ABSTRACT
Objective
To describe the economic burden among VWD patients with angiodysplasia compared to VWD patients without angiodysplasia and the general population.
Methods
This was a retrospective analysis using the Merative MarketScan Commercial and Medicare Databases® (January 2011-September 2020). Selected patients had ≥1 medical claim for VWD or low VWF, ≥1 medical claim for AGD, and ≥3 GI-related bleeding episodes within a year. HCRU and all-cause costs were compared with the VWD (only) and the general cohorts.
Results
The mean total all-cause costs were $150,101 among patients with VWD and angiodysplasia (n = 34), higher compared to $48,249 among matched VWD patients without angiodysplasia (n = 136) and $31,029 among matched individuals of the general population [n = 136; p-value < 0.0001]. The differences in costs between groups were primarily due to inpatient care. During the 12-month follow-up, VWD patients with symptomatic (n = 35), asymptomatic (n = 81), and suspected (n = 378) angiodysplasia had an average of 4.1, 0.6, and 3.8 gastrointestinal (GI) bleeds, respectively. Desmopressin, VWF concentrates, and aminocaproic acid were the most frequent treatments used. The most frequent procedures to treat GI-related bleeding and underlying lesions were blood transfusion and laser therapy.
Conclusions
Despite recent therapeutic advances, there is room for considerable reduction of the disease burden in patients with VWD and angiodysplasia.
1. Introduction
Von Willebrand disease (VWD) is the most common inherited bleeding disorder, characterized by dysfunction or deficiency of von Willebrand factor (VWF) [Citation1,Citation2], a physiological binding protein that plays a crucial role in platelet adhesion and acts as a carrier and stabilizer of factor VIII (FVIII). Lack of properly functioning VWF increases the risk of bleeding symptoms in mucous membranes (epistaxis, heavy menstrual bleeding, and gastrointestinal (GI) bleeding), while the secondary deficiency of FVIII in plasma may result in the occurrence of soft tissue, joint, and post-operative bleeding [Citation3,Citation4]. Further, VWD patients can develop vessel abnormalities, including angiodysplasia, a rare but highly burdensome complication characterized by abnormal, tortuous, dilated small blood vessels, present in the mucosal and submucosal layers of the GI tract [Citation5–7]. Patients with VWD and angiodysplasia experience recurrent hospital admissions due to recurrent bleeding episodes, polypharmacy, and significant morbidity [Citation5,Citation6]. About 2–5% of patients with angiodysplasia experience uncontrollable bleeding episodes which worsen with age [Citation5].
The diagnosis of angiodysplasia in VWD patients is challenging due to complex anatomic accessibility (especially when the lesions are in the small bowel) and the requirement that active bleeding be present at the time of the examination [Citation6,Citation8,Citation9]. The initial tools for diagnosis are endoscopy and colonoscopy [Citation10]. Further, video capsule endoscopy, deep bowel enteroscopy or radionuclide scanning images (radiologic diagnostic tool) are necessary to evaluate small bowel angiodysplasia [Citation6]. If the initial investigation does not reveal a bleeding source, then computed tomography (CT) angiography and magnetic resonance angiography are also useful investigative instruments [Citation6,Citation11]. Furthermore, intraoperative enteroscopy is conducted when both endoscopic and radiological procedures fail to detect the source of bleeding in an active bleeding patient [Citation12,Citation13].
