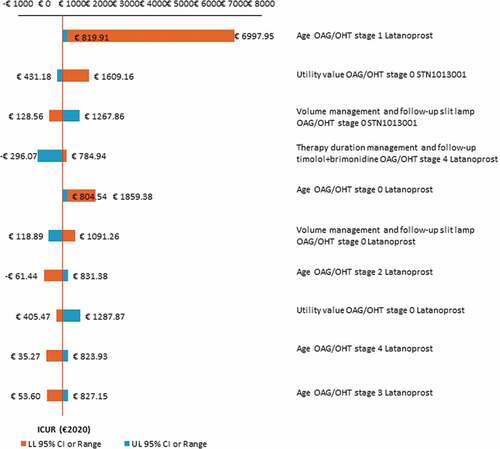
Most of the OWSA results confirm that STN1013001 is highly cost-effective vs. Latanoprost. OWSA shows that the base case ICUR appears most sensitive to changes in age for notional patients on Latanoprost across disease stages 0–4 and utility values for notional patients on STN1013001 (OAG/OHT stage 0) (). Furthermore, OWSA proves the baseline ICUR to be robust to variations in the annual probability of concomitant OSD and OSD-related disutility, as well as in the real social discount rate for costs, LYS and QALYs.
Figure 2. One-way sensitivity analysis - Results concerning the first 10 parameters of the Markov model that causes the widest variation in base case ICUR STN1013001 (€647.65; NE sector of the cost-effectiveness plane) (€2020).a Abbreviations: ICUR, incremental cost-utility ratio; LL 95% CI, lower limit 95% confidenceinterval; NE, north-east; OAG/OHT, open angle glaucoma/ocular hypertension; UL 95% CI, upper limit 95% confidence interval. aY and X-axes intersect at the baseline ICUR.
3.3.2. Probabilistic sensitivity analysis
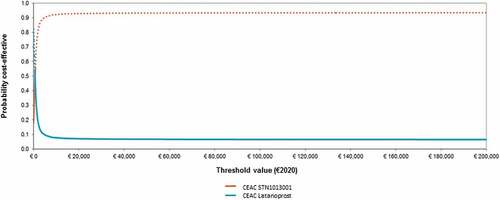
STN1013001 has 16.35% likelihood of being strongly dominant (i.e. less costly and more effective) than Latanoprost (). The probability for STN1013001 to be cost-effective reaches 93.01% and 93.18% at the bounds of the informal willingness-to-pay (WTP) range (€25,000-€40,000) as suggested by AIES for the Italian setting [Citation31] (). The CEAF highlights that STN1013001 is the optimal alternative (i.e. the healthcare program with the highest expected average NMB) from a WTP of €800.80 onwards ().
Figure 3. Probabilistic sensitivity analysis- I - Cost-effectiveness plane (10,000 out of 10,000 Monte Carlo iterationsreported) (€2020).a,b Abbreviations: ΔC, incrementalcost; ΔQALYs,incremental quality-adjusted life years; ICUR, incremental cost-utility ratio; NE, north-east; NW, north-west; SE, south-east; SW, south-west. aBase caseICUR STN1013001: €647.65; NE sector of the cost-effectiveness plane. bNumber of Monte Carlo iterations (%) for each sector of the cost-effectiveness plane: NE=7721 (77.21%); NW=498 (4.98%); SW=146 (1.46%); SE=1635 (16.35%).
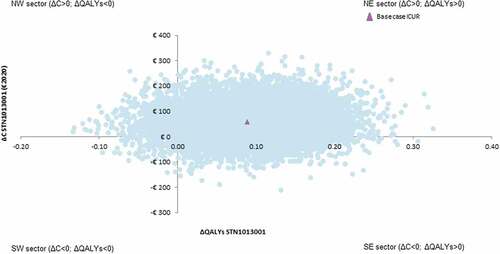
Figure 4. Probabilistic sensitivity analysis - II - Cost-effectiveness acceptability curve and frontier (1000 out of 1000 threshold values reported) (€2020).a,b,c Abbreviations: CEAC,cost-effectiveness acceptability curve; CEAF,cost-effectiveness acceptability frontier; ICUR, incremental cost-utility ratio; NE, north-east; NMB, net monetary benefit; WTP, willingness-to-pay. aBase case ICUR STN1013001: €647.65; NE sector of the cost-effectiveness plane. bThe dotted line represents the CEAF. cCEAF shows that Latanoprost has the highest expected NMB up to a WTP <€800.80 and a decreasing probabilityof being optimal; STN1013001 has the highest expected NMB from a WTP ≥€800.80 and an increasing probability of being optimal.
