ABSTRACT
Background
Urinary retention (UR) caused by non-neurogenic conditions is a frequent disorder often requiring the use of intermittent catheterization (IC). This study examines the burden of illness among subjects with an IC indication due to non-neurogenic UR.
Methods
Health-care utilization and costs were extracted from Danish registers (2002–2016) related to the first year after IC training and compared to matched controls.
Results
A total of 4,758 subjects with UR due to benign prostatic hyperplasia (BPH) and 3,618 subjects with UR due to other non-neurological conditions were identified. Total health-care utilization and costs per patient-year were significantly higher compared to matched controls (BPH: 12,406 EUR vs 4,363, p < 0.000; other non-neurogenic causes: 12,497 EUR vs 3,920, p < 0.000) and driven mainly by hospitalizations. Urinary tract infections (UTIs) were the most frequent bladder complications often requiring hospitalization. The inpatient costs per patient-year for UTIs were significantly higher for cases than controls (BPH: 479 EUR vs 31, p < 0.000; other non-neurogenic causes: 434 EUR vs 25, p < 0.000).
Conclusions
The burden of illness caused by non-neurogenic UR with need for IC was high and essentially driven by hospitalizations. Further research should clarify if additional treatment measures may reduce the burden of illness in subjects suffering from non-neurogenic UR using IC.
1. Introduction
Urinary retention (UR) is one of the most frequent urologic diagnoses [Citation1,Citation2]. When it occurs acutely, there is a sudden inability to urinate, despite having a full bladder, and immediate bladder decompression by catheterization is required [Citation3]. Chronic UR is a persistent inability to fully empty the bladder [Citation3] and may require intermittent catheterization (IC) [Citation4].
Subjects with non-neurogenic UR and the need for IC management constitute a very heterogenic group [Citation5]. Benign prostatic hyperplasia (BPH) with obstruction of the urethra is most common in men [Citation2], whereas detrusor dysfunction plays an important role among women [Citation6]. There is considerable overlap between UR caused by obstruction and detrusor dysfunction, which makes it difficult to establish the underlying cause of the incomplete bladder emptying [Citation5]. The risk of developing UR increases with age, it often develops slowly, it is initially asymptomatic, and most people do not consult a physician until severe urinary complications like acute retention, urinary tract infections (UTIs), or even kidney failure appear [Citation1,Citation5,Citation7]. Alongside this, discrepancies in diagnostic terminology have resulted in unclear prevalence and cost estimates [Citation8].
In cases with UR where there is an indication for IC treatment, the procedure regains control over the bladder emptying pattern [Citation9], and it increases the quality of life due to an increased level of independence and mobility [Citation10]. IC is considered the gold standard for managing chronic UR [Citation4,Citation11]. Even though IC performed several times a day still places the user at risk of urethral trauma and urinary tract infections (UTIs), the risk is reduced compared to a permanent indwelling catheter [Citation4,Citation11]. Hence, whether separately or combined, UR and IC may induce severe and costly UTIs, which frequently require hospitalization or emergency room (ER) visits as well as treatment with antibiotics [Citation12,Citation13]. Thus, in addition to the costs of an underlying condition, UTIs impose a significant economic burden on the health-care sector [Citation14,Citation15].
Non-neurological causes of UR other than BPH have been stated to be under-reported and under-researched, and the characterization of this group of subjects, the personal consequences, and the impact on the health-care sector and the society are inadequately described [Citation5,Citation16]. Furthermore, studies focusing on the economic burden of BPH are still scarce [Citation17–20], and transferability and comparability of these studies are limited because of variations in study design, characteristics of study population, treatment modalities, cost calculation methods, and health systems.
Thus, the objective of this study was to describe the total and complication-specific burdens of UR caused by BPH or other non-neurogenic causes among subjects, who have been trained to perform IC.
2. Methods
This study was a descriptive observational nationwide study based on retrospectively collected data from Danish national registers and using an incidence approach. The study focused on the resource utilization and the direct and indirect costs the first year after IC training among people with UR caused by BPH or other non-neurogenic causes.
