ABSTRACT
Objective
The aim of this study is to assess the budget impact of daratumumab for light-chain amyloidosis in Cyprus
Methods
A budget impact model assessed the cost prior and after the introduction of daratumumab for light-chain amyloidosis. All related costs were set from the perspective of Cyprus NHS. Clinical data were extracted from the published trials. One-way sensitivity analysis was conducted. We reported incremental budget impact, per member per month, per year, and per treated member per month
Results
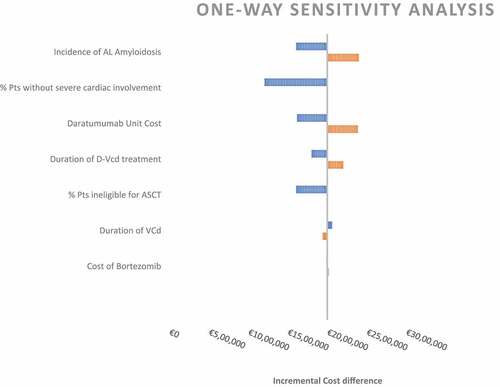
The introduction of D-VCd led to a net budget impact of €254,264 in the first year, which escalated to €497,007 by fifth year. The PMPY was estimated at €0.2893 in the first year, reaching €0.5246 at fifth year, the PMPM were at €0.0241 at the first year escalating to €0.0437 at the fifth year, and the PTMPM costs were €2,379 at the first year and gauged to €4,435 by fifth year. Our results were sensitive to incidence of the disease, percentage of patients without cardiac involvement and daratumumab cost.
Conclusions
The introduction of daratumumab for AL amyloidosis, with a 90% annual uptake over 5 years, leads to a substantial budget impact. Managed entry agreement schemes can be considered in order to mitigate the impact.
1. Introduction
Light-chain Amyloidosis (AL) is a condition defined by the extra-cellular deposition of fibril-forming monoclonal Ig light chains (LC) on several organs and tissues [Citation1]. As these deposits accumulate, they compromise the tissue structure, which leads to organ dysfunction, with the heart being affected the most. The early symptoms of this disease are usually asthenia and dyspnea, an element that complicates its differentiation from other diseases, and further thwarts disease management. The most common symptoms include malabsorption, nephrotic syndrome, and heart failure [Citation2]. Patients are usually diagnosed at the age of 65, while men appear to have a higher risk for disease development.
AL amyloidosis is concurrently the most common and severe form of amyloidosis. Its incidence is low and approximately 11 people per million population in Europe and the United States (US), are affected by the disease.
The focal point of an optimum treatment for AL amyloidosis lies on the suppression of the production of amyloidogenic Ig light-chain formation, while the preservation of organ function comprises a primary target of the treatment plan [Citation3,Citation4].
So far, only a small number of pharmaceutical agents demonstrated efficacy against AL. The combination of bortezomib, cyclophosphamide, and dexamethasone (VCd) emerged as the gold standard in AL treatment, based on a string of clinical trials, which reported a positive efficacy profile [Citation4–8] on the primary endpoint of a swift hematologic complete response (CR) or a very good partial response (VGPR). The extent of hematologic response is assessed by measuring serumfree light-chain (FLC) levels and/or serum and urine immunofixation. In general, the reduction of monoclonal light chains is associated with improved organ function, quality of life, and overall survival [Citation9].
The selection of the treatment modality is contingent to several factors such as patient’s age, comorbidities, extent of organ involvement, and patient preference as well. Prior to the introduction of daratumumab (DARZALEX®), bortezomib, cyclophosphamide, and dexamethasone – agents approved for multiple myeloma (MM) – including corticosteroids, proteasome inhibitors, and immunomodulatory drugs, were used off-label in the treatment of this disease. High-dose melphalan followed by autologous stem cell transplant (ASCT) has also been used to treat patients with AL amyloidosis [Citation4–6]. Nevertheless, the ASCT was embroiled in controversy. Despite the reduction of mortality in the long-term, the absolute mortality rates remained substantial, which mandated the introduction of strict criteria regarding the eligibility for ASCT. Moreover, the comorbidity of patients presenting with AL, partly imputed to a late stage and later life diagnosis, comprises a contraindication for ASCT, which may pertain up to 80% of AL patients. Therefore, the focus is to offer potent pharmacological therapies [Citation9,Citation10].