3.3.3. Scenario sensitivity analysis
The first SSA shows a progressive, time-dependent decreasing ICUR. STN1013001 remains always cost-effective, with the cost per ΔQALY falling well below the lower bound of the informal acceptability range (€25,000-€40,000) suggested by AIES [Citation31] (). From year 10 onwards STN1013001 proves to be strongly dominant vs. Latanoprost.
Table 4. Scenario sensitivity analyses – I – Results (€2020).
The second SSA confirms the cost-effectiveness of STN1013001 even under conservative assumptions concerning OSD ().
Table 5. Scenario sensitivity analyses – II – Results (€2020).
Assuming no OSD-related disutility has a negligible effect on the baseline ICUR (+28.88%) as well as on the probability for STN1013001 to be cost-effective (86.91% and 87.24% for a WTP of €25,000 and €40,000, respectively) or optimal (55.26% from a WTP of €1001 onwards) (). These findings are explained by a reduction in the base case ΔQALYs (−22.47%).
Reversing the annual probabilities of concomitant OSD between STN1013001 and Latanoprost causes the widest variation in the base case ICUR (+370.07%). This result is due to a remarkable increase in ΔC (+121.79%) driven by a reduction in cost-savings for OSD management with STN1013001 (−165.17%), and diminished ΔQALYs (−52.81%). Yet, STN1013001 has a pretty high probability of being cost-effective (73.36% and 74.37% for a WTP of €25,000 and €40,000, respectively) and optimal (50.69% from a WTP of €3203.20 onwards).
The extreme research hypothesis of no OSD for both the monotherapies produces an apparent increase in the baseline ICUR (+123.90) because of increased ΔC (+73.73%) coupled with reduced ΔQALYs (−22.47%). However, the likelihood of STN1013001 to be cost-effective (85.83% and 86.40% for a WTP of €25,000 and €40,000, respectively) and optimal (53.71% from a WTP of €1601.60 onwards) is still relevant.
3.4. Value of information analysis
The EVPI increases for WTP values higher than €8000 and, as expected, reaches a local maximum for a threshold value (€600.60) that approaches the baseline ICUR (€647.65) ().
Figure 5. Value of information analysis - Expected value of perfect information and expectedvalueof parameter perfect information scaled up to a population of 94,342 patients (i.e., 100,000 in total over 5 years discounted at 3%) (€2020).a,b Abbreviations: AE, adverse events; EVPI, expected value of perfect information; EVPPI, expected value of parameter perfect information; FU, follow-up; OAG/OHT, open angle glaucoma/ocular hypertension; OSD, ocular surface disease. aEVPI: 10,000 out of 10,000 Monte Carlo iterations reported. bEVPPI: 100 and 200 Monte Carlo iterations reported for the inner and outer loop, respectively.
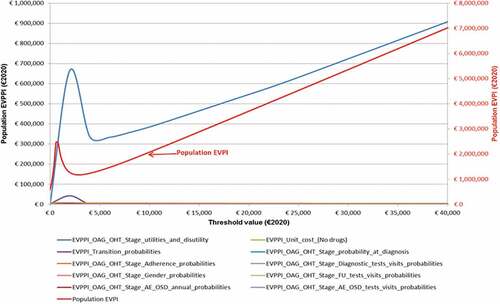
If additional research aimed at reducing the uncertainty surrounding the current information costs €0.97 million it is potentially cost-effective for WTP values greater than €200.20.
The EVPPI functions highlight that, for most of the parameters under consideration, further evidence is not expected to change INHS decision makers’ opinion about the cost-effectiveness of STN1013001 made on the grounds of current information.
For 4 out of the 10 parameters included in VOIA (unit cost for healthcare resources different from drugs; probability of patients’ gender, diagnostic, and OSD-related tests and visits) the EVPPI is zero regardless of the WTP value. Hence, the existing information is sufficient for the INHS to decide to reimburse STN1013001 as a cost-effective healthcare program for OAG/OHT+OSD patients.