The treatment and management of patients with VWD and angiodysplasia is burdensome due to persistent lesions causing periodic episodes of bleeding [Citation14]. Difficulty in identifying the angiodysplastic lesions hinders the ability to effectively manage GI bleeding. VWF replacement therapy has been successful at managing acute GI bleeding, however the relative lack of high molecular weight multimers (HMWM) in plasma derived VWF (pdVWF) concentrates may make them less effective in preventing recurrent GI Bleeding. Although there is no high-quality evidence to support recombinant VWF (rVWF) being a more effective prevention or treatment option for recurrent GI bleeding episodes, the high molecular weight characteristics have been hypothesized to have a beneficial impact [Citation5]. In instances where the lesions can be identified through endoscopy, embolization, or surgical resection can be used to manage the GI bleed. However, this is not as effective when there are multiple diffuse lesions throughout the GI tract. Thalidomide and bevacizumab, neo-angiogenesis inhibitors acting through the suppression of vascular endothelial growth factor, have been somewhat successful in treating angiodysplasia but have been associated with high incidence of adverse events. Octreotide and antifibrinolytic agents, such as aminocaproic acid and tranexamic acid, have utility when given alone or in conjunction with replacement therapies [Citation6,Citation14–16]. High doses of atorvastatin can also be successful in reducing GI bleeding, due to its anti-angiogenic properties [Citation5].
Studies assessing the economic burden associated with major bleeding events and surgeries in patients with VWD have been published [Citation17,Citation18], but the burden of disease-specific to VWD with angiodysplasia has not been evaluated using real-world data. Research highlighting the burden of angiodysplasia in VWD is needed to underscore the unmet need for effective management of the disease. We aimed to understand the healthcare resource utilization (HCRU) and total costs of care in patients with VWD-related angiodysplasia by comparing with two matched control groups: VWD without angiodysplasia and the general population. We also aimed to describe the procedures/treatments used for diagnoses and management (both acutely and preventatively) for VWD-related angiodysplasia in the real-world.
2. Patients & methods
2.1. Study design and population
This was a retrospective, observational study conducted using the Merative MarketScan Commercial and Medicare Databases®, with the study period from 1 January 2011, to 30 September 2020. The database includes de-identified, patient-specific health information of reimbursed healthcare claims from active employees, early retirees, Consolidated Omnibus Budget Reconciliation Act (COBRA) continues, and dependents insured by employer-sponsored health insurance plans in the United States. The database captures utilization and the associated costs of medical (inpatient and outpatient) and pharmacy services, patient enrollment status, and demographics [Citation19]. International classification of Disease codes, Ninth & Tenth Revisions (ICD-9/10), Current Procedural Terminology (CPT) codes, and Healthcare Common Procedure Coding System (HCPCS) codes were used to identify patients and observe outcomes. The study design is illustrated in . Patients were included in the VWD with angiodysplasia group if they had 1) ≥1 medical claims for VWD or low VWF and 2) ≥1 medical claims for angiodysplasia and 3) ≥3 GI-related bleed episodes within a 1-year period (bleed claims that occurred within 7 days from each other were grouped as one episode). The index date was defined as the date of the first GI bleed-related medical claim before or within 30 days of a claim for angiodysplasia; otherwise, the date of the first angiodysplasia-related medical claim was considered as the index date. The study outcomes were measured during the 12-month period following the index date (follow-up period). Further, patients were required to have continuous insurance coverage without a gap longer than 45 days for 6 months before and 12 months after their index date.
Figure 1. Study design schematic: Retrospective observation study conducted using the IBM MarketScan claims databases®.
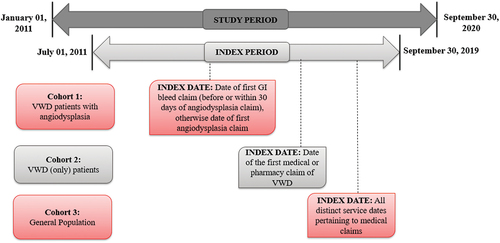
The VWD with angiodysplasia cohort was compared with two matched control groups (VWD and general population) to reduce bias and improve the validity of the statistical estimates. A 1:4 exact matching (without replacement) on age (±5 years), gender, index year (±5 years), and Charlson Comorbidity Index (CCI) score was performed. The VWD control group was required to have a claim for low VWF or VWD during the index period with the date of the first claim determining the index date. For the general population control group, all distinct service dates pertaining to the unique medical claims for a patient within the index period were used to match on index-date. Patients with a claim for VWD or low VWF were excluded from the general population control group. Patients with a diagnosis of angiodysplasia (either a diagnosis or ≥3 episodes of GI bleed within a year) during the study period were excluded from both the control groups. Patients were excluded from the study (reference and control groups) if, during the study period, they had ≥2 claims at least 30 days apart associated with a diagnosis (any position) for hemophilia A, hemophilia B, acquired hemophilia, ulcerative colitis, Crohn’s disease, acquired VWD, or other bleeding disorders.