2.1. Data sources
In Denmark, all social and health-care services are recorded in national registers on an individual level. All Danish residents are, at birth or on immigration, assigned a permanent and unique personal identification number, which allows unambiguous linkage of data from all Danish registers. In this study, data from the Danish National Patient Register [Citation21], the National Health Service Register [Citation22], the Danish Prescription Register [Citation23], the Civil Registration System (CRS) [Citation24], and Statistics Denmark [Citation25–27] were applied. Identifying community-based catheterized people is impossible since this information is not registered by any of the Danish registers. In contrast, receiving IC training in hospital is registered and allows to identify subjects based on this information. The code for IC training was introduced in 2001. Diagnosis of diseases and reasons for hospitalization, ER visits, and mortality were based on ICD-10 codes, IC training was based on SKS codes (procedure codes from the Health Care Classification System, which is a Danish health-care classification system) [Citation28] (Table S1), and information of medication was based on ATC codes (Table S2).
2.2. Study population
The base case population included all subjects, who had been trained in IC and with a diagnosis of BPH and/or UR (ICD-10 code N40.9 and/or R33) during 2000–2016 (). The study period was limited to begin in 2002, and subjects with neurogenic causes of UR, such as spinal cord injury (SCI), multiple sclerosis (MS), or spina bifida (SB), were excluded. To ensure one-year follow-up, subjects were excluded if they were diagnosed with BPH or UR in 2016. Subjects, who had not received IC training (SKS code BJAK02) were also excluded, and to justify that the need for IC training was due to BPH or UR, subjects were excluded if the date of IC training overlapped more than 1 year before or after the last BPH or UR contact. Even though the subjects with BPH were not necessarily diagnosed with UR, the education to perform IC indicated that they did suffer from UR. Subjects were also excluded if they did not appear in CRS, were below 18 years, if they had passed away during hospitalization at index, or if it was unattainable to match a case subject with control subjects. Case subjects were indexed when they received IC training. A pool of controls was created from the general population by excluding subjects, who were diagnosed with, respectively, BPH or UR at any time during 2000–2016. From the pool of controls, one case subject was matched in their index year to possible control subjects on age, gender, marital status, municipality, and educational level, and of these, four controls were randomly selected. The case index date was assigned to the four controls. Thus, we ended up with two case groups: one consisting of subjects with UR due to BPH and trained to perform IC, and one consisting of subjects with UR due to other non-neurogenic causes than BPH and trained to perform IC as well as two matched control groups representing the general population. All subjects were followed for 1 year. The follow-up period was limited to 1 year to minimize the risk of interference from adjacent conditions developing over time. Subjects were censored at death or at the indication of prostate resection in subjects in the BPH group (SKS code KKED).
2.3. Health-care utilization and economic costs
Health-care utilization and costs were calculated as predicted incidents or costs per patient-year in the one-year follow-up period after index based on estimates from regression models (Poisson model and GLM model). Aggregated incidents or costs were divided by the aggregated number of patient-years. Costs related to inpatient admission or outpatient services at the index were not included. Costs were calculated in Danish crowns (DKK) and presented in 2016 values applying the net price index. Based on the average exchange rate in 2016, the costs in DKK were converted to EUR (100 EUR = 745 DKK).
The overall health-care utilization included the number of hospital admissions, number of days in hospital, outpatient visits (including ER visits), 30-day hospital re-admission, and primary health-sector services (one primary health-sector service is not necessarily equal to a contact, as one contact can include more than one service). The overall direct health-care costs contained costs of the primary health sector, outpatient services, hospitalization, and prescription medication (excluding medication given in secondary care).
Utilization and costs in the primary health sector were divided into specialist areas such as general practitioner and on-call doctors. Costs of prescription medication were grouped into clusters including antibiotics with UTI indications, mood disorder medication, antibacterial medication for systemic use, medication for overactive bladder, urgency, and enlarged prostate. Furthermore, the health-care utilization and costs were detailed in relation to bladder complications such as UTI, urosepsis, kidney disease, stones in urinary tract or kidney, urethral stricture, urinary tract obstruction, or hematuria, cancer in the bladder or urethra, dehydration, and sepsis. UTI can be coded with several different ICD-10 codes. Consequently, to ensure that upper, lower, and unspecified UTIs were captured, a UTI cluster was constructed consisting of the respective ICD-10 codes as outlined in Table S1. The health-care utilization and costs of bladder complications included the number of hospital admissions, days in hospital, outpatient visits (including ER visits), as well as costs of inpatient admissions and outpatient services. Top-20 reasons for hospital admissions and ER visits as well as top-5 causes of death for case groups were established based on the frequency of ICD-10 codes.