The EVPPI absolute maxima for 5 parameters (probability of: transition to OAG/OHT stages or death; OAG/OHT stage at diagnosis; adherence to monotherapies; follow-up tests and visits; OSD) ranges between €3635 (probabilities of being classified as OAG/OHT stage 0–5 at diagnosis) and €0.23 million (probability of adherence to monotherapies) for a WTP value of €2000. To the right of those turning points the 5 EVPPI functions decline and reach zero for threshold values varying between €4000 and €8000. These findings prove that, given the current evidence, the economic consequences of reimbursing STN1013001 as a cost-ineffective alternative (opportunity loss) is totally offset by the increasing probability for STN1013001 to be cost-effective at WTP values well below the lower limit (€25,000) of the informal acceptability range for ICUR recommended for Italy [Citation31].
Eventually, OAG/OHT stage-specific utilities and OSD-related disutility EVPPI and population EVPI functions share the very same shape and similar results whether to acquire further evidence for WTP values greater than €200.20.
4. Discussion
Despite currently available glaucoma therapies [Citation11,Citation12], the frequent occurrence of OSD in OAG/OHT patients remains a therapeutic challenge. This research suggests that, thanks to its innovative technology [Citation13,Citation14], STN1013001 could play an important role in managing OSD in OAG/OHT patients and help address a significant unmet need in this patient population in a cost-effective way.
The present study aimed at evaluating the cost-utility of STN1013001 vs. Latanoprost in OAG/OHT+OSD patients from the INHS perspective via an early Markov model [Citation17–23].
Therefore, our predicted results [Citation17,Citation30] should be re-assessed using the data from the currently ongoing Phase III trial investigating the comparative efficacy and safety of STN1013001 vs. Latanoprost (NCT04133311) [Citation16].
In addition, more research is needed to prove the cost-effectiveness profile of STN1013001 in the real-world clinical practice via future CUAs carried out alongside empirical trials aimed at comparing the two monotherapies.
That said, the base case CUA predicts that, when contrasted against the informal acceptability range for the ICUR (€25,000-€40,000) suggested by AIES [Citation31], STN1013001 has the potential to be highly cost-effective vs. Latanoprost across a 5-year time horizon. This finding is partly driven by the fact that, based on experts opinion, HRQoL was higher across disease stages 1–4 in notional patients in the STN1013001 cohort compared to their Latanoprost counterparts. Another important factor contributing to this result was the lower probability of developing OSD in notional patients on STN1013001 vs. notional Latanoprost patients. This finding is in line with previous research, which indicated that glaucoma patients suffering from concomitant OSD reported significantly lower HRQoL compared to glaucoma patients without concomitant OSD [Citation6,Citation9]. This underlines the importance of effectively managing OSD in this patient population.
Being less likely to develop OSD, notional patients on STN1013001 incurred significantly less costs related to the management of this concomitant disease vs. notional patients on Latanoprost.
The baseline findings were substantively confirmed by comprehensive sensitivity analyses. Most of the variations included in OWSA support the evidence that the ICUR of STN1013001 is far below the lower bound of the unofficial WTP range (€25,000) proposed by AIES [Citation31], suggesting that STN1013001 is a highly cost-effective treatment option for patients suffering from OAG/OHT+OSD vs. Latanoprost in Italy. The base case results appeared most sensitive to changes in Latanoprost notional patients’ age, as this parameter influences, via age and gender-specific all-cause mortality probability, and half-cycle correction [Citation19–23], both costs and QALYs.
Additionally, the CEAF indicates that, for the €25,000-€40,000 WTP range [Citation31], INHS healthcare resources misallocation as a result of funding STN1013001 is negligible.
Interestingly, SSA findings confirms that STN101300 has the potential to be highly cost-effective and optimal regardless of remarkable variations in OSD probability and OSD-related disutility.
VOIA findings suggest that collecting further evidence might only be cost-effective for OAG/OHT stage-specific utilities and OSD-related disutility, also due to the lack of researches on this topic for the Italian setting. As recommended by the literature [Citation23,Citation33], the expected value of sample information along with the expected benefit of sampling shall be calculated to investigate whether the upper bound on further research placed by the EVPPI exceeds the expected cost of sampling to obtain more information on OAG/OHT patients’ HRQoL suitable for QALYs calculation.
The shape of the population EVPI and OAG/OHT stage-specific utility and OSD-related disutility EVPPI functions are consistent with the lack of statistical significance in ΔQALYs resulting from the baseline CUA [Citation51]. However, statistical significance of ΔQALYs (and/or ΔCost) is irrelevant to reimbursement decisions, as what matters to decision makers is whether current evidence is sufficient or not to fund a given healthcare technology [Citation23,Citation33,Citation52,Citation53].