Due to interest in better understanding the real-world approaches to diagnosing, treating, and preventing VWD with angiodysplasia, we identified patients who met at least one out of the two inclusion criteria for angiodysplasia and separated them into three groups for clinical description; 1) VWD with symptomatic angiodysplasia (VWD diagnosis, angiodysplasia diagnosis, and recurrent GI bleeds), 2) VWD with asymptomatic angiodysplasia (VWD diagnosis, angiodysplasia diagnosis, and no recurrent GI bleeds), and 3) VWD with suspected angiodysplasia (VWD diagnosis, no angiodysplasia diagnosis, and recurrent GI bleeds). Patient characteristics, treatments received, and diagnostic procedure patterns were described for the three groups. This study was exempt from review by an institutional review board (as defined at 45 CFR 46.102(f)(2)) because IBM® provided de-identified data from the IBM MarketScan Claims Databases®, fully compliant with U.S. privacy laws and regulations, that is, the Health Insurance Portability and Accountability Act (HIPAA).
2.2. Study measures
All-cause and treatment-specific HCRU during the follow-up period were quantified in terms of the number of inpatient visits, inpatient hospital days, emergency visits, ambulatory visits (ambulatory care & outpatient hospital on-campus visits), physician visits, other outpatient services, and pharmacy claims. Total all-cause and treatment-specific costs assessed were those covered by payers as well as the out-of-pocket costs for patients (copayment, coinsurance, and deductibles). The treatment-specific costs included costs pertaining to diagnosis and screening-related procedures for VWD and angiodysplasia and pharmacological treatments and procedures for the treatment of bleeding and underlying lesions. Costs were inflated to 2021 United States dollars (USD) using the annual medical care component of the consumer price index (CPI).
We aimed to identify patients who were using VWF for prophylaxis after being diagnosed with angiodysplasia. The definition we used for prophylaxis use of VWF, was a cumulative annual total dosage of more than 3,120 International Unit [IU]/Kilogram [Kg] VWF concentrate (60 IU/kg/week). To calculate the VWF dispensed during the follow-up, all medical and pharmacy claims for VWF concentrate were identified using HCPCS-J and NDC codes. VWF concentrate claims identified within 3 days of any claim for bleed events in the inpatient, outpatient, or emergency department setting were excluded as they were deemed associated with specific bleeding events. The annual dose (IU/Kg) of VWF concentrate was then calculated using the total units of VWF dispensed based on the age-specific national weight average [Citation20,Citation21].
To calculate the number of bleeds, an event was defined using bleeding-related claims, either inpatient or outpatient, grouped according to body location. These claims were captured using ICD-9/10-CM diagnosis codes in any diagnosis position. All bleeding-related medical claims were extracted using previously finalized diagnosis codes and grouped by locations. All claims in the same location within 7 days were grouped as a single bleeding event (Supplementary Table S2). Bleeding events related to GI hemorrhages were described separately. The time to diagnosis of angiodysplasia on or after the diagnosis of VWD was reported for the VWD and angiodysplasia patients in the study period.