2.4. Societal costs
The societal costs included the direct health-care costs, as well as indirect costs, such as earned income and income transfer payments including unemployment benefits, publicly funded sick pay, disability pension, early retirement pay, and age pension.
2.5. Statistical analysis
The SAS SURVEYSELECT procedure was applied to choose probability-based random control groups. The chi-squared test was used to explore if a variable, which was not part of the matching process, was statistically different between case and control groups. A Poisson model and a GLM model were used to predict incidents and costs per patient-year and to test whether differences between case and control groups were significant. The outcome for health-care utilization is a count variable, and a Poisson model was applied. In the model, case vs. control (case = 1) was included as independent variable, and the estimates from the model were used to predict utilization for case and control groups. The model is suitable for count data with observations with a value of 0. Furthermore, an offset is applied to weigh for exposure time. Regarding the cost estimates, a 2-step one-model generalized linear model (GLM) with gamma distribution and log link function was used, including case vs. control (case = 1), and the estimates from the model were used to predict costs for case and control groups. This model was used since we were estimating the cost, which is a continuous variable, and have 0ʹs in the response variable. To ensure that standard errors were valid, a generalized estimating equation (GEE) model was used instead of the GLM model. The estimates were the same in the two models, but the standard errors were robust in the GEE model. Utilizations and costs were reported as mean and 95% confidence interval (CI). To estimate the model, the costs per year for each subject, who was alive the whole year, were calculated and corrected for exposure time for those who died during the one-year. A p-value < 0.05 was considered statistically significant. Analyses were performed using the statistical package SAS 9.4 (SAS Institute, Cary, NC, USA).
2.6. Ethical considerations
Since this study applied retrospective anonymized register data, ethical approval from the Danish Ethics Committee was not required by Danish law.
3. Results
3.1. Demographic characteristics
shows that 4,758 IC users had BPH and 3,618 other non-neurogenic causes. No imbalances between case and matched control groups were present regarding matching variables as case and control subjects were matched on the individual level. The mean age of IC users with UR due to BPH was 70.4 ± 9.0 years (mean ± sd), while, for those with UR due to other non-neurogenic causes, the age was 64.7 ± 15.5 years of age, and one-third of these were women. In both case groups, around two-thirds to three-quarters were married or co-habitating, and the majority had primary or vocational levels as educational attainment. Significantly more subjects with UR because of non-neurogenic causes other than BPH died (6.3%) during the one-year follow-up period compared to their matched controls (2.8%). Among subjects with UR due to BPH, there was no significant difference in number of deaths between case subjects (3.0%) and their matched controls (3.3%).
Table 1. Demographic characteristics of case and matched control groups at index.
3.2. Health-care utilization and costs
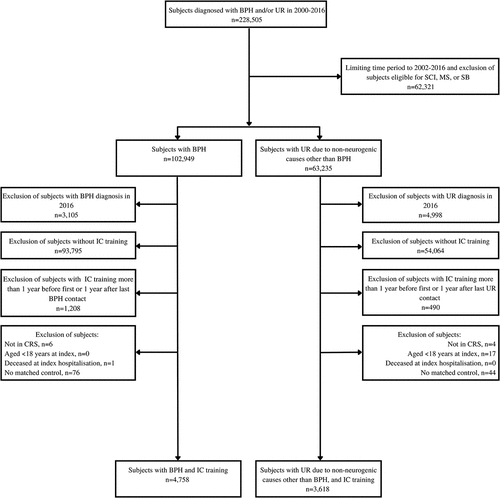
The total health-care utilization and costs per patient-year were significantly higher during follow-up compared to their matched controls ( and ). The total health-care costs amounted to approximately 12,500 EUR per patient-year for both case groups compared to around 4,000 EUR for the control groups. The total health-care costs were primarily driven by hospitalization and to a lesser extent by outpatient services. The costs per patient-year for inpatient admissions and outpatient services were increased 3–5 times for case groups compared to control groups and amounted approximately 6,500 EUR and 4,500 EUR, respectively. The increases for the primary health sector and prescription drugs were more modest, with a two-fold increase for case subjects compared to controls, each reaching roughly between 700 and 1,000 EUR per patient-year.