In this respect, most of the VOIA results clearly indicate that STN1013001 is to be reimbursed by INHS as a cost-effective healthcare technology in the light of existing information [Citation23,Citation33,Citation53].
In the literature, two cost-effectiveness analyses (CEAs) that compared Latanoprost with other active compounds targeting OAG/OHT following the INHS viewpoint were identified [Citation54,Citation55]. One study [Citation54] compared Latanoprost to bimatoprost and timolol separately, whereas, in the second study, the cost-effectiveness of three fixed-dose combination therapies, including Latanoprost+timolol, was investigated in OAG-patients. Both CEAs, adopting relatively short time horizons (3 months [Citation55] and 1 year [Citation54], respectively), used IOP reduction as the effectiveness outcome. Given the chosen comparators and outcome in the identified studies, a meaningful comparison of their results with those of the present research was not possible.
As part of the multinational health economic study that the present study belongs to, the cost-utility of STN1013001 vs. Latanoprost has also been investigated in OAG/OHT+OSD patients in Germany [Citation24] and France [Citation25] adopting the same methodology as the present research.
The French and German CUAs were mainly populated based on the input of local glaucoma experts and were customized to adhere to local unit costs and guidelines for health economic evaluation [Citation56,Citation57]. Therefore, country-specific costing approaches made results pooling unfeasible [Citation51].
The distribution of the notional patients across the 6 OAG/OHT stages in both studies was similar to the present one, with at least 40% of the hypothetical cohorts remaining in OAG/OHT stage 0 over the 5-year time horizon.
STN1013001 proved to be strongly dominant vs. Latanoprost (ΔC = -€141.73; ΔQALY = +0.247) in Germany [Citation24] and highly cost-effective in France (ΔC = €7.39; ΔQALY = +0.348; ICUR = €21.2 per ΔQALY vs. an informal WTP range of €30,000-€50,000) [Citation25,Citation57].
These baseline findings were confirmed by PSA. For the German setting CEAF showed that STN1013001 was the optimal healthcare program regardless of the WTP [Citation24], whereas for France the probability for STN1013001 to be the optimal healthcare program reached 100% from a WTP = €1000 onward [Citation25].
Similar to the present study these results were mainly driven by the lower probability of OSD that, in turn, caused cost-savings for OSD management and higher QALYs for STN1013001.
What are the main limitations of this research?
First, as STN1013001 is currently being investigated in a pivotal phase III trial [Citation16], a health economic evaluation based on empirical data was not possible. As a consequence, at the time of conducting this research, it was unfeasible to incorporate disease stage specific utility values in the model that were collected directly from patients during a head-to-head clinical trial. However, the utility values for stage 0 and 5 were collected from a Dutch cross-sectional study including 537 OAG/OHT patients and were assumed to be a good alternative [Citation38] due to the aforementioned lack of Italian data. For disease stages 1–4, the experts opinion of the 5 Italian ophthalmologists included in our convenience sample [Citation28], was assumed to be a good proxy for patients’ HRQoL, given the experts’ extensive experience with and in-depth knowledge of glaucoma.
The extensive use of experts opinion that supported this research poses a wider methodological issue on the appropriateness of this source of data elicitation for early decision models.
While early assessment of healthcare programs should pave the way to later rigorous empirical trials [Citation17,Citation18,Citation58], experts opinion is justified due to the temporary lack of evidence, provided that a comprehensive set of sensitivity analyses is performed to check their robustness [Citation17,Citation58–60] and VOIA is carried to prioritize further data collection [Citation18].
In an early decision tree-supported CEA aimed at comparing an innovative vascular closure device vs. manual compression in managing bleeding complications following revascularization procedures, due to the lack of published data a German research group elicited from experts the probabilities of treating retroperitoneal hematomas and performing vascular surgery [Citation60].
In a UK study aimed at calculating the headroom of tissue engineering for bladder and urethra, utility values after cystoplasty were elicited from urologists as they cannot be retrieved from literature [Citation61].
However, while the use of experts opinion cannot replace empirical data, it can contribute to populating an early decision model aimed at predicting the future cost-effectiveness profile of the healthcare technology under investigation [Citation17,Citation30].
A second limitation is that country specific OSD-related disutility values were unavailable. Hence, a disutility value equal to severe DED was applied in the model to reflect the loss of HRQoL experienced by OAG/OHT patients suffering from concomitant OSD [Citation39].