Daratumumab is a CD38-targeted human IgG1ĸ monoclonal antibody, which was previously approved for multiple myeloma and its effectiveness in AL was posited through the phase 3 Andromeda clinical trial [Citation11].
ANDROMEDA, a randomized controlled trial (RCT), compared the efficacy and safety of the combination of daratumumab, bortezomib, cyclophosphamide, and dexamethasone (D-VCd) compared with VCd alone in newly diagnosed patients with AL amyloidosis. After a follow-up period of 11.4 months, D-VCd was related to statistically significantly higher hematologic complete response rates and organ (cardiac and renal) response rates compared to patients allocated to VCd. Moreover, D-VCd significantly delayed the start of second-line therapy and major organ deterioration progression-free survival (MOD-PFS; a composite endpoint of major organ deterioration, hematologic progression, or death, whichever occurred first) compared to treatment with VCd. The safety profile of D-VCd was consistent with the known safety profiles for independently administered daratumumab and VCd, with low rates of both systemic administration-related reactions and local injection site reactions reported.
As with all new medicines, their soaring costs is a source of concern for payers worldwide. The economic evaluations of Daratumumab in multiple sclerosis demonstrated a noncost-effective product. Apart from the efficiency, the ability to fund, even cost-effective, treatments with steep prices, emerges as a key factor in Cyprus, global budget regulated Health Care system. Therefore, the scope of this publication is to assess the budget impact of daratumumab in eligible Cypriot patients presenting with AL amyloidosis.
2. Methodology
2.1. Model
We developed a budget impact model (BIM), which abided by the principles of ISPOR, to simulate the flow of AL amyloidosis patients [] [Citation12]. In line with current practices in Cyprus, we set VCd as the comparator agent . Efficacy values informing the model were derived from the ANDROMEDA clinical trial. In our scenario, the daratumumab is added to the current mainstay treatment in Cyprus, that is the triple combination of bortezomib, cyclophosphamide, and dexamethasone. Consequently, we compared the current scenario (treatment with VCd) with the future scenario (assuming the introduction of daratumumab). We estimated the incremental budget impact as the difference between the costs of the regimen D-VCd and the cost of the current therapeutic benchmark regimen, the VCd. Moreover, we reported the costs per member per month (PMPM), per member per year (PMPY) and per treated member per month (PTMPM).
Figure 1. Model Structure. Abbreviations: AE, Adverse events; VCD, Bortezomib, cyclophosphamide and dexamethasone; D-VCD, Daratumumab, bortezomib, cyclophosphamide and dexamethasone
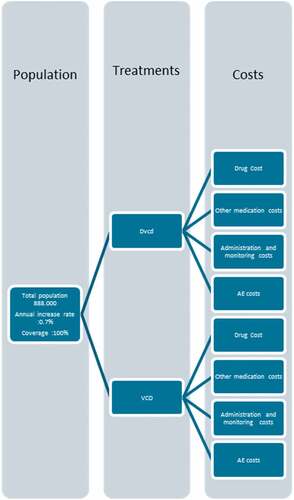
We assumed an initial modeled population of 888,000 people, with an annual population growth of 0.7% annually [] [Citation13]. We adopted a public health-care payer perspective, which provides a 100% coverage for AL. The results were expressed per incremental budget, member per year (PMPY), per member per month (PMPM), and per treated member per month (PTMPM) costs.
Table 1. Population.
2.2. Sensitivity analysis
In order to assess the robustness of our assumption, we performed a one-way sensitivity analysis, with the main variables. The values of each variable were varied by a 20% margin, in order to assess the robustness of the results.
2.3. Costs
We assumed that during the first 1–6 cycles all patients should perform (per cycle)
One physician visit. Currently, HIO reimburses seven outpatient visit types.
10 minutes visit: 125 weight (April value: 10.63 euro)
15 minutes visit: 2 weight (April value 17 euro)
20 minutes visit: 2.1 weight (April value 17.85 euro)
30 minutes visit: 3.05 weight (April Value 25.93 euro)
40 minutes visit: 4.1 weight (April value 34.84 euro)
45 minutes visit: 4.65 weight (April Value 39.53 euro)
60 minutes visit: 5.85 weight (April Value 49.73 euro)
Serum chemistry assessment 46.64 euro
Full hematology assessment, 6.33 euro
Troponin 12.75 euro
Urine and serum disease evaluation 14.50 + 3.18 euro
NT-proBNP 39.91 euro
Serum FLC 15.98 euro
The serum chemistry is performed every 2 weeks. Pregnancy test was calculated for females less than 65 years old.