Figure 2. Predicted total mean healthcare costs in EUR per patient-year for case and matched control groups. All differences between case and matched control group were significant (p < 0.000). Please refer to Table S9 for confidence intervals.
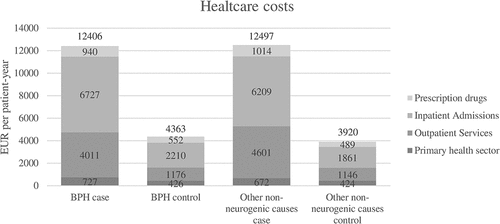
Table 2. Predicted total mean health-care utilization per patient-year for case and matched control groups.
Utilization and costs of general practitioner and on-call doctor were significantly higher for case groups compared to matched controls during the follow-up period as shown in . The highest consumption and costs were recorded in relation to the general practitioner (431 EUR per patient-year for the BPH case group and 381 EUR for the case group with other non-neurogenic causes), which was around a doubling compared to controls.
Table 3. Predicted mean utilization and costs in EUR per patient-year of selected primary health-sector services for case and matched control groups.
Prescription of antibiotics with UTI indication was significantly increased to around 60 EUR per patient-year for both case groups compared to 5 EUR for control groups, which is illustrated in . The costs of medication against overactive bladder, urgency, and enlarged prostate were increased to 136 EUR per patient-year for the BPH case group compared to 22 EUR for the matched control group. In contrast, for the group consisting of subjects with UR due to non-neurogenic causes other than BPH, prescription of mood disorder medication was markedly increased: 98 EUR per patient-year for the case group and 34 EUR for the matched controls.
Figure 3. Predicted mean costs in EUR per patient-year of selected prescription drugs for case and matched control groups. All differences between case and their matched control groups are significant (p < 0.000). Please refer to Table S10 for confidence intervals.
illustrate the health-care utilization and costs per patient-year due to various bladder complications. The UTI cluster had the highest impact on incidence of hospitalization and especially the length of these, and the associated costs of hospitalization resulted in 15–17 times increase for the case groups compared to controls and reaching more than 400 EUR per patient-year. Urosepsis and lower UTIs required relatively long hospital stays, reflected by 13–23 times increased inpatient costs for each complication for case groups compared to controls. Kidney disease did also result in relatively long hospital stays and resulting inpatient costs of 158 EUR per patient-year for both case groups compared to 38 for BPH control group and 23 for other non-neurological causes control group. The incidence of outpatient visits and the derived costs were primarily driven by kidney disease, which resulted in 321 and 248 EUR per patient-year for the BPH case group and the case group with other non-neurogenic causes, respectively.
Table 4. Predicted inpatient and outpatient mean utilization per patient-year of bladder complications for case and matched control groups.
Table 5. Predicted inpatient and outpatient mean costs in EUR per patient-year of bladder complications for case and matched control groups.
3.3. Societal costs
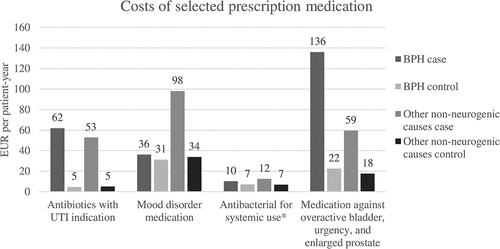
Income transfer payment was significantly higher for case groups compared to controls. For both case and control groups, income transfer payment was driven by disability pension and early retirement benefit as outlined in . Most subjects from the BPH case group and their matched controls were on early retirement, while most people from the group with other non-neurogenic causes and their matched controls received disability pension. The differences between case and control groups in earned income and age pension were relatively small (Table S3).
Figure 4. Predicted mean income transfer payments in EUR per patient-year for case and matched control groups. All differences between case and their matched control groups are significant (p < 0.000; however, for unemployment benefit p < 0.010). Please refer to Table S11 for confidence intervals.