A small number of inner and outer Monte Carlo iterations for EVPPI calculations [Citation23,Citation33] represents the third limitation of this research.
5. Conclusion
STN1013001 is potentially a highly cost-effective and strongly dominant treatment alternative vs. Latanoprost for OAG/OHT+OSD patients from an INHS perspective. These findings should be re-assessed in the light of the data from the currently ongoing Phase III trial comparing the efficacy and safety of STN1013001 vs. Latanoprost (NCT04133311) [Citation16], and confirmed by future health economic evaluations carried out alongside head-to-head comparisons of STN1013001 vs. Latanoprost in real-world clinical practice upon the availability of STN1013001 on the Italian market.
Declaration of interest
C Lazzaro has received an unconditional research grant from Santen GmbH, München,
Germany. Outside this research, in the past three years C Lazzaro has received research grants, speaker or consultancy fees from AstraZeneca S.p.A, Boehringer Ingelheim Italia S.p.A., CSL Behring S.p.A., Ferring S.p.A., Ipsen S.p.A., Roche S.p.A., Sanofi s.r.l., Santen GmbH, Shire S.p.A, Sobi S.p.A. C van Steen is employee of Santen GmbH, München, Germany. G Ghirelli received a research fee for data provision and manuscript reviewing from Santen GmbH, München, Germany. M Sacchi received a research fee for data provision and manuscript reviewing from Santen
GmbH, München, Germany. D Sisto received a research fee for data provision and manuscript reviewing from Santen GmbH, München, Germany. M Uva received a research fee for data provision and manuscript reviewing from Santen GmbH, München, Germany. L Varano received a research fee for data provision and manuscript reviewing from Santen GmbH, München, Germany. L Angelillo is employee of Santen GmbH, München, Germany. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
C Lazzaro: Conceptualization, Data curation, Formal analysis, Manuscript preparation, Manuscript review. C van Steen: Conceptualization, Data curation, Formal analysis, Manuscript preparation, Manuscript review. G Ghirelli: Data curation, Expert suggestions, Manuscript review. M Sacchi: Data curation, Expert suggestions, Manuscript review. D Sisto: Data curation, Expert suggestions, Manuscript review. M Uva: Data curation, Expert suggestions, Manuscript review. L Varano: Data curation, Expert suggestions, Manuscript review. L Angelillo: Conceptualization, Data curation, Manuscript review. All authors read and approved the final version of the manuscript for publication.
Supplemental Material
Download MS Word (75.3 KB)Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14737167.2023.2161515
Additional information
Funding
References
- Allison K, Patel D, Alabi O. Epidemiology of glaucoma: the past, present, and predictions for the future. Cureus. 2020;12(11):e11686.
- Floriani I, Quaranta L, Rulli E, et al. Italian Study Group on QoL in glaucoma. Health-related quality of life in patients with primary open-angle glaucoma. An Italian multicentre observational study. Acta Ophthalmol. 2016;94(5):e278–e286.
- Traverso CE, Walt JG, Kelly SP, et al. Direct costs of glaucoma and severity of the disease: a multinational long term study of resource utilisation in Europe. Br J Ophthalmol. 2005;89(10):1245–1249.
- Mills RP, Budenz DL, Lee PP, et al. Categorizing the stage of glaucoma from pre-diagnosis to end-stage disease. Am J Ophthalmol. 2006;141(1):24–30.
- Koleva D, Motterlini N, Schiavone M, et al. Study Group GLAUCO. Medical costs of glaucoma and ocular hypertension in Italian referral centres: a prospective study. Ophthalmologica. 2007;221(5):340–347.
- European Glaucoma Society. Terminology and guidelines for glaucoma. 5th ed. Savona (Italy): PubliComm; 2020.
- Pisella PJ, Pouliquen P, Baudouin C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol. 2002;86(4):418–423.
- Mylla Boso AL, Gasperi E, Fernandes L, et al. Impact of ocular surface disease treatment in patients with glaucoma. Clin Ophthalmol. 2020;14:103–111.
- Quaranta L, Riva I, Gerardi C, et al. Quality of life in glaucoma: a review of the literature. Adv Ther. 2016;33(6):959–981.
- Kim CY, Park KH, Ahn J, et al. Treatment patterns and medication adherence of patients with glaucoma in South Korea. Br J Ophthalmol. 2017;101(6):801–807.