From Cycle 7 and onwards, the model considers on a bimonthly basis the following: physician visits, troponin test, serum, and urine evaluation, along with serum FLC assessment and NT -proBNP assay. As per the protocol, HBV test were performed in patients with prior exposure to the virus, which was 13% and 14% for D-VCd and VCd, respectively.
Regarding the administration fee, a flat rate of 60 euro for IV therapy was applied. We included the costs for the adjuvant pharmaceutical care and for treating grade ≥3 adverse events. The costs were calculated based on NHS CPT system.
As a second line treatment, patients on D-VCd will receive lenalidomide, while patients on VCd will receive either lenalidomide or D-VCd. ,
Table 2. Drug costs.
Table 3. Key Assumptions.
In addition, we included the end-stage organ failure, hemodialysis, heart, and kidney transplant costs. The cost of heart transplant was €77,112 based on the DRG base rate of April and renal transplantation is €29,000. We applied a once-off subsequent therapy. The Andromeda study did not provide data regarding median time to next treatment; therefore, we used a subgroup analysis of Asian patients. The HR for the endpoint median time to next treatment was 0.10 (0.01–0.79) P = 0.0069 [Citation14]. Therefore, we estimated that these costs will not be captured in Budget impact’s horizon.
Due to lack of local epidemiological data, we extrapolated relevant data from the medical literature. We concluded an incidence of 0.0010% [Citation2,Citation15]. The target population consists of newly diagnosed patients without severe cardiac involvement and are either ASCT-ineligible or have rejected ASCT in spite of being eligible. We assumed that 50% of Cyprus population has cardiac involvement, therefore we included patients without cardiac disease 50% as eligible in our BIA [Citation16,Citation17]. Moreover, we assumed that patients were not eligible for ASCT due to the high contraindication rate and also due to the low penetration of ASCT in Cyprus.
We presumed that in the first year, 50% of patients will receive the D-VCd (). The usual uptake of new agents is usually lower; however, a substantial number of patients is already on D-VCd, through the patient-basis request Committee.
In line with the recommendations of ISPOR, the horizon of the BIM was set at a 5-year duration and not discounting was used. We used the publicly available costs of NHS, both for drug and non-drug costs. We added drug costs, administration monitoring costs, adverse events (AE) management costs, as well as end-of-life costs. Cyprus implements a global budget, with a fluctuating base rate. The costs were calculated with the base rate of April, based on the CPT and DRG rates [Citation18,Citation19].
Table 4. Adverse events costs.
2.4. Treatment dosage and duration of treatment
The dosage was assumed as per the ANDROMEDA clinical trial protocol. The ANDROMEDA trial reported 9.6 months mean duration for the D-VCd and 4.361 months for the VCd arm, respectively. These numbers lead to 4.7 cycles of VCd treatment and 6.00 cycles of D-VCd. Patients on D-VCd will then receive 6,18 more treatment cycles. The cost of D-VCd in year 1 was assessed on 13 cycles, while we pressumed 17 cycles of treatment for VCd, due to its 21-day cycle length.
The median-dose intensity was 84,3% for cyclophosphamide, 77,2% for bortezomib, 72,4% for dexamethasone, and 100% for daratumumab.
For products whose dosage is patient body surface area (BSA) dependent, we considered a BSA of 1.84 m2, while for patient body weight, a weight of 73.4 kilograms (kg) was used. No vial sharing was permitted in the study, therefore we applied a one-time use perspective.
3. Results
The introduction of D-VCd to Cyprus formulary perpetuates to an incremental budget impact €254,264 in the first year, which escalated to €497,007 in the 5th year, compared to the cost of VCd. More specifically, the scenario with VCd only, assumes a budget impact of € 80,554 in the 1st year, € 81,118 in the 2nd year € 81,685 in the 3rd, year € 82,257 in the 4th year and € 82,833 in the 5th year. Correspondingly, the introduction of daratumumab was interlaced with a € 256,924 budget impact in the 1st year, € 362,913 in the 2nd year, € 418,546 in the 3rd year, € 475,075 in the 4th year and € 479,028 in the 5th year. The cost-driver was the cost of daratumumab, which was partially offset by less monitoring costs. THE PMPY were estimated at €0.2893 in the first year, reaching €0.5246 at 5th year. Conversely, the PMPMY was calculated at €0.0241 in the 1st year, escalating to €0.0437 at the 5th year. Finally, the PTMPM costs were €2,379 in the first year and by the 5th year they were approximated at €4,435 [].