2.3. Statistical analysis
Patient characteristics and outcomes measured during follow-up were summarized using descriptive statistics. Patients with and without an outcome of exposure are sampled from an underlying cohort using the exact matching technique such that their distributions of socio-demographic or clinical characteristics are identical [Citation22]. To minimize potential confounding due to sociodemographic and clinical differences (i.e. age [±5 years], gender, index-year [±5 years], and CCI Scores) between the cohorts (i.e. treatment and the control groups), 1:4 exact matching algorithm without replacement was performed. The exact matching algorithm matches every treated unit to four possible control units, based on the values of the covariates, which improves statistical efficiency in adjusted analyses. Exact matches often lead to many individuals not being matched, which can result in a bias. Hence, to get a sizable sample for comparison we allowed ±5 years range for age and index-year [Citation23,Citation24]. The differences in HCRU and costs were compared among the VWD with angiodysplasia cohort, the VWD (only) control group, and the general population control group using ANOVA (Analysis of Variance) for continuous variables and chi-square test for categorical variables. Diagnostic tests, procedures, pharmacologic treatments, and the number of bleeds were described for the VWD and symptomatic angiodysplasia, VWD and asymptomatic angiodysplasia, and VWD and recurrent bleed groups.
3. Results
3.1. Patient inclusion
A total of 35 patients met the VWD with angiodysplasia inclusion criteria for the HCRU and cost comparison with the matched control groups (Supplementary Figure S1). After the 1:4 match, there were 136 patients in both the VWD (only) and general population control groups. The matching procedure resulted in 34 of the 35 VWD with angiodysplasia patients matched to 136 VWD (only) patients and 136 individuals from the general population. The baseline characteristics of the three groups compared are shown in . Most patients (76.5%) were females, and the mean age of patients was 60.0 years in matched individuals from the general population cohort and 61.6 years among VWD with angiodysplasia patients. The CCI did not differ significantly between groups (p-value = 0.1773).
Table 1. Characteristics of VWD with angiodysplasia patients in comparison with matched cohorts of patients with VWD (only) and the general population.
3.2. Matched comparison of all-cause HCRUs and costs
The comparison of mean all-cause HCRUs and costs for the matched control groups is presented in . The number of inpatient hospital days (p-value < 0.001), inpatient visits (p-value < 0.001), emergency visits (p-value = 0.0002) and ambulatory visits (p-value = 0.0045) were significantly higher for the VWD with angiodysplasia cohort as compared to the VWD (only) cohort and the general cohort. The number of pharmacy claims did not differ between the matched cohorts (). The mean total all-cause costs were $150,101 for the VWD with angiodysplasia patients. These were significantly higher compared to $48,249 among patients in the matched VWD (only) cohort and $31,029 among those in the matched general cohort (p-value < 0.0001). The differences in costs between the cohorts were mainly driven by the inpatient costs, which were $106,910 among the VWD with angiodysplasia patients, $16,328 among patients in the matched VWD (only) cohort, and $9,095 among those in the matched general cohort (p-value < 0.0001). Other than costs pertaining to physician’s office visits (p-value = 0.4810) and pharmacy visits (p-value = 0.4869), differences in costs between cohorts for emergency visits (p-value = 0.002), ambulatory visits (p-value = 0.0115) and other outpatient services (p-value = 0.0481) were statistically significant.
Figure 2. All-cause HCRU components for VWD and angiodysplasia cohort, VWD (only) cohort, and general cohort over a 12-month follow-up period.
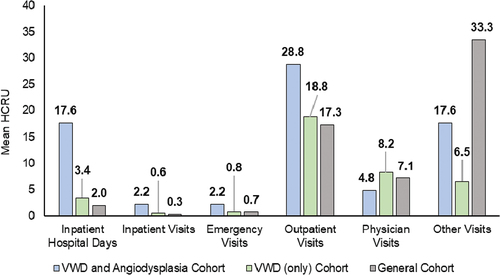
Table 2. All-cause HCRU and costs during the one-year follow-up period for VWD with angiodysplasia patients in comparison with matched patients of the VWD (only) and the general population cohorts.