- Newman-Casey PA, Niziol LM, Gillespie BW, et al. The association between medication adherence and visual field progression in the collaborative initial glaucoma treatment study. Ophthalmology. 2020;127(4):477–483.
- Newman-Casey PA, Niziol LM, Lee PP, et al. The impact of the support, educate, empower personalized glaucoma coaching pilot study on glaucoma medication adherence. Ophthalmol Glaucoma. 2020;3(4):228–237.
- Daull P, Amrane M, Garrigue JS. Novasorb® cationic nanoemulsion and latanoprost: the ideal combination for glaucoma management? [Internet]. J Eye Dis Disord. 2017;2(1):1; [cited 2021 Aug 27]. Available from: https://www.walshmedicalmedia.com/open-access/novasorb-cationicnanoemulsion-and-latanoprost-the-ideal-combination-forglaucomamanagement.pdf14
- Lallemand F, Daull P, Benita S, et al. Successfully improving ocular drug delivery using the cationic nanoemulsion, novasorb. J Drug Deliv. 2012;2012:604204.
- Ismail D, Amrane M, Garrigue JS, et al. A phase 2, randomized study evaluating the safety and efficacy of STN1013001® (unpreserved latanoprost 0.005% emulsion) compared to Travatan Z® in subjects with glaucoma and ocular surface disease. Poster session presented at: Glaucoma. 14th annual meeting of the European Association for Vision and Eye Research; 2011 Oct 5–8; Crete Greece. Available from: https://www.academia.edu/21377543/A_phase_2_randomized_study_evaluating_the_safety_and_efficacy_of_Catioprost_unpreserved_latanoprost_0_005_emulsion_compared_to_Travatan_Z_in_subjects_with_glaucoma_and_ocular_surface_disease
- ClinicalTrials.gov [Internet]. National Library of Medicine (NLM). A phase III multinational multicenter investigator-masked randomised active-controlled trial comparing the efficacy and safety of DE-130A with Xalatan® in patients with open-angle glaucoma or ocular hypertension. NLM identifier: NCT04133311. Bethesda (MD): U.S. NLM; [updated 2019 Oct 21; cited 2021 Aug 27]. Available from: https://clinicaltrials.gov/ct2/show/NCT04133311
- Annemans L, Genesté B, Jolain B. Early modelling for assessing health and economic outcomes of drug therapy. Value Health. 2000;3(6):427–434.
- IJzerman MJ, Koffijberg H, Fenwick E, et al. Emerging use of early health technology assessment in medical product development: a scoping review of the literature. Pharmacoeconomics. 2017;35(7):727–740.
- Drummond MF, Sculpher MJ, Claxton K, et al. Methods for the economic evaluation of health care programmes. 4th. Oxford: Oxford University Press; 2015.
- Neumann PJ, Ganiats TG, Russell LB, et al. editors Cost-effectiveness in health and medicine. 2nd. New York (NY): Oxford University Press; 2016
- Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13(4):322–338.
- Briggs AH. Handling uncertainty in economic evaluation and presenting the results. In: Drummond M, McGuire A, editors. Economic evaluation in health care: merging theory with practice. Oxford: Oxford University Press; 2001. p. 172–214.
- Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006.
- Lazzaro C, van Steen C, Billeit S, et al. Cost–utility analysis of a latanoprost cationic emulsion (STN1013001) versus other latanoprost in the treatment of open-angle glaucoma or ocular hypertension and concomitant ocular surface disease in Germany. Clin Ophthalmol. 2022;16:323–337.
- Lazzaro C, van Steen C, Aptel F, et al. Cost-utility analysis of STN1013001, a latanoprost cationic emulsion, versus other latanoprost formulations (Latanoprost) in open-angle glaucoma or ocular hypertension and ocular surface disease in France. J Ophthalmol. 2022;2022:3837471.
- Sistema Statistico Nazionale - Istituto Nazionale di Statistica [Internet]. Demo-geodemo. Mappe, popolazione, statistiche demografiche dell’ISTAT. Tavole di mortalità della popolazione residente. Ripartizione: Italia - Femmine - Anno: 2018. Rome: Sistema Statistico Nazionale - Istituto Nazionale di Statistica; [updated 2018 May 30; cited 2020 Aug 9]. Available from: http://demo.istat.it.Italian
- Sistema Statistico Nazionale - Istituto Nazionale di Statistica [Internet]. Demo-Geodemo. Mappe, Popolazione, Statistiche Demografiche dell’ISTAT. Tavole di mortalità della popolazione residente. Ripartizione: Italia - Maschi - Anno: 2018. Rome: Sistema Statistico Nazionale - Istituto Nazionale di Statistica; [updated 2018 May 30; cited 2020 Aug 9].Available from: http://demo.istat.it
- Lohr SL. Sampling: design and analysis. 2nd ed. Boston (MA): Brooks/Cole; 2010.