3.4. Top reasons for hospitalization, ER visits, and mortality
The top-20 reasons for hospital admissions and ER visits are shown in Table S4-S7. For the case groups, complications related to the urinary tract are among the most common reasons for hospital admission and ER visits. In addition, complications from indwelling catheter use were also a frequent cause of ER visits. The top-5 causes of deaths are shown in Table S8, which highlights that the main causes of death for the case groups were prostate cancer and other cancers.
4. Discussion
For all we know, this study is the first to estimate the direct and indirect costs of UR due to BPH or other non-neurogenic causes among IC users. Using nationwide registers, we showed that UR caused by BPH or other non-neurogenic causes significantly impacted the subjects’ health as well as the societal economic burden. Outpatient services and especially hospitalization were the primary drivers of elevated health-care costs, and regarding complications, and UTI had the highest negative impact on the health-care costs.
A recent study has been published regarding healthcare use and societal costs of neurogenic bladder dysfunction using a similar methodology as in this study [Citation29]. In that study, approximately 4,000 subjects with UR due to neurogenic bladder were identified, while we identified more than 8,000 subjects with UR due to non-neurogenic causes in the present study. The societal costs per patient-year compared to the matched controls in the present study were substantially lower than in the study regarding neurogenic bladder [Citation29]. This is presumably explained by the fact that neurogenic bladder dysfunction and its underlying causes are typically complex and costly to handle. More so, the subjects in the present study were on average 10–15 years older, which means that their withdrawal from the labor market was mainly due to ordinary retirement rather than sickness. Likewise, their matched controls were correspondingly old and therefore also incurred increased health-care costs and age pension. The prevalence of UR due to neurogenic causes has been shown to be fairly constant over time, while it increases for non-neurogenic causes – especially among older subjects [Citation16]. This will amplify the societal costs of non-neurologic UR in an aging society.
UTIs are of fundamental concern among people with bladder dysfunction. Our observations showed that UTIs often required hospitalizations and use of antibiotics, which is a well-known fact [Citation14,Citation15,Citation30–32]. The number of days in hospital and the associated costs per patient-year due to UTIs in the present study were lower than the aforementioned study focusing on neurogenic bladder [Citation29]. This is in accordance with a novel study, which found that UTIs required longer hospital stays when the bladder dysfunction was of neurogenic origin because it is often more complex to manage than non-neurogenic causes [Citation33]. The present study furthermore confirmed that upper UTI (pyelonephritis) may cause urosepsis, which was responsible for long and costly hospital stays [Citation13,Citation34].
The daily number of catheterizations may vary highly among the subjects in the non-neurogenic population depending on the pathology. However, data regarding the subject’s daily bladder drainage pattern are not available, which could otherwise have nuanced the findings in this study. Likewise, data concerning potential discontinuation of catheterization or switch to other catheter types in the one-year follow-up period are not attainable in the Danish registers. As the top-20 reasons for ER visits are related to indwelling catheters, it demonstrates that alternating catheter procedures during the one-year follow-up have taken place.
The design of this study has important advantages. Results were based on nationwide registers, which ensured a real-world representative sample of subjects, who had received training of IC due to non-neurogenic UR. This decreased the risk of information and selection bias. Each case subject was matched with four controls to ensure a high degree of homogeneity in the control groups and to provide a more balanced comparison to the case groups. We acknowledge that the study also has limitations. The data quality of the registers is dependent on the correct diagnosis coding into the registers. However, the registers are considered valid and are used as background data for political and economic decisions in Danish health-care policies. Costs at the municipality level, such as education, rehabilitation, home care, nursing home, and medical devices, were not captured. Results regarding bladder complications were based exclusively on data from the secondary health-care sector, and since many uncomplicated cases of UTI are treated in primary care, the estimates may be conservative. Since only IC training is registered, we do not have information on whether the identified subjects continued to perform IC, if they switched to other kinds of catheters, or if they stopped using catheters. A societal perspective of the economic costs was included to illustrate the ‘true’ costs and estimates of productivity were based on a very conservative approach, which only included ‘earned income’ instead of applying the more comprehensive ‘Human Capital Approach.’ Non-medical costs like e.g. transportation to and from hospital were not included. Taken together, our cost estimates are conservative. We did not characterize the specific causes for UR. Non-neurogenic causes of UR are generally underactive bladder or chronic outlet obstruction other than BPH, like anterior/posterior urethral valve. Codes for such subjects are difficult to identify, and urethral valve problems are essentially managed in childhood. Subjects with neurogenic bladder due to SCI, MS, or SB were excluded from the case groups. The number of subejcts who perform IC due to UR as a cause of neurogenerative diseases, like e.g. Parkinson´s disease, is low and were not excluded from the case group. However, it seems unlikely that exclusion of these subjects would have changed the results substantially. The nature of the study was observational, and causality cannot be inferred.