3.3. Clinical description of VWD and angiodysplasia
A total of 494 patients with VWD were identified for clinical description with 35 patients classified as symptomatic angiodysplasia, 81 patients with asymptomatic angiodysplasia, and 378 patients with suspected angiodysplasia. The socio-demographic and clinical characteristics of patients with VWD and symptomatic, asymptomatic, and suspected angiodysplasia are described in . In each sub-group, roughly 76% of the patients were females and the majority belonged to 18–64 age-group. Among VWD patients with symptomatic, asymptomatic, and suspected angiodysplasia, the mean age was 59.9, 56.2, and 40.7 years, and the mean CCI score was 2.6, 1.4, and 1.0, respectively. Among the patients classified as symptomatic or asymptomatic angiodysplasia who had a diagnosis of angiodysplasia on or after a diagnosis of VWD, the mean ± SD (median) time from VWD diagnosis to angiodysplasia was 453 ± 496 (249) days.
Table 3. Characteristics of patients included in the clinical and diagnostic investigation of VWD and angiodysplasia.
The treatment and diagnostic-related procedures among the three groups of patients are described in . The percentage of patients with symptomatic angiodysplasia who underwent diagnosis-related procedures was higher compared to those with asymptomatic or suspected angiodysplasia. In the symptomatic angiodysplasia cohort, 14 (40%) patients had claims for video capsule endoscopy, whereas 8 (22.9%) and 3 (8.6%) patients had claims for enteroscopy and angiography, respectively. Among all the VWD and angiodysplasia sub-cohorts, desmopressin, VWF concentrates, and aminocaproic acid were the most frequently used treatments. Only one patient (1.2%) of the 81 patients classified as asymptomatic angiodysplasia received aminocaproic acid. Tranexamic acid was used in 15 (4.0%) patients classified as suspected angiodysplasia and in one patient (1.2%) classified as asymptomatic angiodysplasia. Bevacizumab was used in one VWD patient of the suspected angiodysplasia group (0.3%) and none of those in symptomatic or asymptomatic groups. The most frequent procedures to treat GI-related bleeding and underlying lesions were blood transfusion (20% for symptomatic angiodysplasia, 9.9% for asymptomatic angiodysplasia, and 5.8% for suspected angiodysplasia) and laser therapy (8.6% for symptomatic angiodysplasia, 3.7% for asymptomatic angiodysplasia, and 5.3% for suspected angiodysplasia). Resection of GI tract was identified in two patients with asymptomatic angiodysplasia (2.5%), three patients with suspected angiodysplasia (0.8%), and none of those with symptomatic angiodysplasia. The VWF dose dispensed during the one-year follow-up was higher among patients with symptomatic angiodysplasia (529.0 IU/kg/year) as compared to those with asymptomatic (345.0 IU/kg/year) or suspected angiodysplasia (200.1 IU/kg/year) and none of the patients from the three cohorts were on VWF for prophylaxis. The billed annualized bleed rates during follow-up (overall and GI hemorrhages) for the symptomatic angiodysplasia, asymptomatic angiodysplasia, and suspected angiodysplasia were 4.2 and 4.1, 0.8 and 0.6, and 4.2 and 3.8, respectively ().
Table 4. Treatment and procedures among patients included in the clinical and diagnostic investigation of VWD and angiodysplasia.
4. Discussion
Although the economic burden associated with major bleeding events and surgeries in patients with VWD have been documented in the literature [Citation17,Citation18], there is limited real-world evidence about the burden of disease in VWD patients with angiodysplasia. To the best of our knowledge, this analysis is the first real-world study using a large geographically representative US healthcare claims database, observed from 2011 to 2020, which identified patients with VWD and angiodysplasia in real-life routine care setting. This analysis found significantly higher rates of hospitalizations, inpatient hospital days, emergency visits, and ambulatory visits (p-value < 0.05) in the VWD with angiodysplasia patients compared to the matched VWD (only) patients and general population. As a result, all-cause total costs were significantly higher for patients with VWD and symptomatic angiodysplasia compared to matched VWD (only) and general population cohorts (mean costs of $150,101 versus $48,249 and $31,029, respectively; p-value < 0.0001), underscoring the need for more effective identification, treatment, and prevention of GI bleeding in this population. The cost and HCRU results can be used for cost-effectiveness models to estimate the costs of disease for patients who experience VWD and angiodysplasia.