- Wilkins GA [Internet]. The IAU Style Manual (1989). The preparation of astronomical papers and reports. Transactions of the International Astronomical Union. 1990; Series B: S23; cited 2022 Aug 22. Available from: https://www.iau.org/static/publications/stylemanual1989.pdf
- de Windt TS, Sorel JC, Vonk LA, et al. Early health economic modelling of single-stage cartilage repair. Guiding implementation of technologies in regenerative medicine. J Tissue Eng Regen Med. 2017;11(10):2950–2959.
- Fattore G per Gruppo di lavoro Associazione Italiana di Economia Sanitaria (AIES). Proposta di linee guida per la valutazione economica degli interventi sanitari in Italia. [A proposal of guidelines for the economic evaluation of health interventions in Italy]. Pharmacoeconomics-Ital Res Articles. 2009;11(2):83–93. Italian.
- Agenzia Italiana del Farmaco [Internet]. Linea guida per la compilazione del dossier a supporto della domanda di rimborsabilità e prezzo di un medicinale ai sensi del D.M. 2 agosto 2019. Versione 1.0. Rome: Agenzia del Farmaco, Settembre 2020; [updated 2020 Dec 30; cited 2022 Aug 22]. Available from: https://www.aifa.gov.it/en/-/l-aifa-approva-le-nuove-linee-guida-per-lacontrattazione-dei-prezzi-e-rimborsi-dei-farmaci. Italian
- Claxton K. Exploring uncertainty in cost-effectiveness analysis. Pharmacoeconomics. 2008;26(9):781–798.
- Keeney S, Hasson F, McKenna H. The Delphi technique in nursing and health research. Chichester: Wiley-Blackwell; 2011.
- O’Hagan A, Buck CE, Daneshkhah A, et al. Uncertain judgements: eliciting experts’ probabilities. Chichester: Wiley; 2006.
- Ministero della Salute. Decreto 8 febbraio 2013. Criteri per la composizione e il funzionamento dei comitati etici. (13A03474). Gazzetta Ufficiale della Repubblica Italiana, Serie Generale, n. 96 del 24 aprile 2013; 12–21. Italian.
- Fea AM, Cattel F, Gandolfi S, et al. Cost-utility analysis of trabecular micro-bypass stents (TBS) in patients with mild-to-moderate open-angle glaucoma in Italy. BMC Health Serv Res. 2021;21(1):824.
- van Gestel A, Webers CA, Beckers HJ, et al. The relationship between visual field loss in glaucoma and health-related quality-of-life. Eye (Lond);2010. 24(12): 1759–1769.
- Canadian Agency for Drugs and Technology in Health (CADTH) [Internet]. Pharmacoeconomic review report. Cyclosporine (VERKAZIA). Ottawa: Ottawa: CADTH; 2020; cited 2022 Feb 20]. Available from 2022 Feb 20: https://cadth.ca/sites/default/files/cdr/pharmacoeconomic/sr0615-verkaziapharmacoeconomic-review-report.pdf
- Agenzia Italiana del Farmaco [Internet]. Iopize. Riassunto delle caratteristiche del prodotto. Rome: Agenzia Italiana del Farmaco; [updated 2018 Apr 6; cited 2020 Nov 30]. Available from: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_001300_039972_RCP.pdf&retry=0&sys=m0b1l3.Italian
- Agenzia Italiana del Farmaco. Liste di Trasparenza [Internet]. Rome: agenzia Italiana del Farmaco; [updated 2020 Nov 16; cited 2020 Nov 30]. Available from: https://www.aifa.gov.it/en/storico-liste-di-trasparenza.Italian
- IQVIA. MIDAS Database [Internet]. Danbury (CT): IQVIA (US); [updated 2020 Nov 30; cited 2020 Nov 30]. Available from: https://www.iqvia.com/solutions/commercialization/brand-strategy-and-management/market-measurement/midas
- Ministero della Salute [Internet]. Decreto 18 ottobre 2012. Remunerazione prestazioni di assistenza ospedaliera per acuti, assistenza ospedaliera di riabilitazione e di lungodegenza post acuzie e di assistenza specialistica ambulatoriale. (13A00528). Gazzetta Ufficiale della Repubblica Italiana. Serie Generale, n. 23 del 28 gennaio 2013b. Allegato 3 Prestazioni di assistenza specialistica ambulatoriale; [cited 2020 Nov 30] Available from: https://www.trovanorme.salute.gov.it/norme/renderPdf.spring?seriegu=SG&datagu=28/01/2013&redaz=13A00528&artp=3&art=1&subart=1&subart1=10&vers=1&prog=001.Italian
- Regione del Veneto [Internet]. Decreto n. 47 del 22 Maggio 2013. Aggiornamento, ai sensi della DGR n. 442 del 10 aprile 2013, degli Allegati A e B del Nomenclatore Tariffario Regionale dell’assistenza specialistica ambulatoriale di cui alla DGR n. 859/2011 e successive modifiche e integrazioni. Venice: Regione del Veneto, 2013 May 22; cited 2020 Nov 30] Available from 2020 Nov 30: https://www.aulss3.veneto.it/index.cfm?action=mys.apridoc&iddoc=12647. Italian.