Table 5. RESULTS.
Table 6. BUDGET IMPACT ANALYSIS
4. Discussion
Our study explored the economic consequences of adopting a new technology for AL amyloidosis, and it deduced that the budget impact is substantial. However, we should annotate that we are referring to orphan diseases, whose microenvironment is-understandably- financially unattractive. From this perspective, an equilibrium must be reached between affordability and providing access to essential medicines. The cost-effectiveness analysis (CEA) elucidates only one side of the coin: Policy makers are under pressure for the short-term fiscal robustness of the health system as well.
In this sense, the budget impact presents a more rational and less romantic approach, which diverges from the holistic and social equity-based perspective of the CEA.
Our data, both the incremental budget but also the PMPM indicate that the introduction of Daratumumab for this indication corresponds approximately to 0.5% of the inpatient pharmaceutical budget. We should also comment that the listed prices are the wholesale ones; confidential discounts apply.
In this backdrop, a price reduction of Daratumumab is essential, as it will make the product more affordable, especially with the upcoming introduction of Venetoclax and carfilzomib. This can be achieved in the context of a Managed entry agreement scheme, preferably in the form of performance-based managements, with regards to CR. Conceptual designs in managed entry agreement have been recently developed like the six delta platform for outcome-based contracting for pharmaceuticals. Such designs may enhance patient access to daratumumab in Cyprus public health jurisdictions [Citation20].
Cyprus does not apply differential pricing. Therefore, the price of daratumumab will also apply to the MM. This finding is further compounded by a string of non-cost-effective results of daratumumab economic evaluations in Multiple Myeloma.
In the near future, more therapeutic options are anticipated. Venetoclax, which demonstrated its efficacy in the treatment of CLL, comprises an emerging option for patients with AL, however data are still scarce [Citation21]. Relevant comorbidities include potential contraindications to bortezomib, such as peripheral neuropathy and pulmonary fibrosis. Oral MDex or immunomodulatory drug (ImiD)-based regimens can be also considered for disease management, especially in patients who cannot take bortezomib. In patients presenting with peripheral neuropathy, Carfilzomib is an alternative treatment modality, although its cardiac toxicity mandates close monitoring [Citation22].
5. Limitations of the study
One of the limitations of our study is the interpretation of the data. In contrast, with the CEA and its tools, which have been extensively studied, there is scarcity of data pertinent to the budget impact tools, such as PMPM and PMPY, especially with regards to thresholds. The assessment of BIA and its consequent interrelation with the affordability, extends beyond a merely technical health economic affair and spills over to public governance domain. As such, more data are needed to raise BIA to the same level as CEA. Moreover, the gap in the literature regarding positive CEA and non-optimum BIA must be spanned.
Moreover, a single RCT was used. As in any case, the transferability of data from a single study may construe a source for bias. However, given the orphan status of the disease, we do not foresee significant resources diverted in this therapeutic area. This concern is partially extenuated by the good methodological quality of the study. We also used the official prices, due to confidentiality of agreed prices. We also used data from a subgroup with the potential hazards that this approach entails.
6. Conclusion
The conducted BIA summarizes that daratumumab is associated with a substantial budget impact, in Cyprus health-care sector. To eliminate uncertainty, apart from cost reduction strategies, MEA should be put into perspective.
Declaration of interest
Dr Petrou is an employee of Cyprus Health Insurance Organisation (HIO). The views and opinions expressed in this publication are those of the author. They do not purport to reflect the opinions or views of the HIO. The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
P. Petrou conceived the scope of the paper, performed the litereature review, created the BIA model, analyzed the results, and drafted the final version of the manuscript. P Petrou read and approved the final manuscript to be published.
Additional information
Funding
References
- Desport E, Bridoux F, Sirac C, et al. AL Amyloidosis. Orphanet J Rare Dis. 2012;7(1):54.