In addition to quantifying the economic and HCRU burden in the VWD and angiodysplasia population, we also investigated the clinical journey of these patients to better understand the diagnostic, procedural, treatment, and prevention approaches that were used. Patients who met one out of the two inclusion criteria for angiodysplasia were included in the clinical description to better understand the procedures used in the undiagnosed angiodysplasia patients and to describe the patients diagnosed with angiodysplasia who did not present with recurrent GI bleeding symptoms. The most common diagnostic-related procedures were upper and lower GI screening, colonoscopy, esophagogastroduodenoscopy, and video capsule endoscopy. The VWD with symptomatic angiodysplasia group had higher rates of all diagnostic procedures, and many had multiple diagnostic procedures. This may signal an opportunity to identify diagnostic tests that are effective in finding undiagnosed angiodysplasia patients. Additionally, the majority of the patients with VWD and angiodysplasia received pharmacological treatments during the follow-up period, such as desmopressin, VWF concentrates, and aminocaproic acid. Only one patient received bevacizumab from the VWD and suspected angiodysplasia cohort. Blood transfusion and VWF were used in 20% and 14% of VWD and symptomatic angiodysplasia cohort, respectively. The use of VWF concentrate for long-term prophylaxis is conditionally suggested by the American Society of Hematology (ASH), the International Society on Thrombosis and Haemostasis (ISTH), the National Hemophilia Foundation (NHF), and the World Federation of Hemophilia (WFH) guidelines for the management of VWD for patients with frequent and severe bleeds [Citation25]. However, no patient from the three cohorts was identified to be on routine prophylaxis, i.e. having an annual total dosage of more than or equal to 3,120 IU/kg, during the follow-up period. Patients with symptomatic angiodysplasia also experienced GI bleeding during the follow-up more frequently than those with VWD and asymptomatic angiodysplasia (mean of 4.1 versus 0.6). The rate of GI-bleed was however similar between VWD patients with symptomatic angiodysplasia and suspected angiodysplasia (mean of 4.1 versus 3.8). The VWD and suspected angiodysplasia group had similar rates of GI bleeding compared to the VWD and symptomatic angiodysplasia, likely due to meeting the inclusion criteria of having recurrent GI bleeds. A relatively short time period included in the study (March–September 2020) was impacted by COVID-19 pandemic, and the HCRU during this time period may be different as compared to other time periods; however, since this would impact both the treatment and control groups in a similar manner, the differential bias would be minimal.
A key strength of this study is the use of a large claims database, which provided sufficient number of patients to study this rare disease with respect to healthcare resource use and costs. The longitudinal nature of the data allowed us to identify VWD and angiodysplasia patients and observe related treatment, diagnostic, and procedure patterns over time. Additionally, our results of mean total all-cause costs ($48,249) among VWD (only) patients are consistent with a 2008–2018 study using the US IBM Health MarketScan® database reporting the 12-month adjusted mean total costs in VWD to be $50,734 [Citation18]. Patients with VWD might have a higher burden of indirect costs owing to substantial productivity loss [Citation26]. These results, however, are not included in the current analysis.
Our study has limitations, mainly those inherent to studies that are based on claims databases. For instance, variations in coding and billing practices may lead to inconsistency in diagnosis and procedure codes. This translates to a potential risk for misclassification of patients. Comorbidities, medications, bleeding events, or procedures may not be identified if the associated medical services are not claimed to insurers, or if bleeds are entirely managed at home. As we described the treatments dispensed to patients with VWD and angiodysplasia using pharmacy and medical claims, there is no information about the medications administered during inpatient admissions. The clinical characteristics of a patient prior to the assessment period may have affected the outcomes in the assessment period and may have given rise to history bias. Although the IBM® MarketScan® Claims Databases covers geographically diverse US regions with a broad range of ages, the results may not be generalizable to the entire US population of VWD and angiodysplasia patients. Due to the absence of ICD codes for specific subtypes of VWD during the study period, and the lack of clinical parameters in the administrative claims database, our study findings could not be accurately adjusted for disease severity.