- Agenzia Italiana del Farmaco. Xalatan. Riassunto delle caratteristiche del prodotto [Internet]. Rome: Agenzia Italiana del Farmaco (ITALY); [updated 2018 May 30; cited 2020 Nov 30]. Available from: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_000040_033219_RCP.pdf&retry=0&sys=m0b1l3
- Brouwer W, Rutten F, Koopmanschap M. Costing in economic evaluations. In: Drummond M, McGuire A, editors. Economic evaluation in health care: merging theory with practice. Oxford: Oxford University Press; 2001. p. 68–93.
- Pagano M, Gauvreau K. Principles of biostatistics. 2nd ed. Boston (MA): Brooks/Cole; 2000.
- Black WC. The CE plane: a graphic representation of cost-effectiveness. Med Decis Making. 1990;10(3):212–214.
- Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making. 1998;18(2 Suppl):S68–80.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ. 2001;10(8):779–787.
- Glick HA, Doshi JA, Sonnad SA, et al. Economic evaluation in clinical trials. 2nd. Oxford: Oxford University Press; 2014.
- Claxton K. The irrelevance of inference: a decision-making approach to the stochastic evaluation of health care technologies. J Health Econ. 1999;18(3):341–364.
- Eckermann S, Willan AR. Expected value of information and decision making in HTA. Health Econ. 2007;16(2):195–209.
- Holmstrom S, Buchholz P, Walt J, et al. The cost-effectiveness of bimatoprost, latanoprost and timolol in treatment of primary open angle glaucoma in five European countries. Curr Med Res Opin. 2006;22(5):897–905.
- Hommer A, Wickstrøm J, Friis MM, et al. A cost-effectiveness analysis of fixed-combination therapies in patients with open-angle glaucoma: a European perspective. Curr Med Res Opin. 2008;24(4):1057–1063.
- Hanover Consensus Group, von der Schulenburg Jm G, Greiner W, Jost F, et al. German recommendations on health economic evaluation: third and updated version of the Hanover consensus. Value Health. 2008;11(4):539–544.
- Haute Autorité de Santé (HAS) [Internet]. Choices in methods for economic evaluation. 20202; [updated 2020 Jul 2; cited 2021 Oct 10]. Available from: https://www.has-sante.fr/upload/docs/application/pdf/2020-11/methodological_guidance_2020_- choices_in_methods_for_economic_evaluation.pdf
- Grabowski H. The effect of pharmacoeconomics on company research and development decisions. Pharmacoeconomics. 1997;11(5):389–397.
- Grutters JPC, Govers T, Nijboer J, et al. Problems and promises of health technologies: the role of early health economic modeling. Int J Health Policy Manag. 2019;8(10):575–582.
- Brandes A, Sinner MF, Kääb S, et al. Early decision-analytic modeling - a case study on vascular closure devices. BMC Health Serv Res. 2015;15(1):486.
- McAteer H, Cosh E, Freeman G, et al. Cost-effectiveness analysis at the development phase of a potential health technology: examples based on tissue engineering of bladder and urethra. J Tissue Eng Regen Med. 2007;1(5):343–349.