5. Conclusion
Burden of illness in terms of health-care utilization and costs of UR due to BPH or other non-neurogenic conditions in need of IC were high and essentially driven by hospitalizations. UTIs were also prevalent in this population. Further research should clarify if additional treatment measures can reduce the burden of illness among subjects suffering from non-neurogenic UR using IC.
Declaration of interest
M Lynge Buchter is an employee of Coloplast A/S, Denmark. J Kjellberg is employed by VIVE, the Danish Center for Social Science Research, Denmark, an independent research and analysis centre. VIVE received funding from Coloplast for the contribution to this study. R Ibsen is an employee of i2Minds, an independent data analysis agency, which received funding from VIVE to this study. C Sternhufvud is an employee of Coloplast AB, Sweden. B Petersen is employed by MedDevHealth, Denmark, an independent consultancy, and received funding from Coloplast for the contribution to the study. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
M Lynge Buchter and C Sternhufvud were involved in study design, interpretation and discussion of results, appropriate presentation of results, and revising the manuscript. J Kjellberg and R Ibsen contributed to study design, data collection and the processing and statistical handling of the data to generate the results, verified the underlying data and validated results, as well as editorial support. B Petersen provided clinical input, was involved in study design, interpretation and discussion of results, presentation of results, and revising the manuscript. All authors read the final version of the manuscript critically and approved the final version to be published.
Compliance with ethics guidelines
Under Danish law, no ethics approval is required for register‐based studies.
Supplemental Material
Download MS Word (37.2 KB)Acknowledgments
Writing support was provided by Malene Bagger, MSc, PhD, employed by M Bagger Scientific Writing, which is an independent medical writing agency. The writing support was funded by Coloplast.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14737167.2023.2181793
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Serlin DC, Heidelbaugh JJ, Stoffel JT. Urinary retention in adults: evaluation and initial management. Am Fam Physician. 2018 Oct;98:496–503.
- Selius BA, Subedi R. Urinary retention in adults: diagnosis and initial management. Am Fam Physician. 2008 Mar;77:643–650.
- Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the international continence society. Urology. 2003 Jan;61:37–49.
- Lamin E, Newman DK. Clean intermittent catheterization revisited. Int Urol Nephrol. 2016 Jun;48:931–939.
- Stoffel JT. Non-neurogenic chronic urinary retention: what are we treating? Curr Urol Rep. 2017 Sep;18:74.
- Yang TH, Chuang FC, Kuo HC. Urodynamic characteristics of detrusor underactivity in women with voiding dysfunction. PloS one. 2018;13:e0198764.
- Speakman M, Kirby R, Doyle S, et al. Burden of male lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH) - focus on the UK. BJU Int. 2015 Apr;115:508–519.
- Stoffel JT, Peterson AC, Sandhu JS, et al. AUA white paper on nonneurogenic chronic urinary retention: consensus definition, treatment algorithm, and outcome end points. J Urol. 2017 Jul;198:153–160.
- Seth JH, Haslam C, Panicker JN. Ensuring patient adherence to clean intermittent self-catheterization. Patient Prefer Adherence. 2014 Feb;8:191–198.
- Kessler TM, Ryu G, Burkhard FC. Clean intermittent self-catheterization: a burden for the patient? Neurourol Urodyn. 2009;28:18–21.
- Lapides J, Diokno AC, Silber SJ, et al. Clean, intermittent self-catheterization in the treatment of urinary tract disease. J Urol. 1972 Mar;107:458–461.