- Zhang N, Cherepanov D, Romanus D, et al. Estimating the global epidemiology of amyloid light-chain amyloidosis with an incidence-to-prevalence model. (#SP-232) 17th international myeloma workshop. Boston. 2019 September 12;15:2019.
- Wechalekar AD, Gillmore JD, Bird J, et al. Guidelines on the management of AL amyloidosis. Br J Haematol. 2015;168(2):186–206.
- Palladini G, Milani P, Merlini G. Management of AL amyloidosis in 2020. Blood. 2020;136(23):2620–2627.
- Palladini G, Sachchithanantham S, Milani P, et al. A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood. 2015;126(5):612–615.
- Manwani R, Cohen O, Sharpley F, et al. A prospective observational study of 915 patients with systemic AL amyloidosis treated with upfront bortezomib. Blood. 2019;134(25):2271–2280.
- Kastritis E, Leleu X, Arnulf B, et al. Bortezomib, Melphalan, and Dexamethasone for light-chain amyloidosis. J Clin Oncol. 2020;38(28):3252–3260.
- Zhang KW, Stockerl-Goldstein KE, Lenihan DJ. Emerging therapeutics for the treatment of light chain and transthyretin amyloidosis. JACC Basic Transl Sci. 2019;4(3):438–448.
- Merlini G, Dispenzieri A, Sanchorawala V, et al. Systemic immunoglobulin light chain amyloidosis. Nat Rev Dis Primers. 2018;4(1):1–19.
- National Comprehensive Cancer Network (2020). NCCN clinical practice guidelines in oncology - systemic light chain amyloidosis. 1.2021, 1–26. Available online at: https://www.nccn.org/professionals/physician_gls/. Accessed 2020 Sept 23.
- Kastritis E, Palladini G, Minnema MC, et al. ANDROMEDA trial investigators. Daratumumab-based treatment for immunoglobulin light-chain amyloidosis. N Engl J Med. 2021 Jul 1;385(1):46–58. PMID: 34192431.
- Sullivan SD, Mauskopf JA, Augustovski F, et al. (2014) Budget impact analysis-principles of good practice: report of the ISPOR 2012 Budget Impact analysis good practice II task force. Value Health 17 1: 5–14.
- https://www.cystat.gov.cy/el/default?OpenDocument cited Jul 2022
- Suzuki K, Wechalekar AD, Kim K, et al. Subcutaneous daratumumab (DARA SC) + bortezomib, cyclophosphamide, and dexamethasone (VCd) in Asian patients with newly diagnosed light-chain (AL) amyloidosis: subgroup analysis from the phase 3 ANDROMEDA study. Poster presented at: Virtual 62nd American Society of Hematology (ASH) Annual Meeting & Exposition; Dec 5-8, 2020.
- Quock TP, Yan T, Chang E, et al. Epidemiology of AL amyloidosis: a real-world study using US claims data. Blood Adv. 2018;2(10):1046–1053.
- Dispenzieri A, Lacy MQ, Kyle RA, et al. Eligibility for hematopoietic stem-cell transplantation for primary systemic amyloidosis is a favorable prognostic factor for survival. J Clin Oncol. 2001;19:3350–3356. [PubMed] [Google Scholar].
- Falk RH. Diagnosis and management of the cardiac amyloidoses. Circulation. 2005;112(13):2047–2060.
- https://www.gesy.org.cy/sites/Sites?d=Desktop&locale=el_GR&lookuphost=/el-gr/&lookuppage=hioactivitycatinpatientjune2020 cited Jul 2022
- https://www.gesy.org.cy/sites/Sites?d=Desktop&locale=el_GR&lookuphost=/el-gr/&lookuppage=hioactivitycatos29-07-2019 cited Jul 2022
- Alkhatib NS, Abraham I. The six Delta platform for outcome-based contracting for pharmaceuticals. J Med Econ. 2020;23(11):1209–1214.
- Premkumar VJ, Lentzsch S, Pan S, et al. Venetoclax induces deep hematologic remissions in t(11;14) relapsed/refractory AL amyloidosis. Blood Cancer J. 2021;11:10.
- Cohen AD, Heather Landau EC. Scott, et al (2016) safety and efficacy of carfilzomib (CFZ) in previously-treated systemic light-chain (AL) amyloidosis. Blood. 2016;128:645.