5. Conclusions
This study found that patients with VWD and angiodysplasia were predominantly adult females with high clinical burden and healthcare resource use due to recurrent bleeding episodes. There was evidence of substantial economic and clinical burden, primarily driven by hospitalizations. Further, the diagnostic journey for patients and healthcare use seems to be variable and may benefit from a more structured and empiric understanding of how patients with VWD and angiodysplasia are diagnosed and treated. Despite the recent therapeutic advances, there is an opportunity for considerable reduction of the burden of hospitalizations in VWD patients with angiodysplasia and more evidence is needed to better understand the most appropriate clinical guidance.
Article highlights
Patients with von Willebrand disease (VWD) and Angiodysplasia (AGD) had significant healthcare resource utilization (HCRU) and clinical burden from recurrent bleeding episodes.
There was a substantial financial burden that was predominantly driven by hospitalizations.
Most common diagnostic-related procedures were upper and lower gastrointestinal (GI) screening, colonoscopy, esophagogastroduodenoscopy, and video capsule endoscopy.
The majority of the patients with VWD and angiodysplasia received pharmacological treatments during the follow-up period, such as desmopressin, VWF concentrates, and aminocaproic acid.
There may be an opportunity to reduce healthcare costs and burden of illness for VWD and AGD patients by improving the identification, treatment, and prevention of recurrent GI bleeding.
Declaration of interest
The authors were supported by Takeda Pharmaceuticals U.S.A., Inc., Lexington, MA, U.S.A. N Connell participates in an advisory board and is a consultant to Takeda Pharmaceuticals U.S.A., Inc., Lexington, MA, and current holder of equity in publicly traded companies. J Caicedo, M Bullano, N Nieto, and B Sschultz are employees of Takeda Pharmaceuticals U.S.A., Inc., Lexington, MA, U.S.A, and current holders of individual Takeda stock/stock options. A Hait and A Gupta are employees of Complete HEOR Solutions which has received financial compensation from Takeda for conducting the study analysis. S Chatterjee was an employee of Complete HEOR Solutions when the study was conducted, which has received financial compensation from Takeda Pharmaceuticals U.S.A., Inc. for conducting the study analysis. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
N Connell, J Caicedo, M Bullano, and B Schultz conceptualized and designed the study. N Nieto, S Chatterjee, A Hait, and A Gupta contributed to the data analysis and interpretation of results. All authors contributed to revising the paper critically for intellectual content. All authors approved the final version to be published and agreed to be accountable for all aspects of the work.
Presentation
The abstract and poster of « Real-World Analysis of Healthcare Resource Utilization and Costs Among Patients Diagnosed with Von Willebrand disease and Angiodysplasia » was published by the American Society of Hematology in November 2022
Supplemental Material
Download MS Word (284.9 KB)Acknowledgments
Medical writing support was provided by Martin Senecal, MS, of Complete HEOR Solutions, North Wales, PA, USA, and was funded by Takeda Pharmaceuticals U.S.A., Inc., Lexington, MA, USA.
Data availability statement
The data that support the findings of this study are available from Merative Marketscan Research Databases. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the corresponding author ([email protected]) with the permission of Merative Marketscan Research Databases.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14737167.2023.2211270
Additional information
Funding
References
- Mannucci P. New therapies for von Willebrand disease. Hematol 2014 Am Soc Hematol Educ Program Book. 2019 Dec 06;2019(1):590–595.
- Springer T. von Willebrand factor, Jedi knight of the bloodstream. Blood J Am Soc Hematol. 2014 Aug 28;129(9):1412–1425.
- Mannucci P, Franchini M. Laboratory monitoring of replacement therapy for major surgery in von Willebrand disease. Haemophilia. 2017 Mar;23(2):182–187.
- Mannucc PM. Treatment of von Willebrand disease. Thromb Haemost. 2001;86(07):149–153.
- Selvam S, James P. Angiodysplasia in von Willebrand disease: understanding the clinical and basic science. Semin Thromb Hemost. 2017;43(06):572–580.