- Kannan H, Radican L, Turpin RS, et al. Burden of illness associated with lower urinary tract symptoms including overactive bladder/urinary incontinence. Urology. 2009 Jul;74:34–38.
- Geerlings SE, Mulvey MA, Stapleton AE. Clinical presentations and epidemiology of urinary tract infections. Microbiol Spectr. 2016 Oct;4:1–11.
- Ciani O, Grassi D, Tarricone R. An economic perspective on urinary tract infection: the “costs of resignation.” Clin Drug Investig. 2013 Apr;33:255–261.
- François M, Hanslik T, Dervaux B, et al. The economic burden of urinary tract infections in women visiting general practices in France: a cross-sectional survey. BMC Health Serv Res. 2016 Aug;16:365.
- Berendsen SA, van Doorn T, Blok BFM. Trends in the use and costs of intermittent urinary catheters in the Netherlands from 1997 to 2018: a population-based observational study. Neurourol Urodyn. 2021 Mar;40:876–882.
- van Exel NJ, Koopmanschap MA, McDonnell J, et al. Medical consumption and costs during a one-year follow-up of patients with LUTS suggestive of BPH in six European countries: report of the TRIUMPH study. Eur Urol. 2006 Jan;49:92–102.
- Rencz F, Á K, Brodszky V, et al. Cost of illness of medically treated benign prostatic hyperplasia in Hungary. Int Urol Nephrol. 2015 Aug;47:1241–1249.
- Kovacs A. Cost of illness in benign prostatic hyperplasia: a review. Soc Economy. 2015;37:531–542.
- Ahn HS, Kim SJ, Choi JB, et al. Long-term cost comparison between surgical and medical therapy for benign prostatic hyperplasia: a study using hospital billing data. BJU Int. 2019 May;123:E79–85.
- Schmidt M, Schmidt SA, Sandegaard JL, et al. The Danish national patient registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015 Nov;7:449–490.
- Andersen JS, Olivarius Nde F, Krasnik A. The Danish national health service register. Scand J Public Health. 2011 Jul;39:34–37.
- Pottegård A, Schmidt SAJ, Wallach-Kildemoes H, et al. Data resource profile: the Danish national prescription registry. Int J Epidemiol. 2017 Jun;46:798–f.
- Schmidt M, Pedersen L, Sørensen HT. The Danish civil registration system as a tool in epidemiology. Eur J Epidemiol. 2014 Aug;29:541–549.
- Baadsgaard M, Quitzau J. Danish registers on personal income and transfer payments. Scand J Public Health. 2011 Jul;39:103–105.
- Jensen VM, Rasmussen AW. Danish education registers. Scand J Public Health. 2011 Jul;39:91–94.
- Petersson F, Baadsgaard M, Thygesen LC. Danish registers on personal labour market affiliation. Scand J Public Health. 2011 Jul;39:95–98.
- The Danish Health Data Authority. Sundhedsvæsenets Klassifikations System (SKS). [cited 2022 Feb]. Available from: https://medinfo.dk/sks/brows.php?s_nod=0
- Lynge Buchter M, Kjellberg J, Ibsen R, et al. Burden of illness the first year after diagnosed bladder dysfunction among people with spinal cord injury or multiple sclerosis - a Danish register study. Expert Rev Pharmacoecon Outcomes Res. 2022 Sep;22:919–926.
- Öztürk R, Murt A. Epidemiology of urological infections: a global burden. World J Urol. 2020 Nov;38:2669–2679.
- Foxman B. The epidemiology of urinary tract infection. Nat Rev Urol. 2010 Dec;7:653–660.
- Keating KN, Perfetto EM, Subedi P. Economic burden of uncomplicated urinary tract infections: direct, indirect and intangible costs. Expert Rev Pharmacoecon Outcomes Res. 2005 Aug;5:457–466.
- Welk B, Lenherr S, Santiago-Lastra Y, et al. Differences in the incidence of urinary tract infections between neurogenic and non-neurogenic bladder dysfunction individuals performing intermittent catheterization. Neurourol Urodyn. 2022 Apr;41:1002–1011.
- Bonkat G, Cai T, Veeratterapillay R, et al. Management of urosepsis in 2018. Eur Urol Focus. 2019 Jan;5:5–9.