- Aghighi M, Taherian M, Sharma A. Angiodysplasia. 2019.
- Ramsay D, Buist T, Macleod D, et al. Persistent gastrointestinal bleeding due to angiodysplasia of the gut in von Willebrand’s disease. Lancet. 1976;308(7980):275–278.
- Makris M, Federici A, Mannucci P, et al. The natural history of occult or angiodysplastic gastrointestinal bleeding in von Willebrand disease. Haemophilia. 2015;21(3):338–342.
- Dray X, Camus M, E A Coelho J. Treatment of gastrointestinal angiodysplasia and unmet needs. Digestive Liver Dis. 2011;43(7):515–522.
- Rodrigues J, Chivia J, Figueiredo P. Fleeting Angiodysplasia. GE-Portuguese J Gastroenterol. 2018;25(4):203–204.
- Kuhle W, Sheiman R. Detection of active colonic hemorrhage with use of helical CT: findings in a swine model. Radiology. 2003;228(3):743–752.
- Raju G, Das GLA, Lewis B. American Gastroenterological Association (AGA) Institute medical position statement on obscure gastrointestinal bleeding. Gastroenterology. 2007;133(5):1694–1696.
- Vargo JJ. Clinical applications of the argon plasma coagulator. Gastrointest Endosc. 2004;59(1):81–88.
- Franchini M, Mannucci P. Von Willebrand disease‐associated angiodysplasia: a few answers, still many questions. Br J Haematol. 2013 Apr;161(2):177–182.
- Chen H, Ge Z, E A Liu W. The mechanisms of thalidomide in treatment of angiodysplasia due to hypoxia. Zhonghua Nei Ke Za Zhi. 2009;48(4):295–298.
- Eghbali A, Melikof L, Taherahmadi H, et al. Efficacy of tranexamic acid for the prevention of bleeding in patients with von Willebrand disease and Glanzmann thrombasthenia: a controlled, before and after trial. Haemophilia. 2016 Sep;22(5):e423–6.
- Lu M, Oladapo A, Wu Y, et al. Economic burden of major bleeding events in commercially insured patients with von Willebrand disease based on claims data from the United States. J Managed Care Specialty Pharm. 2021;27(2):175–185.
- Oladapo A, Wu Y, Lu M, et al. Economic burden associated with major surgery in patients with von Willebrand disease: a United States retrospective administrative database analysis. J Blood Med. 2021;12:699–708.
- IBM, [Online]. Ibm. Available from: https://www.ibm.com/products
- Ogden C, Kuczmarski R, E A Flegal K. Centers for disease control and prevention 2000 growth charts for the United States: improvements to the 1977 national center for health statistics version. Pediatrics. 2002;109(1):45–60.
- Fryar CD, Kruszon-Moran D, Gu Q, et al. Mean body weight, weight, waist circumference, and body mass index among adults: United States, 1999–2000 through 2015–2016. Natl Health Stat Report. 2018;(122):1–16.
- Iwagami M, Shinozaki T. Introduction to matching in case-control and cohort studies. Annals of Clin Epidemiol. 2022;4(2):33–40.
- Yoon S, Shin J, Heo S, et al. Irritable bowel syndrome and subsequent risk of Parkinson’s disease: a nationwide population-based matched-cohort study. J Neurol. 2022;269(3) :1404–1412.
- Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci a Rev J Inst Math Stat. 2010;25(1). DOI:10.1214/09-STS313
- James P, Connell N, Ameer B, et al. McRae S. ASH ISTH NHF WFH 2021 guidelines on the diagnosis of von Willebrand disease. Blood Adv. 2021;5(1):280–300. DOI:10.1182/bloodadvances.2020003265
- Schinco P, Cultrera D, Valeri F, et al. Cost–consequence analysis of long-term prophylaxis in the treatment of von Willebrand disease in the Italian context. Clinicoecon Outcomes Res. 2014;7:17–25.
